Long-term follow-up of HCV patients with sustained virological response after treatment with pegylated interferon plus ribavirin
2021-05-19YuanJiMaLingYaoDuLiBoYanJuanLiaoXingChengWuWeiXieHongTang
Yuan-Ji Ma,Ling-Yao Du,Li-Bo Yan,Juan Liao,Xing Cheng,Wu-Wei Xie,Hong Tang
Center of Infectious Diseases, West China Hospital of Sichuan University, Chengdu 610041, China
Keywords:Hepatitis C virus Sustained virological response Hepatocellular carcinoma
ABSTRACT Background: The progress of liver diseases may not stop after viral eradication.This study aimed to provide data on long-term prognosis of patients with hepatitis C virus (HCV) infection who underwent pegylated interferon plus ribavirin (PR) regimen and achieved a sustained virological response 24 weeks post-treatment (SVR24).Methods: Responders to the PR regimen in our hospital from January 2011 to June 2014 were enrolled and prospectively followed up.Baseline characteristics were profiled.The incidence of hepatocellular carcinoma (HCC),progression of liver disease (increase in liver stiffness or occurrence of decompensated complication),and HCV recurrence was all monitored.The accumulative and annualized incidence rates(AIRs) of these adverse events were analyzed,and the risk factors were also examined.Results: In total,151 patients reached a median follow-up time of 103 weeks.Among them,two had an incidence of HCC during the surveillance with AIR of 0.68% (95% CI:0.00-1.63%).Six patients showed progression of liver disease with AIR of 2.05% (95% CI:0.42%-3.68%).Three patients who had risky behaviors encountered HCV reinfection.The cirrhotic patients faced higher risk of poor prognosis than non-cirrhotic patients,including HCC and progression of liver disease (AIR:6.17% vs.1.42%,P=0.039).Conclusions: The incidence of HCC and progression of liver disease was evident in PR responders during the long-term follow-up period,but the risk level was low.Cirrhotic responders were more vulnerable to develop HCC post SVR24 compared with non-cirrhotic ones.HCV recurrence was rare in responders with SVR24 who had corrected their risky behaviors.
Introduction
Pegylated interferon (Peg-IFN) plus ribavirin (PR) regimen used to be the standard therapeutic option for patients with chronic hepatitis C (CHC) in China since the beginning of this century [1].A previous study reported that the therapeutic efficacy of the PR regimen in patients with IL-28 CC genotype reached 80% or more [2].Most patients could achieve a sustained virological response (SVR) if a full therapeutic course was completed [3].
However,the progression of liver disease may not stop after viral eradication,especially in patients with advanced cirrhosis.A previous study also revealed that even in non-cirrhotic patients,risks still existed after an SVR was achieved [4].Therefore,patients with viral eradication are recommended to receive longterm hepatocellular carcinoma (HCC) surveillance.However,the data of long-term follow-up in treated Chinese patients are still lacking.Further investigations are needed in both cirrhotic and non-cirrhotic patients.
In this study,we reported the long-term prognosis of hepatitis C virus (HCV)-infected patients who received a full course of PR treatment and achieved SVR 24 weeks post-treatment (SVR24).
Methods
Study subjects
All subjects who were diagnosed with CHC through a positive serum HCV RNA test and had received PR regimens from January 2011 to June 2014,were recorded and prospectively followed up.The subjects who completed a full therapeutic course and achieved an SVR24 were screened.Of the PR regimens,Peg-IFNα−2a or Peg-IFNα−2b was prescribed according to physical condition and health insurance of patients and availability of drugs.Sanger sequencing with PCR kit (Daan gene,Guangzhou,China)was applied and performed on ABI9700 (Life technologies,Carlsbad,USA) to identify HCV genotype.The SVR24 was defined if the high-precision HCV RNA test was negative through“The COBASR○AmpliPrep/COBASR○ TaqManR○ HCV Test”(Roche Diagnostics,GmbH,Mannheim,Germany) 24 weeks after treatment.Then,a long-term follow-up was conducted every year to learn their prognosis.Written informed consent (Version:2016.03) was acquired from all the patients.This study was approved by the Ethics Committee of West China Hospital [Authorization Number:2016 annual audit (91)].

Fig.1.The study flow diagram to identify the cohort of long-term follow-up.SVR24:sustained virological response 24 weeks post treatment.
Study endpoints
The primary endpoint of this study was the long-term prognosis,including the incidence of HCC,the progression of liver disease(increase in liver stiffness or occurrence of decompensated complication),and HCV recurrence.The secondary endpoint was the difference in long-term prognosis between cirrhotic and non-cirrhotic patients.
HCC was identified by yearly follow-up and tracing the source medical record.The first recorded date of HCC after SVR was considered the incident date.The liver stiffness was evaluated through either transient elastography using FibroScanR○or aspartate aminotransferase (AST)-to-platelet ratio index (APRI) score every year.The increase of FibroScanR○value from<12.4 kPa to>12.4 kPa or APRI from<2.0 to>2.0 was defined as an“increase in liver stiffness”[5].The increase in liver stiffness or the occurrence of cirrhosis-related complications other than HCC was defined as progression of liver disease.
The follow-up ended when HCC was diagnosed,the patient died,or HCV RNA was positive.Data were collected until June 1,2017.
Statistical analysis
Baseline characteristics were analyzed in all cohorts to give a profile of CHC patients.The incidence of HCC,progression of liver disease,and HCV recurrence were compared between cirrhotic and non-cirrhotic patients.
All data were analyzed using SPSS 18.0 (IBM SPSS Statistics,Chicago,IL,USA).The enumeration data were expressed as percentages and analyzed with the Chi-square test or Fisher’s exact test.The measurement data were analyzed for normality first.Normally-distributed data were expressed as mean ± standard deviation (SD) and analyzed with the Student’st-test,while nonnormally-distributed data were expressed as median (interquartile range,IQR) and analyzed with theUtest.A comparison between normally-distributed data and non-normally-distributed data was also conducted via theUtest.The relationship between different indicators was analyzed with correlation or regression analysis.APvalue<0.05 was considered statistically significant.
Results
Baseline characteristics of the patients
Among 220 subjects,41 encountered relapse before achieving SVR24,28 lost to follow up,151 finished long-term follow up (Fig.1).The baseline characteristics of the 151 subjects were shown in Table 1 .Half of them were males.The average age at the initial treatment was 46.0 ± 13.3 years.They were infected by various genotypes of HCV.Among them,genotype 1b HCV was the majority (76.2%),and the viral genotype could not be identified in two patients.The median baseline viral load was 6.02 (IQR 4.86–6.66) logIU/mL,while the baseline alanine aminotransferase (ALT)was 54 (IQR 31–109) IU/L.Although alpha-fetoprotein (AFP) level was elevated in 21.9% of the patients,HCC was excluded through abdominal ultrasound.
All patients were divided into two groups according to their liver stiffness,which was measured by FibroScanR○,or elevated APRI (APRI>2.0).Among them,38 patients (25.2%) were identified as cirrhotics (Table 1).The sex distribution in the two groups showed no significant difference (55.3% vs.50.4%,P=0.607).The cirrhotic patients were significantly older (51.4 ± 8.2 vs.44.1 ± 14.2 years,P<0.001).The proportion of genotype 1b in cirrhotic patients was significantly higher than that in non-cirrhotic patients (92.1% vs.70.8%,P=0.030).ALT,antinuclear antibody,AFP,platelet and neutrophil levels were also significantly different between the two groups (Table 1).
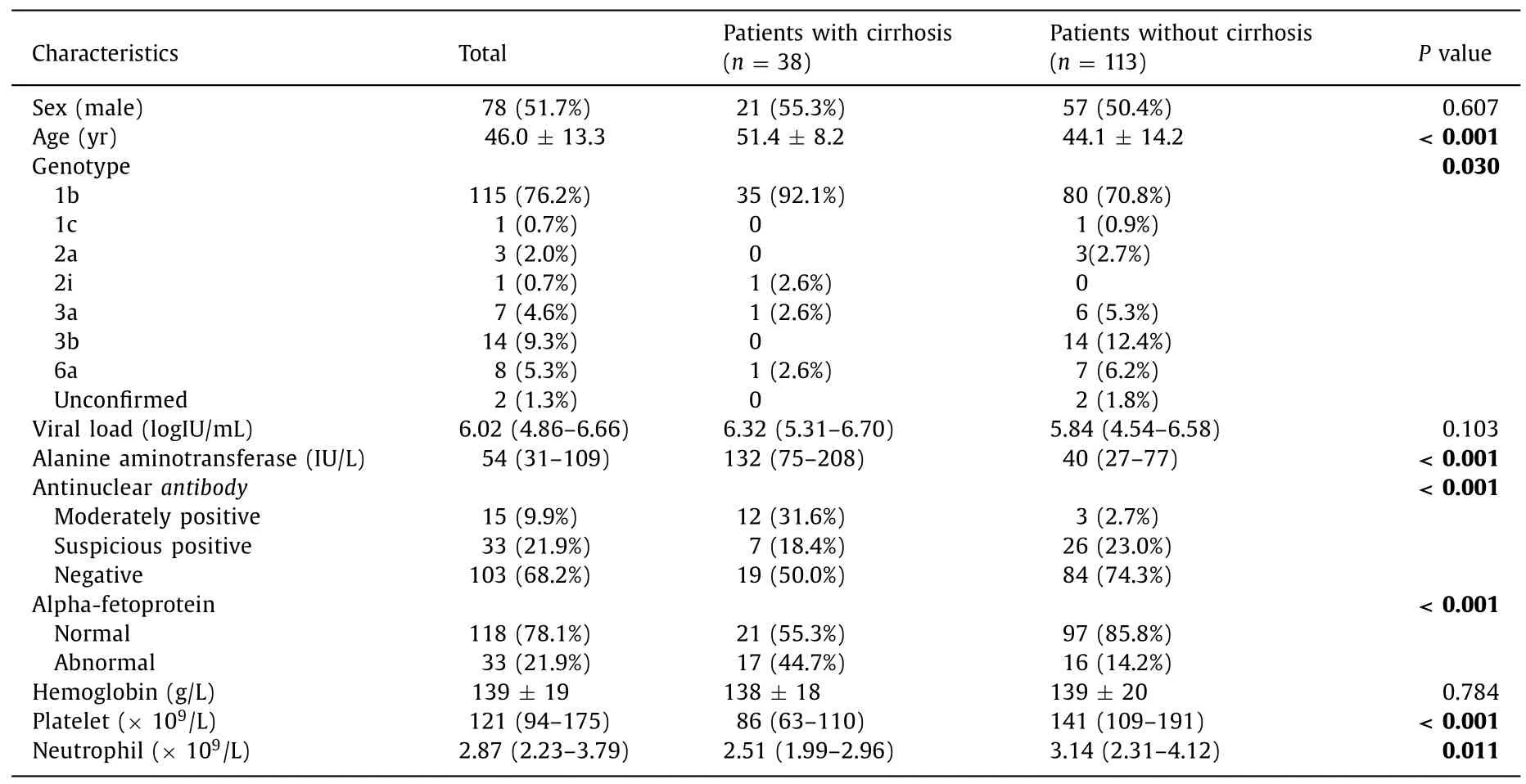
Table 1 Baseline characteristics of the study cohort.

Table 2 The annualized incidence rate (AIR) of adverse events in patients under long-term surveillance after SVR24.

Table 3 Possible risk factors related to HCV reinfection.
Long-term follow-up
Of the recorded 151 patients,38 were cirrhotic responders and 113 were non-cirrhotic ones.The median follow-up time was 103(IQR 77–126) weeks.Two patients had incident HCC during the long-term surveillance.The annualized incidence rate (AIR) of HCC in our cohort was 0.68% (95% CI:0.00 −1.63%) (Table 2).Six patients showed progression of liver disease with AIR of 2.05% (95%CI:0.42% −3.68%).Three patients encountered HCV reinfection with AIR of 1.02% (95% CI:0.00–2.18%).
The AIRs of HCC and progression of liver disease were 0.66%and 1.32% in the patients under the first follow-up year after SVR24,respectively.In the patients under the second follow-up year after SVR24,the AIRs of HCC and progression of liver disease were both 0.93%.In addition,no incidence of HCC was observed in patients followed up for more than 3 years.It may imply that after SVR24 was achieved,the patients would be at a relatively low risk of HCC (Table 2).
According to a previous study,HCV relapse was rare after successful SVR achievement [6].In our cohort,we observed no relapse but 3 cases of HCV reinfection in the long-term follow-up.We retrospectively reviewed the baseline characteristics and therapeutic course in these three patients and found that all had the risk factors of reinfection,among which one had hemophilia and multiple blood transfusions,one had drug injection,and one had risky sexual behavior (Table 3).

Table 4 The annualized incidence rate (AIR) of liver deterioration in cirrhotic patients and non-cirrhotic patients.
As no HCV relapse was found,only the incidence of HCC and progression of liver disease were calculated respectively in cirrhotic and non-cirrhotic patients (Table 4).In addition,we analyzed AIR to balance the difference of follow-up period in the two groups.The AIR of HCC was low in both groups without significant difference (1.24% vs.0.47%,P=0.447).However,the progression of liver disease was different,with marginal significance (4.94% vs.0.94%,P=0.051).Moreover,when the incidence of HCC and progression of liver disease were analyzed together,patients in the cirrhotic groups were more likely to encounter liver deterioration(HCC or progression of liver disease) even after HCV elimination(6.17% vs.1.42%,P=0.039;Table 4).These results implied that although the incidence was low after SVR,the long-term prognosis was still different between cirrhotic and non-cirrhotic patients.
Discussion
HCV-related liver cirrhosis occurs at a rate of 2% −30% depending on age,sex,and transmission route if HCV infection is left untreated [7,8].In addition,once liver cirrhosis is confirmed,the morbidity of the decompensated cirrhosis is 3% −4% yearly and that of HCC is 2% −4% yearly [9].Both statuses are associated with high mortality.Antiviral therapy is aimed to eradicate HCV,ease liver damage,and stop disease progression.
However,eradication of the virus sometimes did not result in a real cure of the disease,and the patients faced a risk of disease deterioration afterward.A retrospective study in Japan showed that the ten-year accumulative incident risk of HCC was up to 4.3% in patients with diabetes mellitus or elevated fibrosis-4 index after SVR was achieved through interferon-based regimens [10].Consistently,we also observed some incident HCC cases.Our accumulative AIR of HCC was relatively lower than the previous reports [9,10].However,when the incidence of progression of liver disease was simultaneously calculated,the accumulative AIR of liver deterioration (HCC or progression of liver disease) was 2.73%.Another large-scale observational study also implied that the risk of HCC remained elevated for several years after SVR12 was achieved through an interferon-based treatment [11].The annual risk of HCC incidence was about 0.3% [11].In our study,the risk of HCC incidence was 0.66% in the first year after SVR24 and 0.93% in the second year,and no more cases were found in the remaining years which may due to limited sample size.However,progression of liver disease was still found in the third and fourth years.
Previous studies [9-11]highlighted that patients with cirrhosis,diabetes or who are elderly have a higher risk of HCC incidence.In addition,cirrhosis is an independent risk factor of HCC incidence and disease progression.It was reported that patients with HCVinduced cirrhosis have an annual risk of 1% for HCC and 2% for disease progression [12].The present study compared the longterm prognosis between cirrhotic and non-cirrhotic patients.Although the incident risk was not significantly different in the two groups,an elevated incidence of liver deterioration (HCC or progression of liver disease) was noted in HCV-induced cirrhotic patients compared with non-cirrhotic ones.Several large trials analyzed the progression of liver disease in previously HCV-infected subjects and found that 1% −14% of them have disease progression [11,13,14].HCV alters glucose metabolism and leads to insulin resistance,sequentially resulting in accelerated fibrogenesis [15].This phenomenon may sustain post SVR.Meanwhile,the hepatic stellate cells,which were reverted to quiescence after SVR,may be reactivated [16].We observed several cases of progression of liver disease.In addition,the incidence of progression of liver disease was correlated with hepatic stellate cells activity in cirrhotic patients.As advanced fibrosis or cirrhosis increases the risk of HCC incidence after HCV eradication [17],the incidence of HCC may increase as the follow-up period is extended.
We have reviewed the latest studies on long-term follow-up of Chinese patients,and the follow-up period of our study is longer than these studies [18–20].Most studies [18,19]reported prognosis at SVR24.In the long-term follow-up with an average period of 4.3 years in“CCgenos”program,334 patients received interferon-based treatment and 71.1% of them achieved SVR24 after treatment [18].In their observation,the risk score of cirrhosis and HCC shifted towards lower among patients who achieved SVR24.The probability for treated patients to develop cirrhosis decreased from 29% to 4%after SVR24.A trend toward reduced probability of HCC progression was also implied from the study although no significance was found.Our long-term follow-up started in patients who achieved SVR24.Extremely low incidence of cirrhosis,decompensated complications and HCC were found in our study which is also consisting with previous publications [18,19].Another study followed 265 patients who achieved SVR24 for over 1931 person-years,which reported that the incidence of HCC was higher in patients with advanced fibrosis (F3-4) [19].Our study also proved that liver cirrhosis was a critical risk factor for HCC in patients who achieved SVR24.
Notably,a previous study reported no recurrence of HCV infection in a cohort of 197 sustained responders [5].These responders experienced a follow-up period for at least 12 months to confirm their SVR and an extra median follow-up period of 17 months.In our cohort with SVR24,we observed no HCV relapse but some cases of HCV reinfection in the long-term follow-up.According to our retrospective review,we found that these patients had potential risks of reinfection (drug injection,risky sexual behavior,or multiple blood transfusions).The recurrent viral genotype in the patient with drug injection was 3b,consistent with our previous publication [20].
Direct-acting antiviral agents (DAAs) application has increased the rate of SVR24 to more than 90%,even close to 100% in certain patients with certain regimens.However,it is still not clear as which regimen,PR or DAAs,would provide a better long-term prognosis [21,22].Interferon has a potential anti-tumor capacity.When therapeutic type I interferon is given,interferon pathways is activated,and the type II interferon from immunocytes is secreted.Previous studies proved that type II interferon directly inhibits cell growth,promotes apoptosis,stimulates the secretion of reactive oxygen species and perforin from natural killer cells and macrophages,and promotes T lymphocyte transformation into Th1 cell and CD8+CTL [23,24].Multiple mechanisms are associated with the anti-tumor effect of interferon.Although DAAs have been approved in most countries in recent years,its long-term follow-up data are still lacking.Most studies [21,22,25]focus on the SVR12 or short-term prognosis within 2 years after treatment.Reddy KR et al.[25]conducted an observational study on clinical trial recipients of daclatasvir-based regimens after SVR12 was achieved and it turned out that the SVR was durable in 99% of recipients.Progression of liver disease and new HCC were found in 15 and 23 patients respectively,indicating that the risk existed but it was infrequent.The result is consistent with ours.It implied that no matter which kind of antivirals was applied,prognosis is improved after viral eradication.We hope comparable follow-up data over 5 years provided more clear evidence.
In conclusion,we reported the long-term prognosis of HCVinfected individuals who experienced a SVR24 after PR treatment.HCC event and progression of liver disease did exist in our cohort,but at a low rate.Cirrhotic patients were more vulnerable during treatment or post SVR.More cirrhotic patients would experience adjusted regimens compared with non-cirrhotic patients.Cirrhotic patients had a higher risk of poor prognosis after treatment.HCV recurrence was rare in responders with SVR24 who had corrected their risky behaviors.
Acknowledgments
We sincerely thank Rui-Chao Yu and Sanin Andres for their help in English revision.
CRediT authorship contribution statement
Yuan-Ji Ma:Formal analysis,Writing– original draft,Writing– review &editing.Ling-Yao Du:Data curation,Formal analysis,Writing– review &editing.Li-Bo Yan:Data curation and Validation.Juan Liao:Investigation.Xing Cheng:Resources.Wu-WeiXie:Project administration.Hong Tang:Conceptualization,Funding acquisition,Writing– review &editing.
Funding
This study was supported by the Research Project of Health Commission of Sichuan Province (No.17PJ006).
Ethical approval
This study was approved by the Ethics Committee of West China Hospital,Chengdu,China [Authorization number:2016 annual audit (91)].
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Practice of precision surgery in primary liver cancer
- Reporting of longitudinal pancreatojejunostomy with partial pancreatic head resection (the Frey procedure) for chronic pancreatitis:A systematic review
- Hepatobiliary&Pancreatic Diseases International
- Presentation and surgical management of xanthogranulomatous cholecystitis
- Transjugular intrahepatic portosystemic shunt is effective in patients with chronic portal vein thrombosis and variceal bleeding
- Laparoscopic hepatectomy is superior to open procedures for hepatic hemangioma
