The immune profiles and“minimizing tacrolimus”strategy for long-term survival recipients after liver transplantation
2021-05-19JunJunJiaShiYuZhangJunYuHaiYangXieLinZhouShuSenZheng
Jun-Jun Jia ,Shi-Yu Zhang ,Jun Yu ,Hai-Yang Xie ,Lin Zhou ,Shu-Sen Zheng ,∗
a Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310 0 03, China
b NHC Key Laboratory of Combined Multi-organ Transplantation, Hangzhou 310 0 03, China
TotheEditor:
Liver transplantation (LT) has become a major and effective therapeutic approach for end-stage liver disease [1].However,10-year graft and patient survival rates remained low with 54% and 61%,respectively [2].Improving the outcome of long-term LT has become a major focus of the transplantation community.
Tacrolimus,a calcineurin inhibitor with strong immunosuppressive ability,has become the cornerstone immunosuppressant in most management protocols [3].Long-term use of tacrolimus can affect kidney function,induce diabetes and hypertension,and increase the risk of cancer and infection.Among them,nephrotoxicity is the most serious complication [4].A recent study [5]showed that after LT,a long-term lower tacrolimus concentration (<3 ng/mL) compared with the recommended drug concentration (>5 ng/mL) will not increase the incidence of immune rejection and mortality.Thus,an accurate and reliable method is needed to guide“minimizing tacrolimus”strategy.
In this study,we enrolled 85 liver transplant patients and all patients received tacrolimus based immunosuppressant regimen.The detail of this immunosuppressant regimen referred to our previous publication [6].According to the follow-up time after LT,they were divided into three groups,which were 1 year (group 2,n=20),5 years (group 3,n=20) and 10 years (group 4,n=45)after LT,and healthy subjects as control (group 1,n=20).Five mL blood was collected from each participant,and the peripheral immune cells were detected by flow cytometry.This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine (2017-595).All participants provided written informed consent forms.Statistical analysis was performed using SPSS 17.0 (SPSS Inc.,Chicago,IL,USA).APvalue<0.05 was considered statistically significant.
After LT,the average blood concentration of tacrolimus declined gradually with the prolonged survival time of patients (Fig.1).At the same time,the proportions of CD3+T,CD4+T and natural killer T cells in the peripheral blood of patients after LT (groups 2-4) were lower than those of healthy subjects (group 1).The dynamic changes of the proportions of CD19+B cells,natural killer cells,Treg cells and Breg cells in peripheral blood of LT patients were similar,which gradually increased as survival time extending(Fig.1).Particularly,the ratio of CD19+B cells,natural killer cells and Treg cells was significantly increased in group 4 compared to group 1 (P<0.05),while the proportion of Breg cells in group 2 was significantly lower than that of group 1 (P<0.05) (Fig.1).
The patient surviving over 10 years after LT (group 4) was defined as a long-term survival recipient,and 45 subjects were enrolled.The average blood concentration of tacrolimus in group 4 was 2.3 ng/mL.Thus,45 subjects were divided into two subgroups based on a cut-off value of 2.3 ng/mL.Twenty-two patients (48.9%)had a tacrolimus concentration of less than 2.3 ng/mL and were assigned to group 4.1,while 23 (51.1%) patients had a concentration of greater than 2.3 ng/mL and were classified as group 4.2.The liver function,peripheral immune profiles and tacrolimusrelated side effects of two subgroups were listed in Table 1,which were not statistically different between two groups (P>0.05).This showed that tacrolimus concentration within 2.3 ng/mL seems safe and practical for long-term survival patients.
Tacrolimus is a double-edged sword,preserving the viability of liver allograft while also bringing side effects [7,8].Immune cell subsets are one of the most important indicators to monitor the immune function.In this study,compared with group 1,the proportion of T-cell subsets in LT patients was obviously lower,which may be due to the targeted inhibitory effect of tacrolimus on T cells.It is notable that the level of CD3+and CD4+T cells in the long-term survival group (group 4) was decreased,which probably was due to the cumulative side effects of long-term use of tacrolimus.What is more,Treg cell is a negative immune regulatory cell and has been proved to play a critical role in the development and maintenance of operational tolerance [9].In our study,the ratio of Treg cells was increased gradually and reached its peak in long-term survival group,which was consistent with its negative immunomodulatory function.The proportions of CD19 +B cells and natural killer cells were gradually ascended following LT,which suggests that these two subgroups may play a protective role in LT recipients,and have an important role in long-term graft survival and even the formation of immune tolerance.Besides,the proportion of Breg cells was firstly reduced and then returned to normal levels in long-term survival group,which kept in line with the results in renal transplantation clinical trials [10].
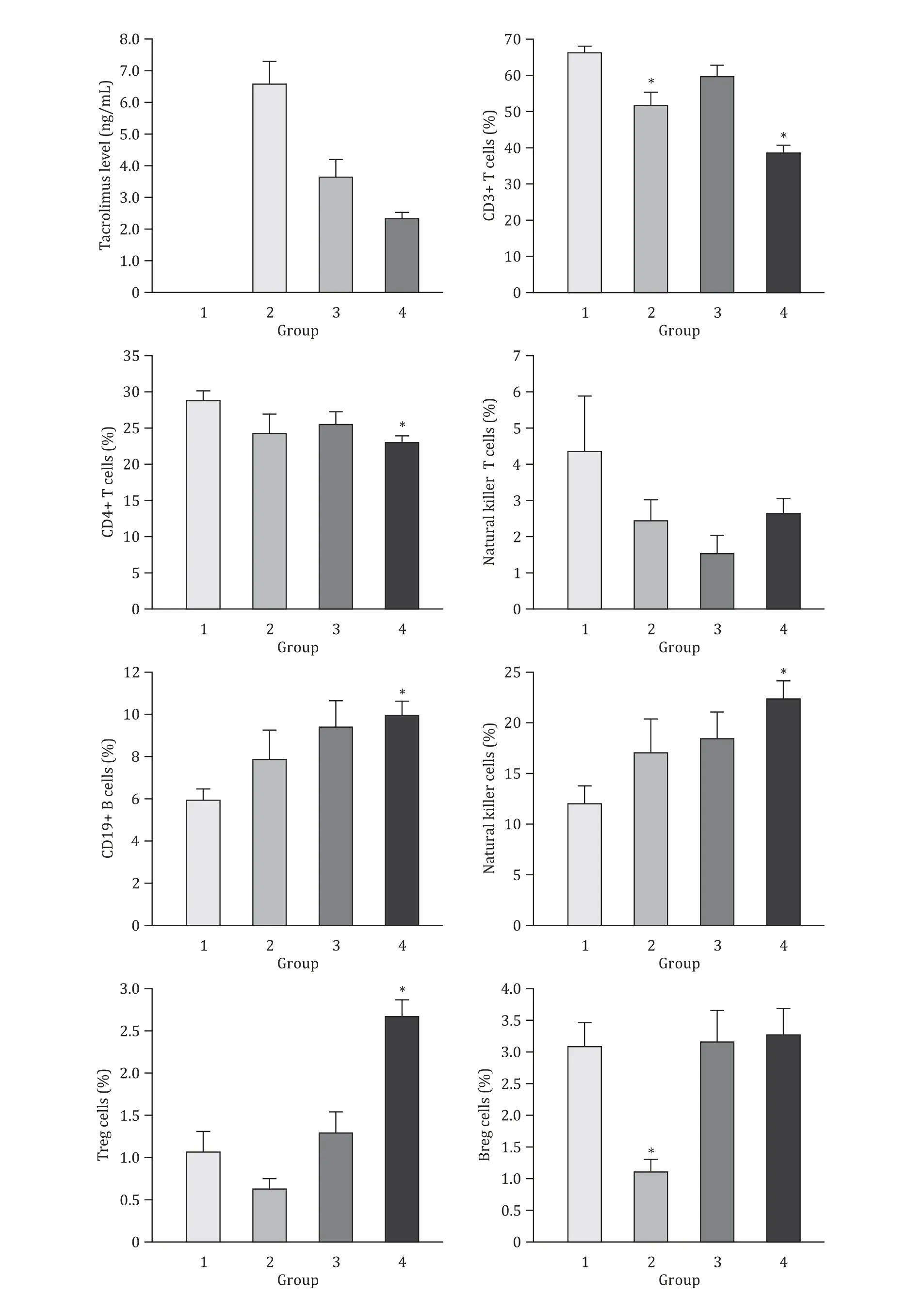
Fig.1.The dynamic changes of peripheral immune cells after liver transplantation.Group 1:healthy subjects;group 2:1 year after liver transplantation;group 3:5 years after liver transplantation;group 4:10 years after liver transplantation.∗P < 0.05,vs.group 1.

Table 1 The liver function,immune status and complications of two subgroups.
“Minimizing tacrolimus”strategy is still in the exploration stage.Our previous study proved that it is safe and feasible to reduce the concentration of tacrolimus from 10–15 ng/mL to 5–10 ng/mL within the first month after LT [6].The present study demonstrated that,for long-term surviving recipients,the“minimizing tacrolimus”strategy of less than 2.3 ng/mL may be practicable.However,more clinical data are needed to support and confirm the conclusion.
Acknowledgments
None.
CRedit authorship contribution statement
Jun-Jun Jia:Funding acquisition,Methodology,Writing -original draft,Writing -review &editing.Shi-Yu Zhang:Data curation,Methodology,Writing -original draft.Jun Yu:Data curation,Writing -review &editing.Hai-Yang Xie:Investigation,Writing -review &editing.Lin Zhou:Conceptualization,Writing -review&editing.Shu-Sen Zheng:Conceptualization,Writing -review &editing.
Funding
This study was supported by grants from Zhejiang Natural Science Foundation (LQ20H030 0 05) and Zhejiang Health Technology Project (2020KY126 and 2019RC153).
Ethical approval
This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine (2017-595).Written informed consent was obtained from all individual participants included in the study.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Practice of precision surgery in primary liver cancer
- Reporting of longitudinal pancreatojejunostomy with partial pancreatic head resection (the Frey procedure) for chronic pancreatitis:A systematic review
- Hepatobiliary&Pancreatic Diseases International
- Presentation and surgical management of xanthogranulomatous cholecystitis
- Transjugular intrahepatic portosystemic shunt is effective in patients with chronic portal vein thrombosis and variceal bleeding
- Long-term follow-up of HCV patients with sustained virological response after treatment with pegylated interferon plus ribavirin
