SOX9 in biliary atresia:New insight for fibrosis progression
2021-05-19HnAhmedElAryMgdyAnwrSerNohMohmedRdwnDohMherTieNerminMohmedAdwyAhmdMohmedSir
Hn Ahmed El-Ary ,Mgdy Anwr Ser ,Noh Mohmed Rdwn ,Doh Mher Tie ,Nermin Mohmed Adwy ,Ahmd Mohmed Sir ,∗
a Department of Pediatric Hepatology, Gastroenterology, and Nutrition, National Liver Institute, Menoufia University, 32511 Shebin El-koom, Menoufia, Egypt
b Department of Pathology, National Liver Institute, Menoufia University, 32511 Shebin El-koom, Menoufia, Egypt
Keywords:Biliary atresia Fibrosis Hepatic progenitor cells Immunohistochemistry Neonatal cholestasis SOX9
ABSTRACT Background: Liver fibrosis is a hallmark determinant of morbidity in biliary atresia (BA) even in successfully operated cases.Responsible factors for this rapid progression of fibrosis are not completely defined.Aberrant expression of the transcription factor SOX9 and hepatic progenitor cells (HPCs) proliferation have roles in fibrogenesis in cholestatic disorders.However,they were not investigated sufficiently in BA.We aimed to delineate the relation of SOX9 and HPCs to fibrosis and its progression in BA.Methods: Forty-eight patients with BA who underwent an initial diagnostic liver biopsy (LB) and consequent intraoperative LB were recruited and compared to 28 cases with non-BA cholestasis that had an LB in their diagnostic workup.Liver fibrosis,tissue SOX9 and HPC expressions were studied in both BA and non-BA-cholestasis cases.Liver fibrosis,SOX9,and HPCs’ dynamic changes in BA cases were assessed.Relation of fibrosis and its progression to SOX9 and HPCs in BA was assessed.Results: SOX9 and HPCs in ductular reaction (DR) form were expressed in 100% of BA and their grades increased significantly in the second biopsy.The rapidly progressive fibrosis in BA,represented by fibrosis grade of the intraoperative LB,correlated significantly to SOX9-DR and HPC-DR at the diagnostic(r=0.420,P=0.003 and r=0.405,P=0.004,respectively) and the intraoperative (r=0.460,P=0.001 and r=0.467,P=0.001,respectively) biopsy.On the other hand,fibrosis,SOX9-DR,and HPC-DR were significantly lower in non-BA cases at a comparable age (P < 0.001,P=0.006,and P=0.014,respectively).Conclusions: Fibrosis in BA is rapidly progressive within a short time and is significantly correlated to SOX9 and HPCs.Assessment of targeting SOX9 and HPCs on fibrosis progression is warranted.
Introduction
Biliary atresia (BA) constitutes the single most common cause of persistent neonatal cholestasis (NC) and the leading indication for liver transplantation in pediatrics.It has a unique feature of rapidly progressive fibrosis that leads to cirrhosis at an early age.Despite early surgical management by Kasai portoenterostomy (KP)with successful biliary drainage,liver fibrosis continues its progression with the development of portal hypertension and its consequent complications [1].
Understanding the changes of fibrosis occurring in a short time interval and the relation of these changes to the presumed orchestrators of fibrogenesis,either on molecular or cellular levels could find a new strategy for effective adjuvant medical therapy.
Portal fibrosis occurring in cholestatic liver diseases is often accompanied by and could be induced by ductular reaction (DR).DR was defined as the presence of increased ductular profiles at the limiting plate or interface [2].DR is the most consistent histological feature noted in BA [3].Reactive cholangiocytes forming DR secrete several proinflammatory cytokines and growth factors that attract and activate hepatic stellate cells (HSCs),leading to collagen deposition [4].
Links between fibrosis,DR,and hepatic progenitor cells (HPCs)have been proposed before [5].HPCs are adult stem cells that can differentiate,under certain conditions,into hepatocytes or biliary epithelial cells.Replicative defects of hepatocytes,induced by inflammation,result in HPCs proliferation which causes not only the regeneration of hepatocytes but also a DR that contributes to periportal fibrogenesis [6].
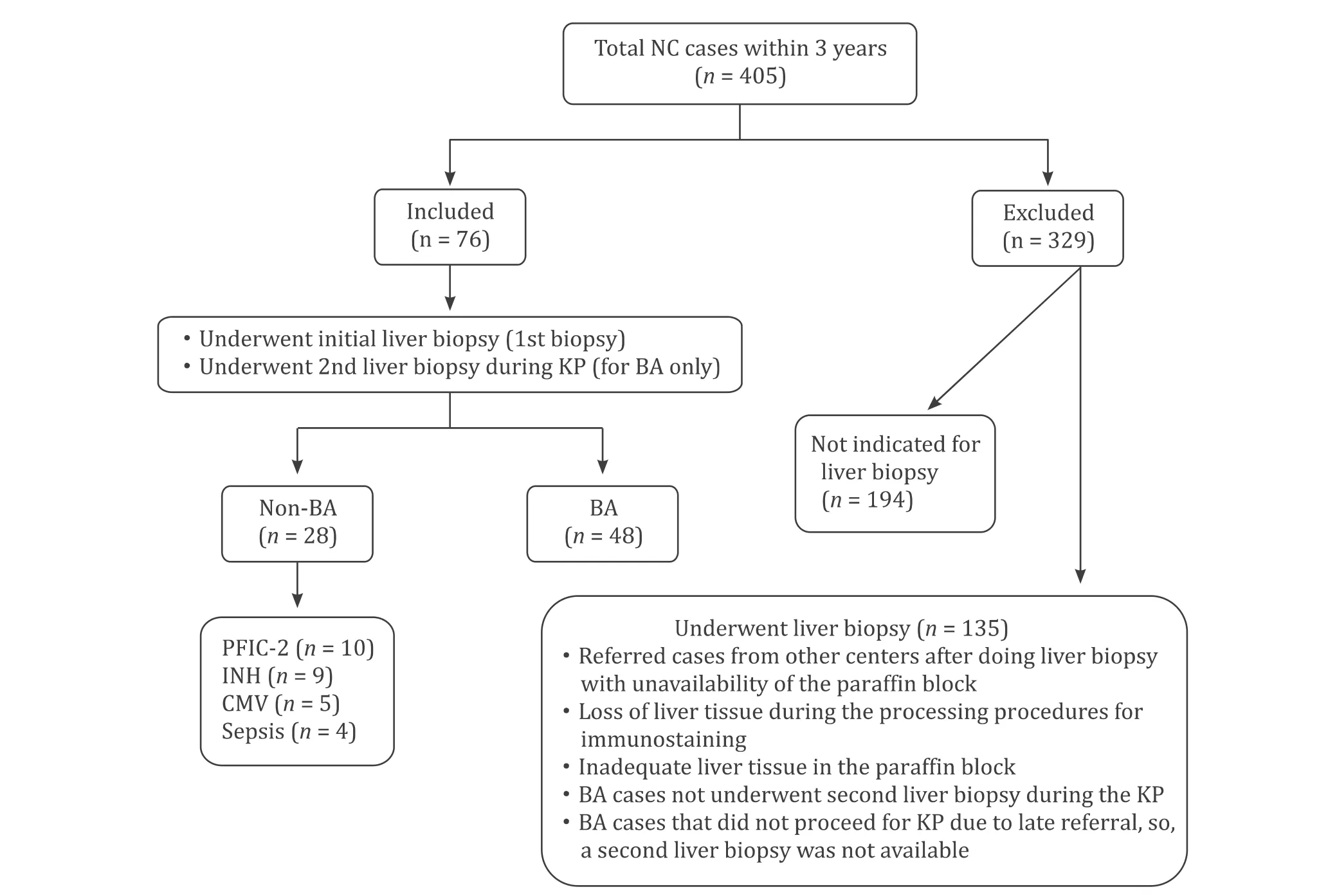
Fig.1.Flow chart for the included and excluded cases within the study.BA:biliary atresia;CMV:cytomegalovirus;INH:idiopathic neonatal hepatitis;KP:Kasai portoenterostomy;NC:neonatal cholestasis;PFIC-2:progressive familial intrahepatic cholestasis type 2.
Molecular elements also play a role in fibrogenesis.Rigorous regulation of gene expression and silencing is important for normal development.Conversely,improper or reactivated gene expression causes disease.Sex-determining region Y-related high mobility group box9 (SOX9) is a transcription factor regulating the development of both the intrahepatic and extrahepatic bile duct system [7].It also contributes to liver regeneration and fibrosis [ 8,9 ].Under normal conditions,SOX9gene keeps silent.When damage occurs in the liver,SOX9 is activated in cells which alters the cell behavior.Ectopic SOX9 expression in HSCs and hepatocytes by liver injury activates extracellular matrix genes resulting in extracellular matrix deposition.
Does fibrosis progression in BA depend just on time? And therefore,the older the child the severer the fibrosis? Are there other drivers for the fibrosis progression,such as SOX9 and HPCs?SOX9 and HPCs and their relation to liver fibrosis have not been investigated sufficiently in human BA [ 9,10 ].In this study,we aimed to describe SOX9 and HPCs expression in BA and investigate their relation to fibrosis and its progression.
Methods
Study population
This observational cohort study included 76 NC cases,in which liver biopsy (LB) was indicated for etiological diagnosis (48 cases of BA and 28 cases of non-BA cholestasis).They were recruited consecutively from September,2015 to August,2018,of 405 NC cases from the Department of Pediatric Hepatology,Gastroenterology,and Nutrition.All included BA cases had two LB;the first one is a diagnostic biopsy and the second one at the time of surgical correction by KP.All inclusion and exclusion criteria were presented in Fig.1 .
After full assessment by history,clinical examination,laboratory investigations,radiological assessment,and LB,infants with NC were classified into BA and non-BA cholestasis,as we reported before [11].Non-BA cholestasis underwent further investigations according to the expected etiology.Their final diagnoses were shown in Fig.1 .This study was approved by the Research Ethics Committee and conforms to the 1975DeclarationofHelsinkiand its later amendments.Informed consent was signed by the parents of each case.
LB
All recruited cases underwent an ultrasound-guided LB using a 16-gauge Tru-cut needle (diagnostic LB) at initial assessment and another LB for the BA cases only,intraoperatively,during the KP(intraoperative LB).
Routine histopathological and fibrosis evaluation
For routine histopathological evaluation,sections were stained with hematoxylin and eosin (HE) and other routine stains.A biopsy with liver tissue containing 10 or more portal tracts was considered adequate [12].
Liver fibrosis was assessed in five grades as reported by Russo et al.[13].Grade 0:absent or fibrous expansion of some portal areas;grade 1:fibrous expansion of most portal areas;grade 2:focal porto-portal bridging;grade 3:marked bridging;grade 4:cirrhosis.
Liver fibrosis assessment in biopsy sections is highly liable to bias,either under-or over-estimation.One of the causes of this biased assessment is the sampling method.The subcapsular area is rich in fibrosis compared to deeper tissue.Padoin et al.showed higher grades of fibrosis when assessed by wedge biopsy due to the inclusion of the fibrosis rich subcapsular area [14].On the other hand,Rawlins et al.reported concordance between the Trucut needle biopsy and wedge biopsy for the grades of fibrosis assessment when the wedge biopsy was sampled 2 mm deeper to the capsule [15].In the present work,all intraoperative biopsies were sampled 5 mm deeper to the capsule to avoid sampling error with the over-estimation of fibrosis in open biopsies.We excluded a few cases of the diagnostic LB due to inadequate tissue with less than 10 portal areas.No case of the wedge biopsies was excluded because of inadequate tissue.
Fibrosis grades were reviewed by two senior pathologists blinded to diagnosis and the timing of the BA patients’ biopsies(diagnostic or intraoperative).Results showed a substantial interobserver agreement with the kappa value of 0.785 for diagnostic biopsy and 0.793 for intraoperative biopsy.
Immunohistochemistry for SOX9 and HPCs
SOX9 determination was performed using an anti-SOX9 antibody.Also,HPCs delineation was performed using anti-cytokeratin(CK) 7 and anti-CK19 antibodies.CK7 and CK19 were considered markers for HPCs in both rat and human tissue studies especially when presenting out of the normal histological sites of the biliary tree [16–18].
For the immunohistochemical (IHC) staining procedure,three serial 4 μm sections were cut from each paraffin block,deparaffinized in xylene,and rehydrated in descending grades of ethanol.The three serial sections from each block were incubated overnight with the primary antibody at 4 °C.One antibody was used for each slide,using rabbit monoclonal anti-SOX9 antibody (Abcam,Cambridge,UK) at a dilution of 1:10 0 0,mouse monoclonal anti-CK7 antibody (Dako,Glostrup,Denmark) at a dilution of 1:500,and rabbit monoclonal anti-CK19 antibody (Abcam) at a dilution of 1:500.
Positive immunostaining was identified by brownish discoloration of the nucleus for SOX9 and the cytoplasm and/or cell membrane for the HPCs.
All IHC stains were assessed blindly by a single senior pathologist (Taie DM),who was unaware of the diagnosis of the patients or the type of biopsy,three times,one month apart.Finally,the median values were reported.Intraobserver agreement with the kappa value for different IHC parameters ranged from 0.83 to 0.96.
Liver fibrosis and IHC stains were compared between the following groups;(i) between the diagnostic biopsy in BA group and LB in the non-BA-cholestasis group (age was significantly younger in BA);(ii) between the intraoperative BA biopsy and the non-BA cholestasis biopsy (comparable for age);(iii) between the diagnostic and intraoperative BA biopsies to assess dynamic changes of fibrosis,SOX9,and HPCs in BA.
Evaluation of HPCs
HPCs were identified as DR (HPC-DR) and as isolated hepatic progenitor cells (IHPCs).DR as we defined before [17]is irregular cords of small cuboidal or elongated cells with or without poorly discernible lumina,located at the portal-parenchymal interface with or without some extension to the periphery.DR was assessed semiquantitatively on a three-grade scale,based on its extent around the limiting plate at high-power fields (HPFs) × 100 using an Olympus Microscope (Tokyo,Japan) [17].IHPCs were observed in the parenchyma.IHPCs were similar to the solitary cells of DR [17].In the parenchyma,IHPCs were assessed as either absent or present.
Evaluation of SOX9
The SOX9 was evaluated according to the site of expression.When it is expressed in the interface,it was presented in three grades as the HPC-DR grading system mentioned before and termed SOX9-DR.When it is present in the parenchyma,it was evaluated as either absent or present and termed SOX9-isolated parenchymal (SOX9-IP).
Statistical analysis
Statistical analysis was performed using SPSS version 16 (SPSS Inc.,Chicago,IL,USA).Quantitative data were expressed as mean ±standard deviation (SD) or median (range),and compared by either independent samplest-test or by Mann-WhitneyUtest according to the nature of the data.Qualitative data were presented as number (percentage) and compared by Chi-square test or Fisher’s exact test.Correlations were analyzed by Spearman’s test.The kappa coefficient was calculated to measure the intraobserver and interobserver agreement.This kappa coefficient was interpreted as excellent (≥0.80),good (0.60–0.79),moderate (0.40–0.59),poor (0.20–0.39) and very poor (<0.20) concordance.Results were considered significant if thePvalue was<0.05.
Results
Demographic, clinical, laboratory, and radiological data
The age of the non-BA cholestasis (70.6 ± 17.2 days) was significantly older (P=0.002) than the BA age at diagnostic LB(59.6 ± 12.2 days) and comparable (P=0.290) with the BA age at the intraoperative LB (74.2 ± 12.0 days).The median interval between the diagnostic and intraoperative LB in the BA group was 14 days,ranging from 5 to 31 days.Females represented 52% of the BA group and 43% of the non-BA cholestasis group (P=0.438).Compared to infants with non-BA cholestasis,those with BA had rare positive family history (2.1% vs.17.9%,P=0.025),lower level of AST (183 vs.284 U/L,P=0.017),higher level of GGT (775 vs.176 U/L,P<0.001),and lower gallbladder length (16 vs.24 mm,P=0.001).Other clinical,laboratory and radiological data were shown in Table 1 .
Liver fibrosis in BA and non-BA cholestasis
Fibrosis grading within the diagnostic LB of BA was significantly higher than that within the non-BA cholestasis group (P=0.003),despite significantly lower age of the BA group at diagnostic LB.When fibrosis was compared between the BA group at the intraoperative LB and the non-BA cholestasis group at a comparable age,the fibrosis grading was also significantly higher in BA than non-BA cholestasis (P<0.001) (Table 2).
On the other hand,liver fibrosis of the BA group showed significant temporal increase within a short time interval from the diagnostic LB to the intraoperative LB (P<0.001);seven cases developed cirrhosis and all other cases showed advanced fibrosis grades(Table 2).Fifteen cases (31.3%) showed no change of fibrosis grade,28 (58.3%) increased one grade,and 5 (10.4%) increased 2 grades.
SOX9 and HPCs expression in BA and non-BA cholestasis
Diagnostic LB of the BA group in comparison to that of non-BA cholestasis showed that SOX9 and HPCs were expressed in DR form in 100% of BA and 96.4% of non-BA cholestasis with different grades (Table 2 and Fig.2) with no statistically significant differences (P=0.562 andP=0.573,respectively).On the other hand,SOX9-IP (41.7%) and IHPCs (45.8%) forms were present in fewer cases than the DR forms (Table 2).

Table 1 Demographic,clinical,laboratory,and radiological findings of the groups studied
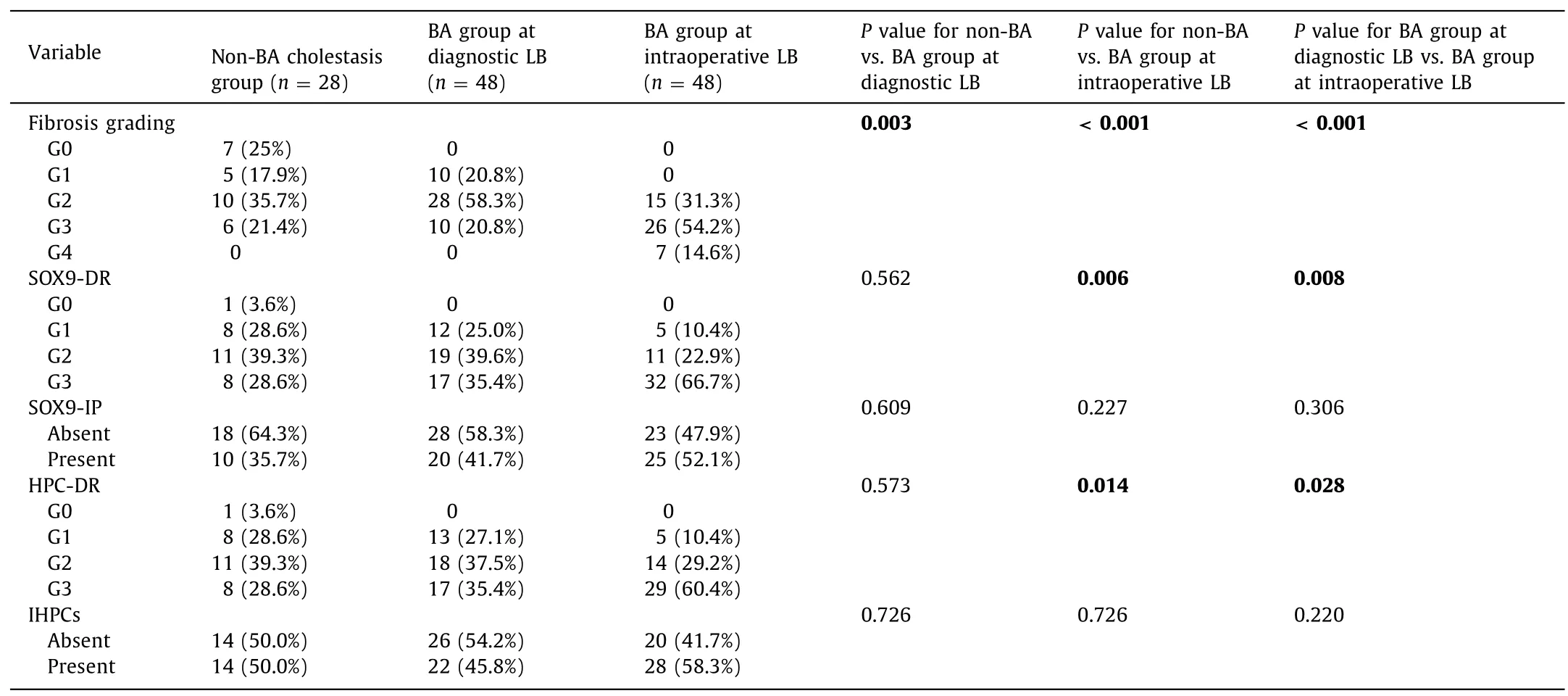
Table 2 Fibrosis and immunohistochemical findings of the groups studied

Fig.2.SOX9 expression and hepatic progenitor cell (HPC) expansion pattern in biliary atresia (BA) and non-biliary atresia cholestasis (original magnification × 100).A,C,E:Three serial sections of liver tissue in an infant with BA showing SOX9 expression (A) in DR pattern (grade 3) and isolated intraparenchymal pattern (arrows) and HPC-DR with positive cytoplasmic expression of CK7 (C) and CK19 (E) in grade 3.B,D,F:Three serial sections of liver tissue,in an infant with non-BA cholestasis showing SOX9 expression (B) in DR pattern (arrows) and HPC-DR with positive cytoplasmic expression of CK7 (D) and CK19 (F) (arrows) in grade 1 for all.CK:cytokeratin;DR:ductular reaction;HPC-DR:hepatic progenitor cells-ductular reaction;SOX9-DR:SOX9-ductular reaction.
Intraoperative LB of BA group in comparison to LB of non-BA cholestasis showed that SOX9-DR and HPC-DR were significantly higher in intraoperative biopsy of BA cases (P=0.006 andP=0.014,respectively).On the other hand,no significant difference was present regarding SOX9-IP and IHPCs (P=0.227 andP=0.726,respectively) (Table 2).
Temporal changes of SOX9 and HPCs expression in BA
There was a significant increase of SOX9-DR and HPC-DR from diagnostic to intraoperative LB of BA cases (P=0.008 andP=0.028,respectively) with no significant difference regarding SOX9-IP and IHPCs forms (Table 2 and Fig.3).Seventeen cases(35%) of BA showed a temporal increase in SOX9-DR expression and HPC-DR expansion pattern from diagnostic to intraoperative LB (Fig.4 A).
Relation of fibrosis to other parameters
The final grade of fibrosis in BA represented by that assessed in the intraoperative LB showed a significant positive correlation to SOX9-DR and HPC-DR expression either in the early stage of the disease (diagnostic LB) (r=0.420,P=0.003 andr=0.405,P=0.004,respectively) or those expressed at the later stage of the disease (intraoperative LB) (r=0.460,P=0.001 andr=0.476,P=0.001,respectively).On the other hand fibrosis grade was not correlated to either age at the intraoperative LB (r=0.192,P=0.191) nor the interval between the diagnostic and intraoperative LB (r=-0.056,P=0.704) (Fig.5).
The temporal increase of fibrosis from the diagnostic to the intraoperative LB showed a significant positive correlation to the temporal increase of SOX9-DR (P=0.038) and temporal increase of HPC-DR (P=0.008) (Fig.4 B).
Correlation of outcomes of KP with SOX-DR, HPC-DR, fibrosis at the time of the operation

Fig.3.Temporal changes of SOX9 expression and hepatic progenitor cell (HPC) expansion in biliary atresia (BA) from diagnostic to intraoperative biopsy (original magnification × 100).A,C,E:Three serial sections of liver tissue from the diagnostic liver biopsy of an infant 68 days old with BA showing SOX9-DR (arrow) grade 2 with some intraparenchymal expression (SOX9-IP) (A) and HPC-DR grade 2 stained for CK7 (C) and CK19 (E) (arrows).B,D,F:Three serial sections of liver tissue of the same infant at 85 days old from intraoperative liver biopsy performed during the Kasai portoenterostomy showing an increase of the SOX9-DR to grade 3 (B) and an increase of the HPCDR to grade 3 stained for CK7 (D) and CK19 (F).Stars denote cirrhotic nodule.CK:cytokeratin;HPC-DR:hepatic progenitor cells-ductular reaction;SOX9-DR:SOX9-ductular reaction;SOX9-IP:SOX9-isolated parenchymal.
Correlating the outcome of KP to the fibrosis,HPC-DR,and SOX9-DR assessed at the time of the operation was performed for the results of 27 cases.The other 21 cases included 4 died early postoperatively with sepsis,2 died with pneumonia and the remaining 15 lost to follow-up with no available data about their outcome.Outcome was defined as successful when bilirubin decreased below 1.5 mg/dL within 6 months of the KP and unsuccessful or failure when this level was not achieved within the defined duration [19].Of the 27 cases with follow up,12 had successful outcome and 15 had failed outcome.Results showed a significant negative correlation of the SOX9-DR with the outcome of KP (r=−0.471,P=0.013).On the other hand,no significant correlation was found for fibrosis or HPC-DR with the outcome(r=−0.240,P=0.228 andr=−0.359,P=0.0 6 6,respectively).
Discussion
Liver fibrosis is the scarring process of liver injury of different etiologies.BA has uniquely progressive fibrosis.If not operated at the optimal time,liver failure will ensue with death around two years of age.Even after timely operated BA cases with successful biliary drainage,fibrosis can still be progressive at variable rates with the development of portal hypertension [1].As fibrosis is a significant determinant of morbidity [13],many studies are concerned with its driving factors [ 20,21 ].
In the present study,liver fibrosis in early stages of BA and its progression in later age,were significantly higher than those in other NC disorders.Kim et al.[22]reported that in BA,liver fibrosis may progress rapidly and even cirrhosis may ensue as early as 2 months of age.Rastogi et al.[23]and Russo et al.[13]also reported high grades of fibrosis at the early age of BA.Our study also showed that a high grade of fibrosis is a feature of BA.More interestingly,our study showed that this fibrosis progression was not correlated with age at intraoperative LB,which means that a younger infant could have a higher grade of fibrosis.Therefore,fibrosis progression in BA is not a matter of aging.Rather,unique factors could drive this rapid progression.
The present study found that within a short time interval (5–31 days;median 14 days),there was a significant temporal increase of fibrosis by more than 60%.Zhang et al.[24]reported a temporal increase in fibrosis grade from intraoperative LB to postoperative LB.Moyer et al.[25]noticed that the predominance of inflammation rather than fibrosis was evident at an early stage of BA which decreases by age with a predominance of fibrosis.DR is proposed to be formed from HPCs.Studying the cellular constituents (HPCs)of DR and molecular driving for its development (the SOX9) could imply future therapeutic interventions.
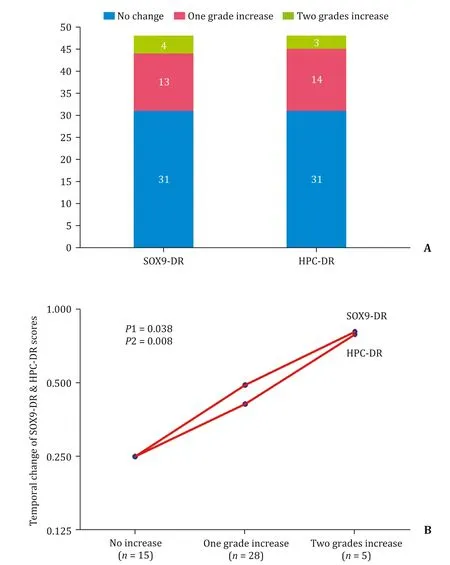
Fig.4.Temporal immunohistochemical changes and relation to fibrosis changes in biliary atresia (BA).A:Temporal changes in SOX9-DR expression and HPC-DR expansion pattern from diagnostic biopsy to intraoperative biopsy in BA cases.B:Relation of fibrosis changes to the changes of SOX9-DR and HPC-DR from diagnostic liver biopsy to intraoperative liver biopsy of BA cases.HPC-DR:hepatic progenitor cells-ductular reaction;SOX9-DR:SOX9-ductular reaction.
Previous studies [ 4,24,26 ]proposed that portal fibrosis occurring in cholestatic liver diseases appears to be induced by the cells that form the DR.It has been shown that ductal epithelium expresses proteins that attract and activate HSCs,leading to collagen deposition.The activation of HSCs is considered an important event in liver fibrogenesis.Many studies proved the relation of DR and the activation of these HSCs [ 27,28 ].Clouston [6],Fabris [29],Zhang and their colleagues [30]reported that the severity of fibrosis was significantly correlated to the grade of DR.
It has been shown that DR is formed by reactive cholangiocytes,the source of which is the HPCs [26].HPCs are quiescent stem cells that are hardly detectable in normal liver.However,in liver injury,they start to proliferate and expand with bipotential power for giving hepatocyte and biliary lineage [31].Another important component that drives the formation of DR,on a molecular level,is the nuclear transcription factor,SOX9 [ 10,30 ].
The present study demonstrated the role of cellular and molecular components in the rapid fibrosis progression in BA,and we found that HPCs expanded in all cases of BA expressed as DR and in nearly half of the cases as isolated form.HPC-DR form showed a significant increase within a short time interval reaching a significantly higher level in BA than an age-comparable non-BA cholestasis.Mavila et al.[32]reported increased HPCs expansion in animal models of BA and human BA.
Like HPCs,The SOX9 as the molecular component of DR showed also expansion in all cases of BA in SOX9-DR form and 41.7% of the BA cases in SOX9-IP form.SOX9 also showed a rapid expansion in the DR form within the same short time interval between the diagnostic and intraoperative biopsy.It was significantly higher than age-comparable non-BA cases.Suda et al.[10]reported that SOX9 expression in DR was increased in BA patients at the early stages of the disease.
In the present study,there was a significant temporal increase of HPC-DR and SOX9-DR in BA.This increase would be the driver of progressive fibrosis.Moreover,there was a significant positive correlation of the ultimate grade of fibrosis in BA cases (those assessed at the intraoperative LB) to the cellular component of the DR (HPC-DR) and the molecular components (SOX9-DR) at all stages of the disease,early (in diagnostic LB) and late (in intraoperative LB).A dynamic relationship,the temporal increase of fibrosis grade was significantly associated with the temporal increase of SOX9-DR and HPC-DR.The more the increase of the SOX9-DR and HPC-DR,the severer of the liver fibrosis.
Zhang et al.[30]reported a temporal increase in SOX9-DR and HPC-DR in an animal model of BA at different time points.They also reported a positive correlation of this temporal increase to fibrosis progression.Previous studies showed that fibrosis grade was correlated to HPCs expansion pattern in human liver diseases [ 5,17,33 ].Also,previous studies showed that SOX9-dependant process is implied in liver fibrogenesis,through activation of HSCs [ 9,34 ].
Our study showed that the higher the grade of SOX9-DR at the time of KP,the less the chance for successful biliary drainage.It seems that besides the presumed effect of SOX9 on fibrosis progression,it also has an impact on the progressive postoperative obliterative process with consequent defective biliary drainage.Studies on larger numbers and trials for targeting SOX9 are necessary to uncover its role for postoperative biliary drainage.
A limitation of this study is the small sample size due to the rarity of the disease.Comparing a diagnostic Tru-cut biopsy to an intraoperative wedge biopsy is another limitation because wedge biopsy may over-estimate the fibrosis due to inclusion of the subcapsular fibrotic tissue.However,we avoided this by sampling the wedge biopsy 5 mm deeper to the capsule to exclude the subcapsular area with possible more fibrosis.Rawlins et al.[15]found that sampling a wedge biopsy 2 mm deeper to the capsule will be concordant to the Tru-cut needle biopsy as regards fibrosis grading.
Assessment of the changes of fibrosis within a short time interval is considered strength of the present study.It gives an idea about the early changes and consequently the possible interventions that could be applied early to avoid fibrosis progression.Our study in this aspect is superior to other studies [ 24,26 ]which assessed changes after a long term interval.
In conclusion,this study showed a novel insight regarding the orchestrators of the rapid fibrosis progression in BA.It showed that fibrosis progression is not simply a matter of aging;rather it correlates to the molecular (SOX9) and cellular (HPCs) components of DR and their temporal changes.Targeting both SOX9 and HPCs on fibrosis progression in BA might be another therapeutic strategy.
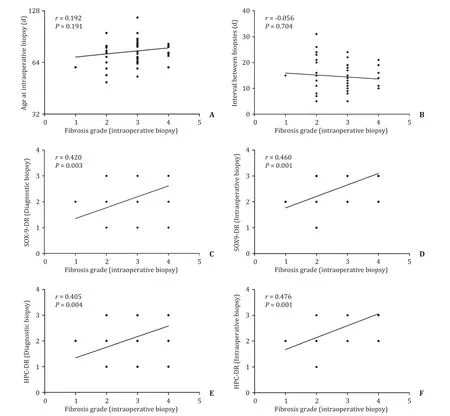
Fig.5.Correlation of fibrosis grade of intraoperative liver biopsy of biliary atresia cases to other parameters.No significant correlation between fibrosis grade and age at intraoperative biopsy (A) or the interval between the diagnostic and intraoperative biopsies (B).Significant positive correlation of fibrosis grade at intraoperative biopsy with SOX9-DR expression and HPC-DR expansion in diagnostic and intraoperative liver biopsies (C-F).HPC-DR:hepatic progenitor cells-ductular reaction;SOX9-DR:SOX9-ductular reaction.
Acknowledgments
None.
CRediT authorship contribution statement
Hanaa Ahmed El-Araby:Conceptualization,Investigation,Methodology,Supervision,Writing -original draft,Writing -review&editing.Magdy Anwar Saber:Conceptualization,Supervision,Writing -review &editing.Noha Mohamed Radwan:Data curation,Funding acquisition,Resources,Software,Writing -original draft.Doha Maher Taie:Conceptualization,Data curation,Investigation,Resources,Supervision,Writing -review &editing.Nermin Mohamed Adawy:Formal analysis,Investigation,Methodology,Writing -original draft.Ahmad Mohamed Sira:Conceptualization,Data curation,Formal analysis,Investigation,Methodology,Resources,Software,Supervision,Writing -original draft,Writing -review &editing.
Funding
This study was supported by a grant from the National Liver Institute,Menoufia University,Egypt (002/2016/NLI).
Ethical approval
This study was approved by the Research Ethics Committee of the National Liver Institute,Menoufia University and in accordance with theDeclarationofHelsinki.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Practice of precision surgery in primary liver cancer
- Reporting of longitudinal pancreatojejunostomy with partial pancreatic head resection (the Frey procedure) for chronic pancreatitis:A systematic review
- Hepatobiliary&Pancreatic Diseases International
- Presentation and surgical management of xanthogranulomatous cholecystitis
- Transjugular intrahepatic portosystemic shunt is effective in patients with chronic portal vein thrombosis and variceal bleeding
- Long-term follow-up of HCV patients with sustained virological response after treatment with pegylated interferon plus ribavirin
