Effects of warming,wetting and nitrogen addition on substrate-induced respiration and temperature sensitivity of heterotrophic respiration in a temperate forest soil
2021-04-14MohammadMOONISJongKyuLEEHyojinJINDongGillKIMandJiHyungPARK
Mohammad MOONIS,Jong-Kyu LEE,Hyojin JIN,Dong-Gill KIMand Ji-Hyung PARK
1Department of Forest Environment Protection,Kangwon National University,Chuncheon 24341(Republic of Korea)
2Department of Environmental Science and Engineering,Ewha Womans University,Seoul 03760(Republic of Korea)
3Wondo Genet College of Forestry and Natural Resources,Hawassa University,P.O.Box 128,Shashemene(Ethiopia)
ABSTRACT Soil heterotrophic respiration and its temperature sensitivity are affected by various climatic and environmental factors.However,little is known about the combined effects of concurrent climatic and environmental changes,such as climatic warming,changing precipitation regimes,and increasing nitrogen(N)deposition.Therefore,in this study,we investigated the individual and combined effects of warming,wetting,and N addition on soil heterotrophic respiration and temperature sensitivity.We incubated soils collected from a temperate forest in South Korea for 60 d at two temperature levels(15 and 20 ◦C,representing the annual mean temperature of the study site and 5 ◦C warming,respectively),three moisture levels(10%,28%,and 50%water-filled pore space(WFPS),representing dry,moist,and wet conditions,respectively),and two N levels(without N and with N addition equivalent to 50 kg N ha−1 year−1).On day 30,soils were distributed across five different temperatures(10,15,20,25,and 30 ◦C)for 24 h to determine short-term changes in temperature sensitivity(Q10,change in respiration with 10 ◦C increase in temperature)of soil heterotrophic respiration.After completing the incubation on day 60,we measured substrate-induced respiration(SIR)by adding six labile substrates to the three types of treatments.Wetting treatment(increase from 28%to 50%WFPS)reduced SIR by 40.8%(3.77 to 2.23µg CO2-C g−1 h−1),but warming(increase from 15 to 20 ◦C)and N addition increased SIR by 47.7%(3.77 to 5.57µg CO2-C g−1 h−1)and 42.0%(3.77 to 5.35µg CO2-C g−1 h−1),respectively.A combination of any two treatments did not affect SIR,but the combination of three treatments reduced SIR by 42.4%(3.70 to 2.20µg CO2-C g−1 h−1).Wetting treatment increased Q10 by 25.0%(2.4 to 3.0).However,warming and N addition reduced Q10 by 37.5%(2.4 to 1.5)and 16.7%(2.4 to 2.0),respectively.Warming coupled with wetting did not significantly change Q10,while warming coupled with N addition reduced Q10 by 33.3%(2.4 to 1.6).The combination of three treatments increased Q10 by 12.5%(2.4 to 2.7).Our results demonstrated that among the three factors,soil moisture is the most important one controlling SIR and Q10.The results suggest that the effect of warming on SIR and Q10 can be modified significantly by rainfall variability and elevated N availability.Therefore,this study emphasizes that concurrent climatic and environmental changes,such as increasing rainfall variability and N deposition,should be considered when predicting changes induced by warming in soil respiration and its temperature sensitivity.
Key Words: carbon dioxide,decomposition,global warming,soil moisture,soil organic carbon,soil respiration,Q10
INTRODUCTION
Soil heterotrophic respiration through decomposition of soil organic carbon(C)is a critical component for studying the exchange of carbon dioxide (CO2) between the atmosphere and terrestrial ecosystems(Allisonet al.,2010;Bond-Lambertyet al., 2018). The effect of temperature on soil heterotrophic respiration has been investigated to enhance understanding of soil respiration and its response to climatic warming(Davidson and Janssens,2006;Zhonget al.,2016).Climatic warming can affect soil microbial activity and its temperature sensitivity, which can directly and indirectly affect the functioning of a wide range of ecosystems(Classenet al.,2015;Dutta and Dutta,2016).On a global level,subtle changes in the temperature sensitivity of soil heterotrophic respiration can have a significant effect on future climatic changes because CO2released from the soil under the influence of warming may provide positive feedback to the warming(Kirschbaum,2000).The temperature dependence of soil organic matter decomposition, which is the major source of soil heterotrophic respiration,has been the subject of intense scientific debate(Kirschbaum,2006).Many studies have shown that warming over periods of several months or years can lead to a temporary decrease in temperature sensitivity(Q10,change in respiration with 10◦C increase in temperature)of soil heterotrophic respiration(Lloyd and Taylor,1994;Vanhalaet al.,2011)and subsequent return to the pre-warming state,because of either substrate reduction or acclimation of soil biota(Melilloet al.,2002;Knorretal., 2005; Bradford, 2013). Varying responses of soil microbes to warming may result from differences in microbial community composition as well as soil type,C and nitrogen(N)availability,and soil moisture conditions.
In addition to climatic warming,changes in soil moisture because of changing precipitation can also affect the overall rate of soil heterotrophic respiration and itsQ10(Moyanoet al.,2013).However,studies on the effects of soil moisture onQ10have produced variable results.For example,Jianget al.(2013)observed a reduction in the temperature sensitivity of soil respiration in a mixed broad-leaved forest when simulated precipitation was doubled from ambient levels.Craine and Gelderman(2011)found that the highest temperature sensitivity occurred at intermediate soil moisture levels in an upland prairie soil(45%water-holding capacity).Nevertheless,they also reported a positive linear relationship betweenQ10of soil respiration and soil moisture of the same soil sampled from a lowland prairie area.
Nitrogen deposition has increased in many parts of the world (Kanakidouet al., 2016; Ackermanet al., 2019),and increased availability of N in the soil coupled with climatic change can further alter the soil C dynamics and soil heterotrophic respiration (Janssenset al., 2010). For example, in a weathered tropical soil from Puerto Rico,N-fertilization initially increased theQ10of labile C pools,whereas warming increased theQ10by initiating the decomposition of recalcitrant C pools(Cusacket al.,2010).Increased availability of N derived from chronic atmospheric deposition can slow the microbial decomposition of soil organic matter(SOM)due to reduced microbial activity and potential changes in organic matter composition(Ågrenet al.,2001;Park,2008),which also affects the rate of soil CO2efflux(Bowdenet al.,2004).Although many studies have found that chronic N deposition can substantially reduce SOM decomposition (Janssenset al., 2010), few studies have investigated its effect on theQ10of soil heterotrophic respiration under a warming environment.
Soil microbial heterotrophs,the main players of soil heterotrophic respiration,are sensitive to abiotic factors,such as temperature,moisture,and nutrient availability(Bissettet al.,2013).Large seasonal variations in soil temperature or smaller increases in annual temperature caused by global warming may shift the microbial community structure and alter microbial physiological functions (Schindlbacheret al.,2011;DeAngeliset al.,2015).Climatic warming may create conditions that favor certain groups of microorganisms,reshaping the entire soil microbial community structure (Deslippeet al., 2012; Ruiet al., 2015). Changes in physiological activities in those microorganisms may result in altered rates of respiration,affecting the gaseous exchange between the atmosphere and soil(Allisonet al.,2010;Bond-Lamberty and Thomson,2010;Bond-Lambertyet al.,2018).Microorganisms can adjust their physiology to warming,by reducing metabolic costs,reducing plant C inputs,or shifting the microbial community composition under a warmer environment(Allisonet al.,2010).For example,Freyet al.(2008) reported increased species diversity and a shift in the microbial community structure in a laboratory warming experiment with soil from a mixed deciduous forest. Soil water content can also have a strong effect on microbial function through soil aeration and substrate availability(Manzoniet al.,2012;Moyanoet al.,2013).In addition,changes in microbial functional capacity and activity were found in soils exposed to chronic N deposition(Chunget al.,2007;Freyet al.,2008).Previous studies have addressed the main factors controlling theQ10of soil heterotrophic respiration,such as temperature,soil moisture,and nutrient availability.Few studies have assessed the combined effects of multiple factors on theQ10of soil heterotrophic respiration (Davidsonet al., 1998; Wanget al., 2017). A meta-analysis reported a smaller combined effect of temperature and precipitation on soil respiration compared with that of a single factor(Wuet al.,2011).
From 2006 to 2024,the Korean Meteorological Administration projected that the average temperature of the Korean Peninsula may rise by 0.5◦C (±0.3◦C) in the winter as well as in the summer.Between 2025 and 2049,the average temperature has been projected to rise by 1.2◦C (±0.7◦C) in the summer and 1.2◦C (±0.8◦C) in the winter(KMA,2014).South Korea is located in East Asia,which experienced 10%—20%increases in precipitation between 1960 and 2003(Goslinget al.,2011).Scientists predict that annual, summer, and winter precipitation in South Korea may increase by 19.1%,20.5%,and 33.3%,respectively,in the later stages of the 21st century(Ohet al.,2016).On the global level,land is estimated to sequester 11%(10%—13%)of annual N inputs(from a baseline of 128 Tg N year−1)(Leeet al., 2019). The annual mean total wet N deposition for 1994—1998 was estimated to be about 10.6 kg N ha−1year−1in South Korea(Park and Lee,2002).The projections suggest that it is important to understand the effects of elevated N on the terrestrial ecosystems in the Korean Peninsula.
This study investigated the combined effects of temperature,soil moisture,and N availability onQ10and substrateinduced respiration(SIR)under controlled laboratory conditions.Incubation experiments under controlled laboratory conditions allowed us to study the combined effects of these three selected factors onQ10,which cannot be tested under field conditions due to the interference of other interacting factors.Therefore,a laboratory incubation experiment was performed using soil collected from a temperate forest in South Korea to investigate the response of SIR andQ10to concurrent changes in temperature, soil moisture, and N addition.
MATERIALS AND METHODS
Study site and soil sampling
Soil samples were collected from a deciduous forest dominated by Mongolian oaks (Quercus mongolica) in Bukhan Mountain,South Korea(37◦43′27′′N,126◦59′96′′E, and 243 m above sea level). The sampling site has an annual mean air temperature of 12.9◦C and annual precipitation of 1 464 mm(Lee,2011).The soil type was characterized as acidic sandy loam in the surface soil horizon up to 10 cm;soil pH was 4.8,soil organic C content was 8.8%,and total N content was 1.4 g gk−1(Baeet al,2008).Water-extractable organic C of the soil sample was 4.28µg g−1soil.
To obtain representative soil samples from the hilly study site,sampling was conducted on a relatively steep(>25◦)slope,which is typical in the area.Samples were collected from a top,a mid-slope,and a foothill location of a slope at an equal distance of 5 m. A metal frame with an open inner square(25 cm×25 cm)was placed on the forest floor.The litter material, composed of freshly fallen or slightly decomposed litter (Oi), moderately decomposed material(Oe), and completely decomposed material (Oa), was cut inside the frame and then collected by hand. Mineral soil in the A-horizon was ploughed to a depth of 10 cm using a trowel.For additional measurements,including bulk density,intact soil samples were collected from the same locations using a soil corer(59 mm in diameter and 61 cm in length)(Giddings Machine Company,USA).Top mineral soils up to 10 cm below the forest floor of the collected intact soil cores were measured for volume and dry weight to calculate bulk density(Nhantumbo and Bennie,2001).
All soil samples were kept in an icebox during transportation to the laboratory.The litter material was cut using scissors and sieved through a 5-mm mesh. Soils from the three sampling points were mixed thoroughly and sieved through a 2-mm mesh to generate one composite sample for the incubation experiment.Sieved soils were kept at 4◦C before starting the incubation.
Soil incubation experiment
The experiment included two incubation temperatures(15◦C,close to the annual mean temperature at the sampling site(T1)and 20◦C,5◦C warming(T2)); three soil moisture conditions measured in water-filled pore space(WFPS)(10%,dry(W1);28%,moist(W2);and 50%,wet(W3));and two N treatments(0(N0)and 59.5 mg N kg−1soil (N1)), totalling 12 treatments, T1N0W1, T1N0W2,T1N0W3, T1N1W1, T1N1W2, T1N1W3, T2N0W1,T2N0W2, T2N0W3, T2N1W1, T2N1W2 and T2N1W3.The treatment T1N0W2 was considered the control. Based on the measured ratio of organic litter material to the mineral soil,2 g of organic horizon materials(Oeand Oamixed together)underlain by 20 g of mineral soils(dry mass basis)was placed in 120 mL glass vials. Seven replicates per treatment were incubated at two different incubation temperatures:15 and 20◦C.Soil moisture levels were set up with deionized water,and WFPS(%)was calculated as follows(Linn and Doran,1984):
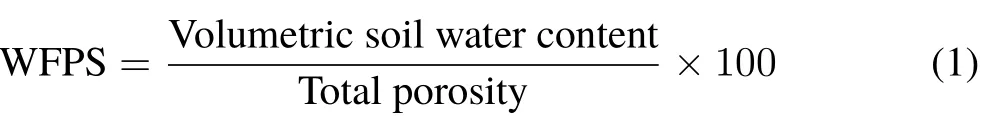
where total porosity was calculated as:

where soil particle density takes the default value of 2.65 g cm−3.
The two levels of N were created by adding 5 mL water or a 5-mL solution containing 3.4 mg NH4NO3to each soil sample(20 g on dry soil basis).The amount of N added for N1 was equivalent to 50 kg N ha−1year−1based on the surface area of the incubated soil and the incubation period(60 d out of 365 d per year),and it is also approximately five times the global average annual atmospheric N deposition(9—10 kg ha−1year−1)projected for 2050(Lamarqueet al.,2005;Kanakidouet al.,2016).
Throughout the incubation,the moisture level of each soil was maintained constant by adding water when needed.On the 30th day of incubation,seven replicates of each treatment incubated at two temperatures(15 and 20◦C)were randomly placed in incubators set at five different temperatures(10,15, 20, 25, and 30◦C) for 24 h to determine short-term changes in the temperature sensitivity of soil heterotrophic respiration.Based on the method of Craineet al.(2010)with modification,three replicates per treatment were maintained under an appropriate incubation temperature(15 or 20◦C)to determine the accuracy of the measurements(three replicate measurements per incubation temperature), whereas only one soil was incubated at the four other measurement temperatures.The incubation temperatures enabled the microbes to respond to two different temperatures for 30 d.Furthermore,higher than normal temperatures are expected under the effects of climatic warming during the summer months.Therefore,30 d(equivalent to one month)allowed the microbes to acclimatize to temperatures before they were subjected to short-term temperature treatments(for the estimation ofQ10).In other soil incubation studies investigating temperature sensitivity,soil samples were often incubated at five or more different temperatures for 24 h(Meyeret al.,2018). According to Meyeret al. (2018), the first 12 h of incubation allowed for equilibrium. Soil microbes need a few hours to adapt to new temperatures.The duration varies between studies, ranging from 2 h (Kochet al., 2007) to 24 h(Vanhalaet al.,2011).This design also enabled us to confirm the measurement accuracy within the given time limits.The initial CO2efflux from the control soils(without N and at 28%WFPS)incubated at 15 and 20◦C was 25.82 and 37.38 mg CO2-C m−2h−1,respectively.
Gas sampling and analysis and Q10 determination
At the end of the 24 h incubation of the soils at 10,15,20,25,and 30◦C,gas samples were collected twice from the headspace:immediately after closing the incubation vial and 30 min later. Concentration of CO2was determined with a gas chromatograph(7890A,Agilent,USA)fitted with a Supelco Hayesep Q column(injector: 200◦C;detector:250◦C;oven:60◦C;and makeup gas:N2).The standard gases used to calibrate the gas chromatograph included four different CO2concentrations in the N2makeup gas. The standard gas with CO2concentration close to the sample range was repeatedly analyzed after every five samples to ensure proper functioning of the gas chromatograph.
The relationship between temperature(T)and soil heterotrophic respiration(SR)can be expressed using an exponential equation:

where SRTis soil respiration at temperatureTandαandβare constants.
Then,Q10was calculated as(Lloyd and Taylor,1994):

Determination of SIR
To determine the effect of warming coupled with N addition under different soil moisture conditions on the physiological profile and substrate utilization pattern of soil microbial community, soil samples were collected at the end of the 60-d incubation and measured for SIR using MicroRespTMtechnique(Macaulay Scientific Consulting Ltd., UK)as described by Campbellet al. (2003). A preincubation period allows a steady state of microbial activity to be established in the soil(Franzluebberset al.,1999).Previous soil incubation experiments have utilized pre-incubation periods ranging from 3 d to 4 weeks,which enable the reliable determination of SIR from homogenous soil(Creameret al.,2014).However,an extended pre-incubation can reduce the reliability of the steady state of microbial activity,due to a decrease in respiration intensity(Smirnovaet al.,2016). Six C substrates were selected: glucose, fructose,galactose, oxalic acid, citric acid, and tyrosine based on their widespread availability in soil environments and their lability,which permits an immediate physiological response of active groups in the soil microbial community(Glanvilleet al., 2012). MicroRespTMconsisted of a reaction plate with deep wells,which was filled with soil and C sources,and a detector plate,which detected CO2released from the reaction plate.Approximately 0.35 g of soil was put in each well of the reaction plate,and C substrates were added to the wells at a concentration of 30 mg mL−1soil water.A separate control group was included, with only deionized water added.The SIR was calculated as the difference between the respiration of the soil with substrate added and that of the control.
Statistical analysis
Regression significance for the relationship between temperature and respiration was tested using SigmaPlot 10.0 (Systat Software Inc., UK). As only oneQ10value was obtained per treatment,treatment effects onQ10were analyzed using single-factor analysis of variance(ANOVA)of SPSS 20 (IBM Inc., USA). For MicroRespTM-based analysis of SIR,one-way ANOVA followed by Tukey’sposthoctest (α= 0.05) at each WFPS level (10%, 28%, and 50%)under various combinations of incubation temperature and N addition was used.
RESULTS
Treatment effects on SIR
Significant differences(P <0.001)in SIR were found among the 12 treatments(Table I).Increasing the WFPS from 10%to 28%increased the overall SIR by 7.0%; however,increasing WFPS from 28% to 50% reduced the SIR by 41.0%.The warming treatment increased the average SIR by 47.7%(from 3.77 to 5.57µg CO2-C g−1h−1). The N addition increased the SIR by 42.0%(from 3.77 to 5.35µg CO2-C g−1h−1).When oxalic acid or citric acid was used as substrate,SIR in the W3 treatment was significantly lower(P <0.05)than those in the W1 and W2 treatments for a same temperature and N combination.
Wetting(W3)coupled with warming(T2)did not significantly affect the overall SIR.However,in the T2N0W3 treatment,SIR was significantly higher(P <0.05)than the control(T1N0W2)when glucose and galactose were used as substrate.Warming(T2)coupled with N addition(N1)did not affect the overall SIR, either. However, high SIR was observed when oxalic acid,glucose,and fructose were used as substrate.Nitrogen addition coupled with wetting did not affect the overall SIR.However,SIR was significantly reduced under warming coupled with wetting (T2N0W3)compared to the control(T1N0W2)when oxalic acid and citric acid were used as substrate (from 5.18 to 1.62 µg CO2-C g−1h−1for oxalic acid;from 7.26 to 1.87µg CO2-C g−1h−1for citric acid) and significantly increased whengalactose was used as substrate(from 2.23 to 5.22µg CO2-C g−1h−1). Warming coupled with N addition and wetting reduced the overall SIR by 42.4%(Table I).Significant reductions were observed on oxalic acid(from 5.18 to 1.72µg CO2-C g−1h−1)and citric acid(from 7.26 to 1.93µg CO2-C g−1h−1)under the treatment of warming coupled with N addition and wetting.

TABLE I Soil substrate-induced respiration measured using six substrates in the treatments of different combinations of incubation temperature(T),nitrogen addition(N),and water-filled pore space(W)
Treatment effects on Q10
Warming had a negative effect onQ10(Fig.1,Table II).The warming treatment reducedQ10by 37.5%(from 2.4 to 1.5)and by 23.0%(from 3.0 to 2.3)for soils with 28%and 50% WFPS, respectively. A negative effect of N addition was also observed.Under incubation at 15◦C,the addition of N reduced theQ10by 16.7%at 28%WFPS(2.4 to 2.0)and reducedQ10by 33.3%at 50%WFPS(from 3.0 to 2.0).In contrast,wetting increased theQ10by 25%(from 2.4 to 3.0,P <0.05,Table II)while 10%WFPS reduced theQ10by 58%(2.4 to 1.0).Increasing the moisture from 10%to 50%WFPS increased theQ10by 200%(1.0 to 3.0).
The addition of N coupled with wetting(from 28%to 50%WFPS)did not affect theQ10(Table II).Wetting(from 28%to 50%WFPS)coupled with the warming treatment did not affect theQ10(4%decrease).Wetting treatment(from 28%to 50%WFPS)coupled with the N addition reduced theQ10.The combination of warming,wetting(from 28%to 50%),and N addition increased theQ10by 12.5%compared with the control(T1N0W2)(P <0.05).
DISCUSSION
Responses of SIR to the different treatments
Warming increased SIR,with significantly higher individual SIRs for the simple sugars,such as glucose,galactose,and fructose, compared with the control (T1N0W2). Generally,microbes can acclimatize to warming(Bradfordet al.,2008);therefore,the catabolic properties adjust to the warmed environment.However,microbes can be reactivated in response to the increased availability of externally added labile substrates(Bradford,2013;Freyet al.,2013).
After a 60-d incubation,SIR decreased under the wettest condition (50% WFPS) compared with the control moisture condition(28%WFPS).The wettest condition in our study may have hindered the diffusion of gas produced by decomposition of the added substrates.The addition of the labile substrates(glucose,galactose,fructose,oxalic acid,citric acid, and tyrosine) may have increased respiration to the maximum rate through a priming effect. However,the added substrate solution coupled with the already wet condition(50%WFPS)appears to have reduced the rate of gas diffusion,limiting O2availability for aerobic respiration(Lin and Brookes,1999).
In our study, we found that N addition increased the overall SIR. Previous studies have shown that N addition increased short-term microbial activity (Crecchioet al.,2001).In a nutrient-poor mineral forest soil,roots provide nutrients such as N, which enhance the SIR of microbes in the planted areas (Prihaet al., 1999). It was reported that N addition stimulates microbes to efficiently use the recalcitrant C pool once the labile C pool is depleted(Jianget al., 2014). In our study, we also observed a significant increase in the utilization of simple sugars, like glucose,galactose, and fructose. Following the addition of simple sugars, C accessibility increases, leading to changes in microbes or microbial activity (Kuzyakovet al., 2000).Microorganisms whose activities are limited by N availability might become stimulated following the addition of N;the stimulated microorganisms can then decompose labile C substrates,such as glucose(Freyet al.,2013;Liet al.,2017).
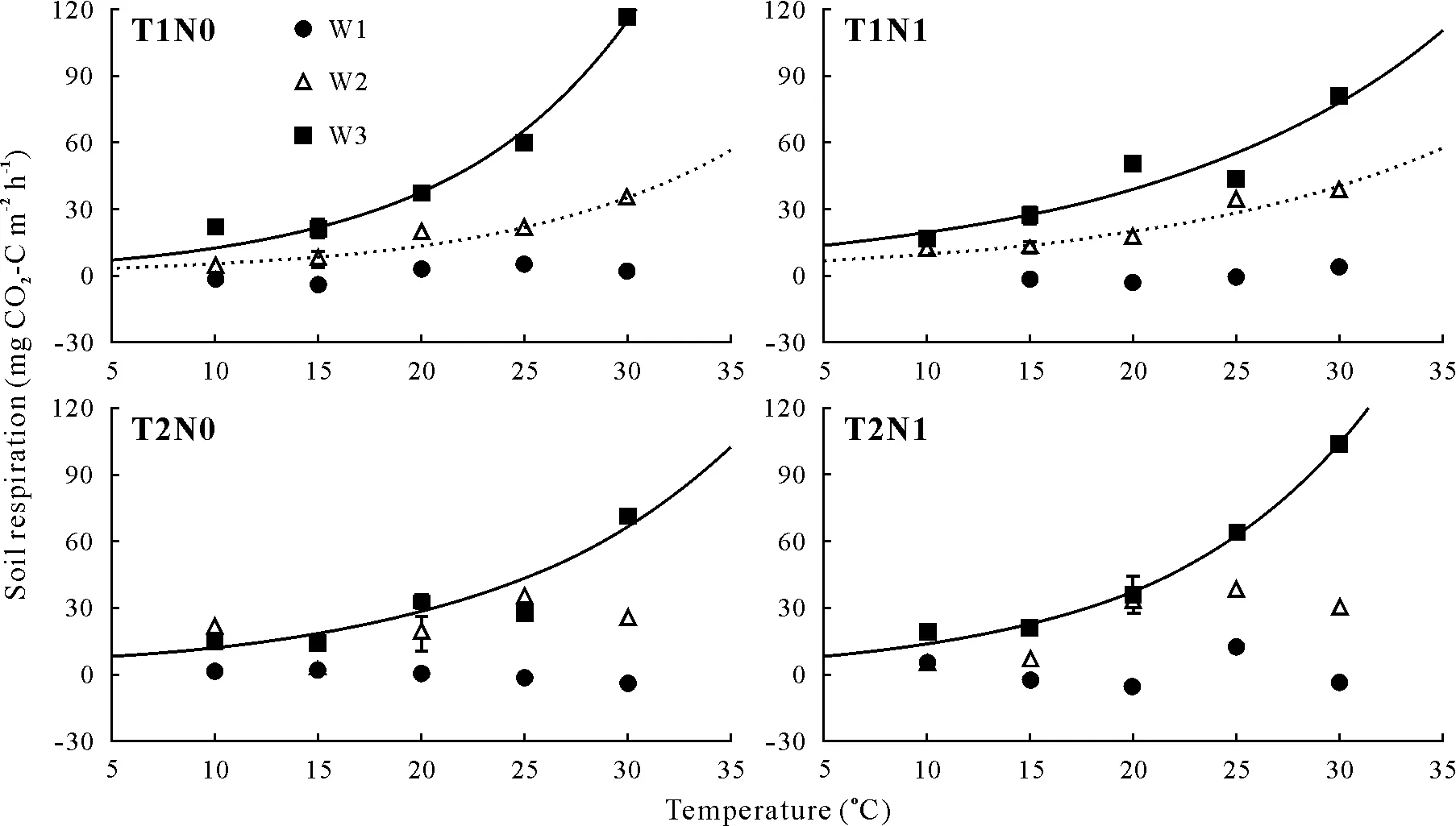
Fig.1 Short-term(24 h)temperature response of soil respiration on the 30th day of incubation at different combinations of temperature(T),nitrogen addition(N),and water-filled pore space(W).Error bars shown for two measurements in each subfigure indicate standard deviations(n=3).Only significant(P <0.05)regressions are indicated by best-fit lines.T1=15 ◦C;T2=20 ◦C;N0=0 mg N kg−1 soil;N1=59.5 mg N kg−1 soil;W1=10%water-filled pore space(WFPS);W2=28%WFPS;W3=50%WFPS.
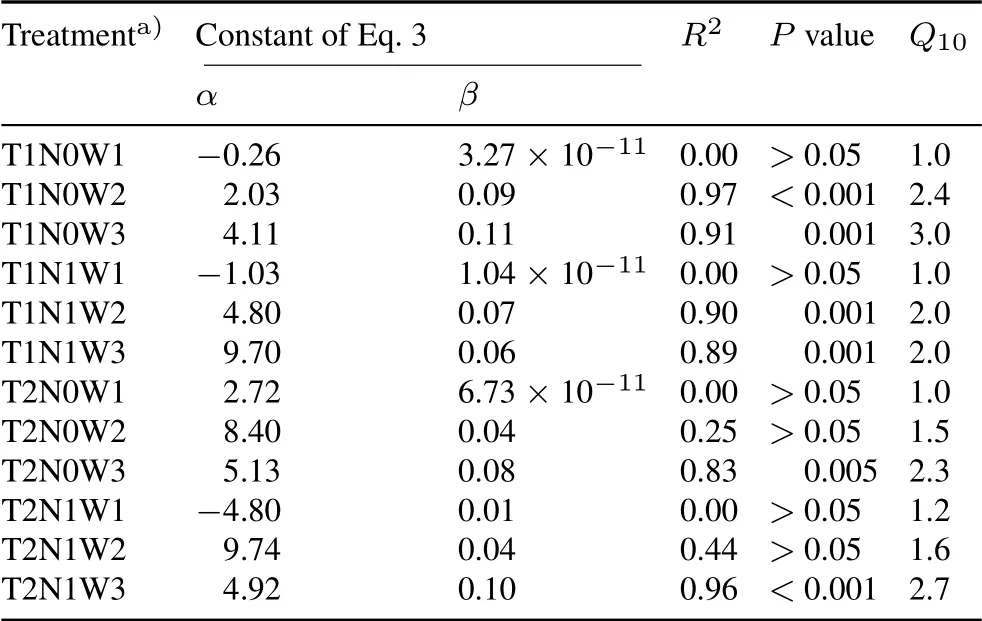
TABLE II Relationships(Eq.3)between soil heterotrophic respiration(SR)and incubation temperature (T) and temperature sensitivity (Q10, change in respiration with 10 ◦C increase in temperature)of SR at different combinations of incubation temperature(T),nitrogen addition(N),and water-filled pore space(W)
The combined treatment of wetting, N addition, and warming reduced the overall SIR.The results suggest that moisture confounded the effect of N availability and temperature on SIR.Soil moisture controls substrate availability in soil aggregates, further constraining the activity of the microbial population(Chenet al.,2007).For example,Zhouet al.(2013)found that six years of warming and elevated precipitation reduced SIR. They also found a marginally positive effect of N availability on SIR. Although in our study the addition of labile substrates may have increased the respiration to the maximum rate through a priming effect,the added solution coupled with the already wet condition(50%WFPS)appears to have reduced the rate of gas diffusion,leading to the limited availability of O2for aerobic respiration(Lin and Brookes 1999).The effect of this combined treatment was particularly pronounced in the case of carboxylic acids,i.e.,oxalic acid and citric acid.Carboxylic acids are low molecular labile C sources in forest soils, which are released as root exudates and during decomposition of leaves and other litter(Strobel,2001)and function as a source of labile C for soil heterotrophs(Van Heeset al.,2003a,b;Datéet al.,2018).As noted,the addition of these labile organic acid substrates might have enhanced respiration,leading to a reduction in gas diffusion.
Although only six C substrates were utilized in our study,all three major groups of C sources have been included:carbohydrates,amino acids,and carboxylic acids.A duration of 6 h was used for SIR measurement using the MicrorespTM,which may not be long enough to detect any long-term changes in catabolic diversity(Datéet al.,2018).A longer duration of MicroRespTMassessment may reveal a shift in the catabolic profile of soil microbial communities.
Responses of Q10 to the different treatments
We found that warming had a negative effect onQ10(Table II),consistent with the results of previous studies(Knorret al., 2005; Subke and Bahn, 2010). A review summarizing 27 warming experiments found inconsistent responses of soil respiration, but a decline inQ10at temperatures above 25◦C(Careyet al.,2016).Although warming briefly enhances soil respiration (Subke and Bahn, 2010), after the initial phase, soil microorganisms become acclimated to the warmer temperature and less temperature-sensitive(Crowther and Bradford,2013).Decrease in microbial enzyme activities, once their physiological thermal limit is reached,may explain the reducedQ10under warmer conditions(Careyet al.,2016).Conantet al.(2008)found that the effect of warming onQ10may change over time,because the decomposition of the labile C pool is less sensitive to temperature than that of the recalcitrant C pool(Conantet al.,2008).Therefore,the negative warming effects onQ10may indicate that the labile C pool is sufficient for fueling heterotrophic respiration in the warmed soil.
Similar to the effects of warmings, N addition also reducedQ10. Previous studies have reported contrasting effects of N addition onQ10in soils from various ecosystems.For example, in an incubation experiment with a pastoral soil from a Tibetan alpine meadow (Songet al., 2010),N addition did not affect theQ10, while in a laboratory incubation with tropical soil from Puerto Rico,N addition enhanced theQ10of both labile and recalcitrant C pools(Cusacket al.,2010).In both a wetland soil and a cultivated soil, a high N level (240 kg N ha−1) reduced theQ10(Jinet al.,2010).The contrasting findings of the previous studies could be attributed to the heterogeneity of biome types, N application rates, soil conditions, environmental conditions,and experimental methods(Zhonget al.,2016).The addition of N can change the quantity and quality of substrate inputs, soil environmental conditions, and the activity of soil microbial communities (Liuet al., 2016).After N addition,less N-demanding microbial species are replaced by more N-demanding species (Janssenset al.,2010).Nitrogen becomes so abundant that N becomes a less limiting factor for soil microbial organisms (Schimel and Weintraub,2003).The shifting of the microbial community composition towards more N-demanding species could be one of the reasons for the observed reduction inQ10,because N-demanding species might require less energy to obtain N in N-enriched soils (Janssenset al., 2010). Weiet al.(2017) observed a decrease inQ10under N-fertilization of a soil from a broad-leaved forest and suggested that recalcitrant organic materials are the major N sources for soil microorganisms. The addition of N inhibited specific microbial groups and changed the substrate preference from recalcitrant organic matter to labile organic matters.This led to a decrease inQ10due to the lower temperature sensitivity of labile organic materials.
The present results are consistent with those of previous studies, since wetting increased theQ10. Generally, soil microbial activity increases with WFPS increase from 30%to 60%(Linn and Doran,1984;Winkleret al.,1996;Qiet al.,2002).Linn and Doran(1984)found that soil respiration rate had a linear relationship with soil moisture at 30%—60%WFPS.Below 30%and over 60%WFPS,soil respiration decreased. Generally, soil respiration is most sensitive to temperature at intermediate moisture levels and becomes less sensitive at low and high moisture levels(Illeriset al.,2004;Reichsteinet al.,2005;Gabriel and Kellman,2011).
Our study found that N addition coupled with war-ming reduced theQ10.Liuet al.(2016)found a negative effect of N and temperature onQ10in a cold temperate forest in northeastern China.The authors stated that in the summer season,microbes responsible for heterotrophic respiration compete with roots for available nutrients,including N.This leads to the suppression of microbial respiration and its temperature sensitivity. In an incubation experiment, Weiet al. (2017) reported reduction inQ10due to N addition(100 kg N ha−1).Treseder(2008)argued that reduction in substrates due to thermal acclimation under warming could be compensated by the availability of externally added N.
In our study,the combined treatment of wetting,warming,and N addition increased theQ10.Considering that both N addition and warming reduced theQ10,while wetting increased theQ10,our results suggest that the effect of wetting might be greater than that of warming and N addition.The overriding effect of wetting can be explained as discussed above.Since N addition may increase the availability of labile substrates,soil moisture may facilitate substrate accessibility(Suseelaet al.,2012).Soluble and labile organic compounds are products of the microbial decomposition of soil organic matter.The higher availability of N can induce significant changes in the soil microbial activity and thus,increase C mineralization per unit of microbial biomass(Lagomarsinoet al.,2006).
Overall,the results of our study suggest that soil moisture may become increasingly important forQ10when climatic warming coincides with changes in precipitation(Hurshet al.,2017).In addition to forest soils,a strong influence of soil moisture onQ10has also been reported in grassland soils(Craine and Gelderman,2011).Given the synergistic effects of soil moisture on the response ofQ10to warming and N enrichment,more attention should be paid to widely varying soil moisture conditions when assessing the effects of warming and N enrichment on theQ10of forest soils under monsoon climates.Moreover,we used a well-drained sandy loam soil from a steep slope, which usually has a WFPS lower than 50%.The results from this experiment may not be applicable to soils that have greater pore-spaces and therefore hold more water.However,in the short-term,even a welldrained soil might possess soil moisture exceeding 50%WFPS, especially during recurrent extreme precipitation events. Our study focused on short-term changes in the temperature sensitivity of soil heterotrophic respiration under varying soil moisture conditions.The number of days with intense rainfalls has increased by 17%per century and 38%over the 227 years of instrumental recorded history in Seoul,South Korea(Wanget al.,2006).The recorded data show that there is a strong positive relationship between soil moisture level and precipitation during the summer months(Choet al.,2016).
CONCLUSIONS
Although this short-term laboratory study cannot fully represent complex field conditions,it provides some insights into the differential short-term responses of SIR andQ10to the individual and combined effects of warming, wetting,and N addition. Notably, the results reveal the concurrent moderating effect of soil moisture and N availability on the warming-induced alteration of SIR andQ10.The overall results suggest that considering warming effects alone might result in substantial errors in the estimation of soil heterotrophic respiration under climate change scenarios,because the actual rates of SIR andQ10can be substantially affected by concurrent environmental changes,including soil N enrichment and changing soil moisture regimes.While we acknowledge that results obtained under controlled laboratory conditions cannot be applied directly to more complex field conditions, we predict that warming coupled with N addition may sustain soil heterotrophic respiration at higher rates compared to the individual effects of warming and N addition,particularly under favorable soil moisture conditions(e.g.,50%WFPS in this experiment),which often occur during the summer monsoon period in forest ecosystems across East Asia.Further studies including multiple climatic and environmental factors under controlled conditions are required to investigate the effects of temperature on soil respiration for better prediction of site-specific soil microbial heterotrophic respiration. In addition, longer experiments should be conducted with various soils of different textures to understand variations in the composition and activity of microbial communities in response to changing environmental conditions.As indicated by the altered SIR rates at the end of the incubation,which did not reflectQ10changes in the middle of incubation,simultaneous measurements of soil microbial properties,such asQ10,SIR,and soil microbial biomass,are essential to understand and corroborate concomitant changes in the microbial physiological profile and temperature sensitivity of soil respiration.
ACKNOWLEDGEMENTS
The study was funded by the National Research Foundation of Korea (No. 2017R1D1A1B06035179) and the Seoul Green Environment Center(SGEC).We are grateful to Tanya Kreutzer Sayyed,Communications Consultant and Researcher, South-South and Triangular Cooperation, for constructive and valuable comments on an earlier version of this manuscript.D.-G.K.gratefully acknowledges support from the International Atomic Energy Agency(IAEA),Vienna,Austria,through a Coordinated Research Project(No.CRP D1 50.16).
杂志排行
Pedosphere的其它文章
- Yield-scaled nitrous oxide emissions from nitrogen-fertilized croplands in China:A meta-analysis of contrasting mitigation scenarios
- Optimizing the use of open chambers to measure ammonia volatilization in field plots amended with urea
- A simple and easy method to measure ammonia volatilization:Accuracy under field conditions
- Nitrification inhibitor nitrapyrin does not affect yield-scaled nitrous oxide emissions in a tropical grassland
- Decreased nitrous oxide emissions associated with functional microbial genes under bio-organic fertilizer application in vegetable fields
- Nutrient cycling and greenhouse gas emissions from soil amended with biochar-manure mixtures
