Effects of the nitrification inhibitor nitrapyrin and the plant growth regulator gibberellic acid on yield-scale nitrous oxide emission in maize fields under hot climatic conditions
2021-04-14KhadimDAWARKamilSARDARMohammadZAMANChristophLLERAlbertoSANZCOBENAAamirKHANAzamBORZOUEIandAnaGabrielaREZCASTILLO
Khadim DAWARKamil SARDARMohammad ZAMANChristoph MÜLLERAlberto SANZ-COBENAAamir KHANAzam BORZOUEI and Ana Gabriela PÉREZ-CASTILLO
1Department of Soil and Environmental Science,University of Agriculture Peshawar,Peshawa 25000(Pakistan)
2Soil and Water Management&Crop Nutrition,Joint FAO/IAEA Division of Nuclear Techniques in Food&Agriculture,P.O.Box 100,Vienna A-1400(Austria)
3Institute of Plant Ecology,Justus Liebig University,Heinrich-Buff-Ring 26,Giessen D-35392(Germany)
4School of Biology and Environmental Science,University College Dublin,Belfield,D04 V1W8(Ireland)
5Management of Environment and Agricultural Risks(CEIGRAM),ETSIAAB,Universidad Poliécnica de Madrid,Madrid 28040(Spain)
6Agricultural Research School,Nuclear Science and Technology Research Institute,P.O.Box 31465/1498,Karaj(Iran)
7Environmental Pollution Research Center(CICA),University of Costa Rica,Montes de Oca 11501(Costa Rica)
ABSTRACT Nitrification inhibitors are widely used in agriculture to mitigate nitrous oxide(N2O)emission and increase crop yield.However,no concrete information on their mitigation of N2O emission is available under soil and environmental conditions as in Pakistan.A field experiment was established using a silt clay loam soil from Peshawar,Pakistan,to study the effect of urea applied in combination with a nitrification inhibitor,nitrapyrin(2-chloro-6-tri-chloromethyl pyridine),and/or a plant growth regulator,gibberellic acid(GA3),on N2O emission and the nitrogen(N)uptake efficiency of maize.The experimental design was a randomized complete block with five treatments in four replicates:control with no N(CK),urea(200 kg N ha−1)alone,urea in combination with nitrapyrin(700 g ha−1),urea in combination with GA3 (60 g ha−1),and urea in combination with nitrapyrin and GA3.The N2O emission,yield,N response efficiency,and total N uptake were measured during the experimental period.The treatment with urea and nitrapyrin reduced total N2O emission by 39%—43%and decreased yield-scaled N2O emission by 47%—52%,relative to the treatment with urea alone.The maize plant biomass,grain yield,and total N uptake increased significantly by 23%,17%,and 15%,respectively,in the treatment with urea and nitrapyrin,relative to the treatment with urea alone,which was possibly due to N saving,lower N loss,and increased N uptake in the form of ammonium;they were further enhanced in the treatment with urea,nitrapyrin,and GA3 by 27%,36%,and 25%,respectively,probably because of the stimulating effect of GA3 on plant growth and development and the reduction in biotic and abiotic stresses.These results suggest that applying urea in combination with nitrapyrin and GA3 has the potential to mitigate N2O emission,improve N response efficiency,and increase maize yield.
Key Words: fertilizer use efficiency,greenhouse gas emission mitigation,N response efficiency,N uptake efficiency,N2O flux,plant growth hormone,urea
INTRODUCTION
The global climate is changing rapidly,leading to food insecurity and increasingly extreme weather events.A major cause of these extreme weather events is the rising temperature of the atmosphere, driven by increasing emissions of greenhouse gases(GHGs),which absorb heat in the atmosphere.Sustainable intensification of cropping systems through conservation agriculture practices(Liet al.,2016)and strategic use of soil nutrients and water resources has been identified as a potential solution to increase food production with less environmental impact(Garnett,2011).In Pakistan, impacts of an increase in the human population from 164 to 194 million over the past decade(SDGP-PBS,2016),urbanization,and climate change on agro-ecosystems are putting increasing pressure on food production systems and on dwindling land and water resources.National arable cropping systems receive most of their nitrogen(N)input from the application of synthetic fertilizers,predominantly urea,farm yard manure,plant residues,and some municipal wastes(MINFAL,2012).
Urea has been shown to have lower N use efficiency than other types of N fertilizers under many soil conditions,with only 5%—56%of the applied N absorbed by crop.Rather,it is lost to the atmosphere as ammonia(NH3)(Dawaret al.,2011;Zamanet al.,2013),nitrous oxide(N2O)(Zamanet al., 2008; Schlesinger, 2009; Zaman and Nguyen, 2012),nitric oxide(NO),and nitrogen gas(N2)(Sanz-Cobenaet al.,2012;Saggaret al.,2012).Unless urea is immediately incorporated into the topsoil mechanically or with the proper irrigation(Sanz-Cobenaet al.,2011;Zamanet al.,2013),N loss of up to 50%can occur.This leads to both environmental and agronomic losses(Gallowayet al.,2008;Schlesinger,2009;Zamanet al.,2013).
New management practices and technologies therefore need to be developed in order to optimize N utilization and avoid N loss to the atmosphere, streams, and lakes. One of the most promising new methods of reducing N loss is applying urea coated with N process inhibitors(Zamanet al.,2008).Urea applied together with nitrification inhibitor(NI) has been shown to be highly effective in reducing N fertilizer loss(Majumdaret al.,2002;Zamanet al.,2008;Cuiet al., 2010, 2011; Moiret al., 2012) and increasing productivity under a range of cropping systems(Linquistet al.,2013;Zhanget al.,2015;Sunet al.,2015)and pasture systems(Zamanet al.,2009).
Many NIs, such as dicyandiamide (DCD) and 3,4-dimethylpyrazol-phosphate(DMPP),are commonly used in agricultural systems,especially pasture and arable cropping systems.The NIs such as nitrapyrin or N-serve(2-chloro-6-(tri-chloromethyl)pyridine)effectively reduce N2O emission and leaching nitrate(NO−3)loss while also increasing yield and fertilizer use efficiency(Maet al.,2013;Zhanget al.,2015;Sunet al.,2015)at relatively low levels(approximately 0.24%the rate of urea N applied).
Similarly,fertilizer use efficiency could be further improved by co-application of urea and plant growth regulators(PGRs)(Aulaket al.,1991;Sturmet al.,1994;Bronson and Fillery,1998;Díaz-Zoritaet al.,2001;Kurepinet al.,2014;Zamanet al.,2014).Nitrogen applied to the soil is not fully utilized by plant,and it is estimated that about half of the applied N often remains unavailable (Greenwood, 1982),which causes increased NO−3leaching losses and enrichment of water bodies(Carvalho and Basch,1995).It is thought that gibberellic acid (GA3) affects N metabolism and N redistribution in plant and improves fertilizer use efficiency by increasing the utilization of soil-derived N.There are also several other theories as to how GA3affects N metabolism.For example,GA3enhances plant growth,which then leads to greater utilization of soil N,and spraying fertilizer during the pre-flowering stages may help to redistribute photoassimilates towards seeds. These processes may enhance N utilization,leading to increased crop yield.
Developing best soil,nutrient,and water management practices is key to enhancing crop production as well as to mitigating N2O emission.However;no published work is available on the effect of urea treated with NI and/or plant growth hormone(GA3)on plant productivity and N2O emission in an arable agricultural system under hot climatic conditions as in Pakistan.Therefore,the objectives of this study were to investigate the effect of applying urea together with NI alone or in combination with GA3on N2O emission,crop productivity,and N response efficiency.
MATERIALS AND METHODS
Experimental site
A field experiment was established at the National Institute of Food and Agriculture near Peshawar,Pakistan(34◦01 N,71◦71 E,350 m above sea level)in June 2015.The soil was silt loam, with a low organic matter content (4.70 g C kg−1), pH of 8, total N of 1.0 g N kg−1, and electrical conductivity of 0.16 dS m−1.The study area is classified as semiarid,with an average annual air temperature of 23◦C and average annual rainfall of 384 mm,most of which occurs in July and August.May and June are the hottest months,with mean temperatures of 39 and 41◦C,respectively.This arable site has been under an irrigated maize-wheat crop rotation system for more than 10 years.
Experimental design and management
The soil was cultivated with a mould board plough to a depth of 0.30 m,followed by two cultivations and planking.Seeds of the maize variety Pioneer 3025 were sown on June 24,2015.During the maize growing season,six irrigation events were applied, with each event being equivalent to 75 mm,except the first(pre-planting)which was equivalent to 100 mm. The plot size was 5 m×3 m, and each plot contained six rows,with each row being 5 m long,a distance of 75 cm between rows,and a distance of 20 cm between plants. In each plot, a 0.5-m2area was allocated to soil sampling in order to measure ammonium(NH+4)and nitrate(NO−3)contents.
Before sowing, basal doses of phosphorus (P) at 90 kg P2O5ha−1in the form of single superphosphate and potassium(K)at 60 kg K2O ha−1in the form of potassium sulphate were applied and incorporated into the soil. The experimental design was a randomized complete block,consisting of the following five treatments in four replicates:control with no N(CK),urea(200 kg N ha−1)alone,urea(200 kg N ha−1)in combination with nitrapyrin(700 g ha−1),urea(200 kg N ha−1)in combination with GA3(60 g ha−1),and urea(200 kg N ha−1)in combination with nitrapyrin(700 g ha−1) and GA3(60 g ha−1). Urea was applied in two split applications,one half during first irrigation(July 7, 2015) and the other half when the maize plants were at knee height (August 15, 2015). Nitrapyrin and GA3were applied at a rate of 0.35%and 0.03%of the applied N(weight/weight),respectively,and the mixtures were obtained by dissolving urea with nitrapyrin and GA3in water.For a uniform application and to reduce NH3volatilization in the applied urea, nitrapyrin and GA3were dissolved in water 30 min before application, and the solutions were surface applied by hand at 90 L per plot.All other practices such as hoeing,weeding,and insect control were carried out on all plots uniformly. Soil moisture (water-filled pore space(WFPS),%)and temperature(◦C)were measured in the top 10 cm of soil by inserting appropriate probes;precipitation(mm)was measured using an on-site rain gauge.
Soil and plant analyses
Before starting experiment,four composite soil samples(0—10 cm depth) were taken using a soil core from the experimental site and passed through a 2-mm sieve.This was to analyse key soil properties including mineral N(Mulvaney,1996), organic matter (Nelson and Sommers, 1982), and texture(Gee and Bauder, 1986). Total N in soil and plant samples were determined by the Kjeldahl method described in Bremner and Mulvaney(1982).
Crop harvesting, yield measurement and N response effi-ciency calculation
Mature maize plants were harvested on October 15,2015, and various agronomic parameters including plant height, number of leaves per plant, number of grains per ear,100-grain weight,biomass yield,grain yield,and stover yield were recorded in two sub-plots (2 m×2 m). Plant biomass yield was separated into two components:grains and above-ground plant tissues(i.e.,shoot and leaves).Plant samples were washed with tap water and deionized water,after which they were dried at 65◦C for seven days.
Nitrogen response efficiency was calculated by subtracting the biomass yield of CK from those of individual fertilizer treatments and dividing the result by the amount of fertilizer N applied.
Measurements of N2O emission
The N2O fluxes were measured in each plot using the modified static chamber method of Saggaret al. (2004,2007).After closing the chambers,three gas samples taken after 0,30,and 60 min were taken from each chamber using a 50-mL polypropylene syringe.The temperature inside the chamber was recorded by placing a thermometer inside the chamber.Gas samples from syringes were transferred to preevacuated 20-mL vials(with gray molded PTFE/black butyl septum,Agilent Technologies,Santa Clara,USA),and were then analysed using an Agilent 7890A gas chromatograph(Agilent Technologies, Santa Clara, USA) equipped with an electron capture detector.The equipment was calibrated with certificated analytical grade standard N2O,CH4,and CO2and with N as a balance gas as well as pure nitrogen gas(ultra-high purity).The concentrations of the standard gases had an uncertainty of 5%.Concentrations were evaluated by a linear fit curve with four points from 0.3—3.0µL L−1.The third standard was prepared by diluting a specific volume of the more concentrated standard.The volume was measured with a gastight syringe which was injected into a vial filled with nitrogen gas at the atmospheric pressure.A vial filled with another standard gas with a concentration around 1µL L−1was placed in each group of 15 vials.These standards were analyzed as if they were samples, and deviations of the obtained concentration from the certified value must be less than 20%in order for the equipment to be utilized.If this requirement was not fulfilled,a new calibration curve was measured.On average,the actual deviations were found to be 8.5%±4.5%.In addition,a new set of standards for every concentration used in the first calibration curve and a blank filled with urea-ammonium phosphate were placed after every 48 h of continuous injection,as well as in the end of the injection.
Basal levels of N2O emission were measured one day before N fertilizer application,and then N2O measurements were made after every fertilizer application from July 7 to October 7,2015.The accuracy of the gas chromatographic data at ambient concentrations was 1%or better.The N2O concentrations within the chamber headspace have been reported to increase linearly(R2>0.90)with time(Zamanet al.,2009).The average rate of change in N2O concentration was,therefore,determined using linear regression,and N2O fluxes(F,mg m−2h−1)were calculated using the ideal gas law:

whereρis the density of N2O(mg m−3),Vis the volume of the chamber(m3),Ais the base area of the chamber(m2),∆c/∆tis the average rate of change of concentration with time(mL L−1h−1), andTis the temperature(◦C)in the chamber.
The yield-scaled N2O emission was calculated as the amount of N emitted as N2O divided by the total N uptake by the aboveground biomass(Van Groenigenet al.,2010).
Statistical Analyses
Analysis of variance(ANOVA)was calculated to compare fertilizer treatments with respect to various measured parameters. When significant effects of treatments were found,adjusted LSD values of Turkey’s test were calculated to make comparisons among the different fertilizer treatments.Minitab(version 12)was used to perform statistical analyses.
RESULTS
Soil and weather variables
Soil temperature and moisture data at a depth of 10 cm,as well as precipitation during the experimental period,are shown in Fig.1.The average air temperature was approximately 34◦C,and soil temperature ranged from 23 to 35◦C from June to September. Soil moisture content changed temporally with rainfall and irrigation events.The minimum amount of precipitation was 8 mm and occurred in June.In August,the precipitation peaked,averaging 68 mm.

Fig.1 Soil temperature(0—10 cm depth)and moisture(water-filled pore space(WFPS),0—10 cm depth)and monthly average precipitation during the experimental period.The solid and dotted arrows indicate the timing of N fertilization and irrigation events,respectively.
Soil NH+4content increased significantly (P <0.05)after urea application(Fig.2a).Urea applied alone or together with nitrapyrin produced significantly (P <0.05) higher(7—9 mg N kg−1)soil NH+4content relative to CK on day 1.Soil NH+4content in the treatment with urea alone reached its maximum on day 7,after which it decreased.Soil NH+4content was significantly (P <0.05) higher (60 mg N kg−1)on day 14 in the treatments with nitrapyrin,compared to the treatment with urea alone. The first application of urea yielded a higher soil NH+4content than the second application.Soil NO−3content was significantly(P <0.05)lower(13 mg N kg−1)in treatments with nitrapyrin,relative to treatment with urea alone(Fig.2b).
N2O emission
Nitrous oxide fluxes varied temporally throughout the maize growing season and were significantly (P <0.05)higher in the treatments with urea,regardless of nitrapyrin application,relative to CK(Fig.3).Three substantial N2O flux peaks appeared in the treatment with urea alone on days 15,35,and 49.The N2O fluxes were always lower in the treatment with urea and nitrapyrin than in the treatment with urea alone,and these differences were significant.The application of urea in combination with GA3had no effect on N2O flux.
The total N2O emission was significantly(P <0.05)higher(2.3±0.41 kg N2O-N ha−1)from the treatment with urea alone than from the treatment with urea and nitrapyrin(1.4±0.21 kg N2O-N ha−1) (Table I). The addition of urea in combination with nitrapyrin significantly(P <0.05)reduced cumulative N2O emission, with a 39%reduction relative to the treatment with urea alone.
Yield-scaled N2O emission
The yield-scaled N2O emission,which was based on the cumulative N2O emission and the aboveground N uptake(i.e.,uptake by grain and straw),ranged from 17.1 to 8.1 g N2O-N kg−1over the entire experimental period (Table I). Urea applied in combination with nitrapyrin significantly(P <0.05)decreased the yield-scaled N2O emission by 47%—52%relative to urea alone across the entire experimental period.
Maize biomass and grain yields,N response efficiency,and N uptake
Maize biomass and grain yields,N response efficiency,and total N uptake all varied significantly(P <0.05)when urea was applied in combination with nitrapyrin and also with GA3(Table II).Maize biomass and grain yields were significantly(P <0.05)greater(23%and 17%)when urea was applied in combination with nitrapyrin relative to the treatment with urea alone.The yields increased further in the treatment with urea,nitrapyrin,and GA3in combination by 27%and 36%,respectively,compared to the treatment with urea alone.Total N uptake by maize was also significantly(P <0.05)greater(14%or 25%)in the treatments with urea in combination with nitrapyrin or with GA3relative to the treatment with urea alone(Table II).Over the growing period,the aboveground maize biomass constituted 155 and 169 kg N ha−1under the treatments with urea in combination with nitrapyrin and urea in combination with nitrapyrin and GA3,respectively,compared to 135 kg N ha−1under the treatment with urea alone. Similarly to total N uptake, N response efficiency was also significantly(P <0.05)greater in the treatment with urea in combination with nitrapyrin and was further increased in the treatment with urea,nitrapyrin,and GA3in combination(Table II).The N response efficiency values were 9, 16, 24, and 28 kg kg−1in the treatments with urea alone, urea in combination with GA3, urea in combination with nitrapyrin,and urea in combination with nitrapyrin and GA3,respectively.Plant height,number of leaves per plant,number of grains per ear,100-grain weight,and leaf area were also influenced by the application of nitrapyrin and GA3(Table III).Urea applied in combination with nitrapyrin and/or GA3significantly enhanced growth,yield,and yield components in maize.

TABLE I Total N2O emission,percentage of N emitted as N2O of the applied N,and yield-scaled N2O emission as affected by application of urea alone and urea in combination with nitrapyrin(Ni)and/or gibberellic acid(GA3)

TABLE II Biomass and grain yields,total N uptake,and N response efficiency as affected by application of urea alone and in combination with nitrapyrin(Ni)and/or gibberellic acid(GA3)
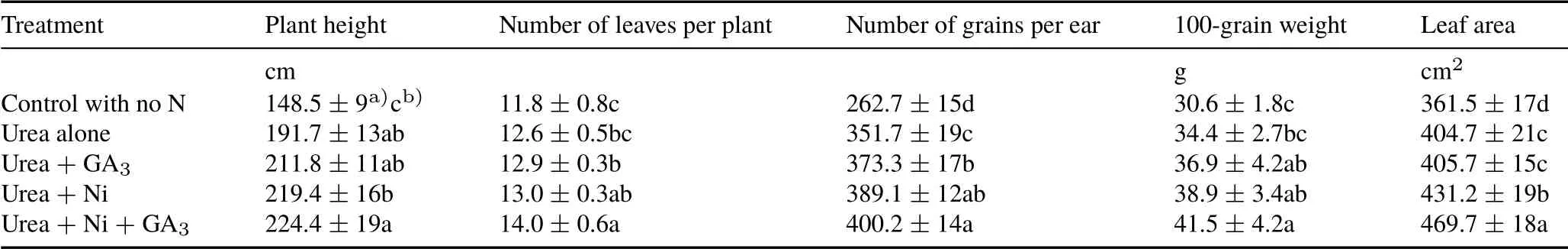
TABLE III Yield components of maize as affected by application of urea alone and in combination with nitrapyrin(Ni)and/or gibberellic acid(GA3)
DISCUSSION
During the experimental period,an impact of treatment type on N2O emission trend was recorded (Fig. 2). Urea applied alone or in combination with nitrapyrin was rapidly hydrolysed immediately after application (Zamanet al.,2008,2013;Dawaret al.,2011;Sanz-Cobenaet al.,2011),as exhibited by the signifciantly higher content of soil NH−4from urea,regardless of nitrapyrin application,during the first seven days(Fig.2).From days 7 to 28,urea applied in combination with nitrapyrin created significantly(P <0.05)more soil NH+4than urea alone, which is evidence of the inhibitory effect of nitrapyrin on nitrification,as observed in previous studies using the same inhibitor(Liet al.,2015;Sunet al., 2015; Wanget al., 2015; Zhanget al., 2015).Retention of NH+4reduces the risk of soil N being lostviaNO−3leaching and N2O emission(Gioacchiniet al.,2002;Macadamet al., 2003; Chenet al., 2010; Abaloset al.,2012),but could increase the risk of NH3volatilization.
Across all treatments,the first prominent N2O peak was observed on day 14, probably due to nitrification(Zaman and Nguyen,2012),since nitrification produces N2O as a byproduct(Inubushiet al.1996)and the emission is directly related to the amount of NO−3in soil (Dinget al., 2015).In contrast, the N2O emission peaks on days 35 and 49 may be attributed to the combination of abundant NO−3(Fig.2b)and high soil moisture from rainfall or irrigation events(Fig.1); both are conducive to high denitrification rates(Tiedje,1988;Bremner,1997;Scholefieldet al.,1997;Delauneet al.,1998).Lower NO−3and higher NH+4contents in soil after the addition of nitrapyrin(Fig.2a,b)thus likely reduced N2O losses from both nitrification (Zaman and Nguyen,2010)and denitrification(Firestone and Davidson,1989).
Total N2O emissions ranged from 0.80 to 2.3 kg N2O-N ha−1under CK and the treatment with urea alone.Applying urea in combination with nitrapyrin significantly reduced total N2O losses by 39%—43%compared to the treatment with urea alone(Table.I).These results suggest that under nitrifying conditions such as those in the present study with WFPS ranging from 40%to 50%,nitrapyrin reduced nitrification,possibly by suppressing NH3-oxidizing bacteria and other relevant soil enzymes,thus effectively reducing N2O emission.This agrees with the findings of other studies(Maet al.,2013;Xionget al.,2013;Liet al.,2015;Zhanget al., 2015; Martinset al., 2017) focusing on vegetable and arable fields, which have shown that nitrapyrin can significantly reduce N2O emission(by 32%—49%).
The yield-scaled N2O emission in the present study was within 5—15 g N2O-N kg−1estimated by others(Van Groenigenet al.,2010;Caiet al.,2013).In the present study,application of urea in combination with nitrapyrin significantly reduced yield-scaled N2O emission by 47%—52%compared to application of urea alone(Table I).These results are in accordance with previous studies on yield-scaled N2O emission in cropping systems(arable and vegetable)(Van Groenigenet al., 2010; Weiet al., 2010; Ventereaet al., 2011; Maet al., 2013; Bellet al., 2015; Liet al.,2015;Zhanget al.,2015;Guardiaet al.,2017).Yield-scaled N2O emission was significantly negatively correlated with N response efficiency,indicating that agronomic practices aiming to increase fertilizer N response efficiency can be directly linked to minimizing N2O emission.
The application of urea in combination with nitrapyrin or GA3significantly increased yield, total N uptake, and N response efficiency compared to the treatment with urea alone.These results are in line with those of other studies(Liuet al.,2013;Maet al.,2013;Zhanget al.,2015).These increases are likely due to increases in mineral N in the form of NH+4rather than NO−3for several days after urea and nitrapyrin application, thus increasing N uptake and crop yield(Aulakhet al.,2001).
The observed improvements in maize yield caused by coating urea with NI could be due to N saving because of lower N loss.In less fertile soils with low organic matter,as in the present experimental field,N retained in soil is used by plants, which leads to increased crop yield. Additionally,increases in crop yield could be due to NH+4retention by nitrapyrin and its subsequent uptake by maize, which leaves plants with additional energy from a nutritional view point.Ammonium retention in soil as a result of urea and nitrapyrin applied in combination not only provides environmental benefits by reducing N2O emission and NO−3leaching(Gooding and Davies,1992;Kettlewell and Juggins,1992;Janzenet al.,1999),but also offers agronomic and economic benefits by increasing N response efficiency,especially in N-deficient soils.
Similarly,N response efficiency was further improved when GA3was applied.Pant growth regulators such as GA3are known to improve plant growth through stimulating both cell division and elongation, reducing biotic and abiotic stresses,and enhancing crop production and N uptake(Boseet al., 2013; Kurepinet al., 2014; Zamanet al., 2014).Thus,maize production was significantly increased in the treatments with GA3.
CONCLUSIONS
Under the semiarid and hot climatic conditions of Pakistan,which are favourable to gaseous N loss from ureabased fertilizers, direct N2O and also yield-scaled N2O emissions were effectively reduced by the addition of nitrapyrin. On the other hand, urea applied in combination with nitrapyrin and/or GA3improved both biomass and grain yields of maize.The combination of nitrapyrin and/or GA3enhanced N response efficiency and N uptake compared to the use of urea alone.In conclusion,combining urea with nitrapyrin and/or GA3was likely to be a significant step toward mitigating N2O emission from typical N fertilization practices in maize-producing areas in Pakistan,while improving N response efficiency as well as biomass and grain yields.Further long-term field research is,however,required under a wide range of soil and environmental conditions to evaluate the performance of GA3and to better understand the effect of nitrapyrin addition on N2O emission and crop biomass production in Pakistan.
ACKNOWLEDGEMENT
This work was funded by the International Atomic Energy Agency through a Coordinated Research Project(CRP D1.50.16)“Minimizing Farming Impacts on Climate Change by Enhancing Carbon and Nitrogen Capture and Storage in Agro-Ecosystems”(18595)of Soil and Water Management and Crop Nutrition Section, Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture,Department of Nuclear Sciences and Applications.
杂志排行
Pedosphere的其它文章
- Notes to Authors
- Mitigating greenhouse gas emissions from croplands and pasturelands—climate-smart agriculture
- Effects of warming,wetting and nitrogen addition on substrate-induced respiration and temperature sensitivity of heterotrophic respiration in a temperate forest soil
- Long-term(42 years)effect of thinning on soil CO2 emission in a mixed broadleaved-Korean pine(Pinus koraiensis)forest in Northeast China
- Larger floods reduce soil CO2 efflux during the post-flooding phase in seasonally flooded forests of Western Amazonia
- Characteristics of greenhouse gas emissions from rice paddy fields in South Korea under climate change scenario RCP-8.5 using the DNDC model
