Complete genome analysis of bacteriochlorophyll a-containing Roseicitreum antarcticum ZS2-28T reveals its adaptation to Antarctic intertidal environment
2021-04-10ZengYinxinYuYongLiHuirongLuoWeiDingHaitao
Zeng Yinxin, Yu Yong, Li Huirong, Luo Wei & Ding Haitao
• Article •
Complete genome analysis of bacteriochlorophyll-containingZS2-28reveals its adaptation to Antarctic intertidal environment
Zeng Yinxin, Yu Yong, Li Huirong, Luo Wei & Ding Haitao
MNR Key Laboratory for Polar Science, Polar Research Institute of China, Shanghai 200136, China
Aerobic anoxygenic phototrophic bacteria (AAPB) are photoheterotrophic prokaryotes able to use both light and dissolved organic matter as energy sources.ZS2-28was isolated from intertidal sediment in the Larsemann Hills, Princess Elizabeth Land, Antarctica, and was able to produce bacteriochlorophyll. It is the type strain of the sole species within the genus. The complete genome sequence of the bacterium was determined using Illumina HiSeq X and PacBio RSII systems. The genome ofZS2-28was 4253095 bp and consisted of one chromosome and four plasmids. A number of genes related to the bacteriochlorophyll, photosynthetic reaction, cold adaptation, salt adaptation, ultra-violet resistance and DNA damage repairing were found in the genome. In addition to genomic islands and type IV secretion systems, genes related to gene transfer agents were detected in the genome ofZS2-28, suggesting that this bacterium can adapt to its environment by acquiring exogenous nucleic acids. The annotated complete genome sequence provides genetic insights into the environmental adaptation and ecological function ofZS2-28in Antarctic coastal area.
, complete genome, adaptation, gene transfer, strain, intertidal sediment, Antarctica
1 Introduction
Aerobic anoxygenic phototrophic bacteria (AAPB) are bacteriochlorophyll-containing bacteria with the capability of photoheterotrophy; they appear to play a unique role in the ocean’s carbon cycle (Swingley et al., 2007; Tang et al., 2010; Graham et al., 2018). Heterotrophy usually is the main system of energy gain of AAPB (Beatty, 2002), and phototrophy is minimal (Ferrera et al., 2017). However, light can enhance the growth rates of AAPB (Ferrera et al., 2017; Piwosz et al., 2018). AAPB are widely distributed in open and coastal oceans (Jiao et al., 2007), including Arctic and Antarctic marine environments (Boeuf et al., 2013; Zeng et al., 2016). AAPB are phylogenetically diverse and include members of the-,-and(Imhoff et al., 2017; Lehours et al., 2018; Auladell et al., 2019). Physiological constraints play an important role in structuring AAPB assemblages at a global scale, and salinity seems to favor lineage-specific adaptations of AAPB (Lehours et al., 2018). AAPB belonging toanddominate the offshore and river-influenced surface waters in the western Arctic Ocean, respectively (Boeuf et al., 2013). Represented by the ordersand(Imhoff et al., 2017), members of theAAPB are widespread and abundant in Arctic and Antarctic environments (Koh et al., 2011; Boeuf et al., 2013; Lehours and Jeanthon, 2015; Zeng et al., 2016).
The genusof the familywithin the classwas proposed by Yu et al. (2011) with one species,ZS2-28. The bacterium was isolated from sandy intertidal sediment samples collected from the coastal regions of the Chinese Antarctic Zhongshan Station in the Larsemann Hills, Princess Elizabeth Land, Antarctica, during the 23rd Chinese National Antarctic Research Expedition in March 2007 (Yu et al., 2011). Phylogenetic analysis based on 16S rRNA gene sequences indicated that strain ZS2-28formed a distinct evolutionary lineage within the clade containing members of the genera,, andof the class(Yu et al., 2011).ZS2-28contained bacteriochlorophyllwas obligately heterotrophic, strictly aerobic, non-motile, and moderately halophilic (Yu et al., 2011). The phenotypic and chemotaxonomic characteristics ofZS2-28were reported by Yu et al. (2011). However, the complete genomic information ofZS2-28,which would be helpful for us to understand the adaptation of the bacterium to Antarctic environment and provide further insight into its ecological functions, was not reported. Thus, we performed genome sequencing and present the complete genome sequence ofZS2-28in this study by using Illumina HiSeq X and PacBio RSII systems. At the time of writing,ZS2-28is the type strain of the sole species with a valid published name within the genus. Therefore, this is also the first complete genome sequence of astrain.
2 Materials and methods
ZS2-28was isolated from sandy intertidal sediment samples in the Larsemann Hills, Princess Elizabeth Land, Antarctica (Yu et al., 2011).The bacterium was deposited into the China General Microbiological Culture Collection Center (CGMCC) with the accession number CGMCC 1.8894and the Belgian Coordinated Collections of Micro-organisms (BCCM) with the accession number LMG 24863. The complete chromosome sequence and four plasmid sequences of.ZS2-28have been deposited in GenBank under the accession numbers CP061498, CP061499, CP061500, CP061501 and CP061502, respectively.
The general features ofZS2-28and MIGS mandatory information are listed in Table 1. Genomic DNA was extracted from overnight cultures using a MagAttract HMW DNA Kit (Qiagen, Germany) according to the manufacturer’s instructions. The harvested DNA was visualized on 1% (w/v) agarose gels, and DNA concentration and purity were measured with a Qubit 2.0 Fluorometer (Life Technologies, USA). Purified DNA was used to construct an Illumina standard shotgun library with an insert size of 300–400 bp followed the NEB Next Ultra DNA Library Prep Kit for Illumina (New England Biolabs, USA), and then was sequenced using the Illumina HiSeq X platform using the PE150 model. A 10-kb DNA library was constructed by the PacBio SMRTbell 10 kb Library preparation Kit (Pacific Biosciences, USA) according to the manufacturer’s instructions. Library construction and sequencing were performed at Sangon Biotech Co. Ltd (Shanghai, China). The whole genome sequencing was performed using Illumina HiSeq X (Illumina, USA) and PacBio RSII (Pacific Biosciences, USA) systems.
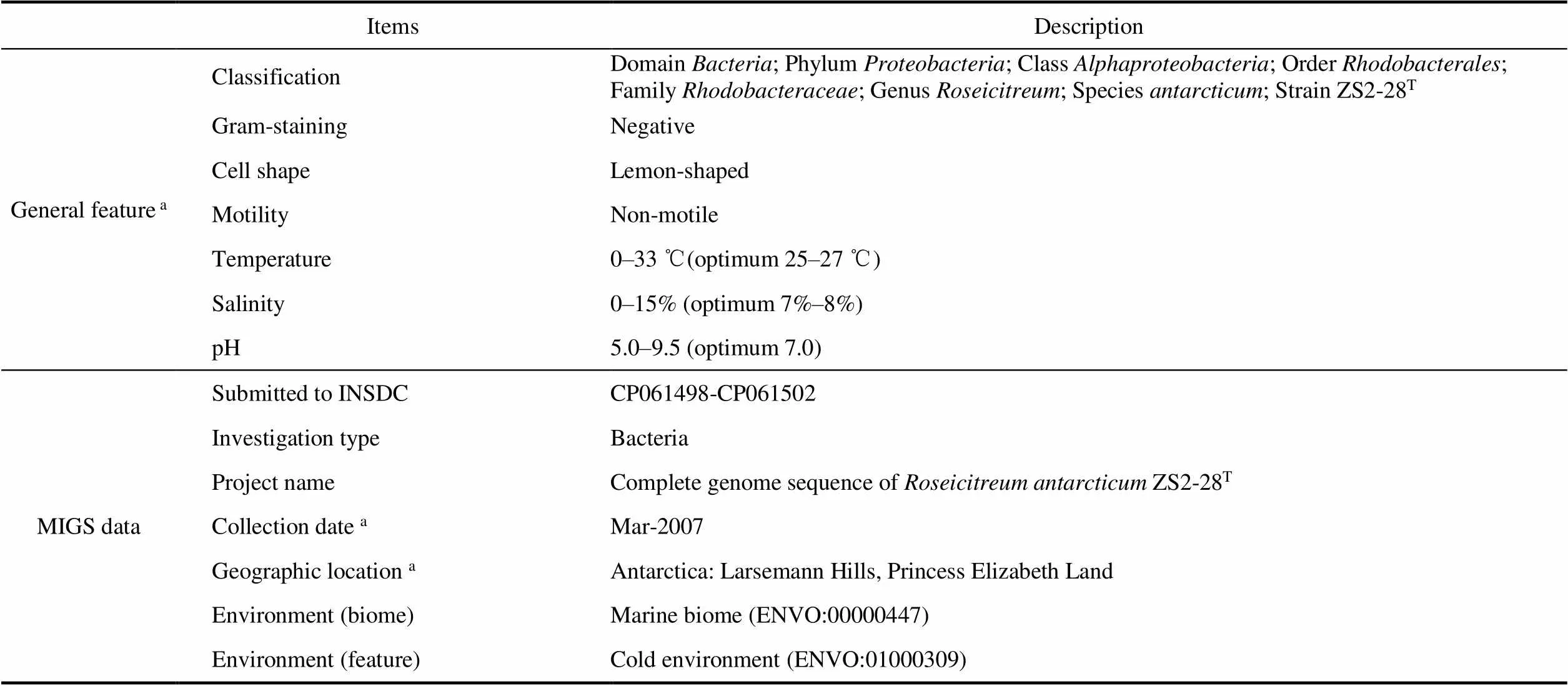
Table 1 General features of Roseicitreum antarcticum ZS2-28T and MIGS mandatory information
Continued
Note:Data from reference (Yu et al., 2011).
genome assembly was performed using continuous long reads following the Canu workflow v1.3 (Koren et al., 2017), and then Pilon v1.23 was engaged to correct assembled contigs with Illumina reads (Walker et al., 2014). Annotation of the genome was generated by using Prokka v1.10 (Seemann, 2014) to predict coding sequences, ribosomal RNA genes, and transfer RNA genes. A whole genome Blast (v2.2.28) search was performed against the databases CDD, PFAM, COG, NR, Swiss-Prot, and TrEMBL. KEGG ontology was identified by submitting predicted peptides to the KAAS server (http://www. genome.jp/tools/kaas/). GO was detected from Swiss-Prot and TrEMBL annotation results. Four additional databases PHI, VFDB, CARD, and CAZy, were used to annotate peptides. Signal peptides were detected on the genome assembly by SignalP v4.1 (Petersen et al., 2011). Transmembrane proteins were detected by TMHMM v2.0 (Möller et al., 2001). Lipoproteins were detected with LipoP v1.0 (Juncker et al., 2003). Repeat regions within the genome were detected with RepeatModeler (http://www. repeatmasker.org/RepeatModeler/) and RepeatMasker (http://repeatmasker.org), and CRISPRs were detected with CRISPRCasFinder (https://crisprcas.i2bc.paris-saclay.fr). Genomic islands were predicted using IslandPath-DIOMB (Bertelli and Brinkman, 2018). The prophage regions were detected with PhiSpy (Akhter et al., 2012). Architecture of the photosynthesis gene cluster was produced using gggenes v0.4.1 (https://wilkox.org/gggenes/). Multiple sequence alignments (MSAs) were produced with ClustalW algorithm implemented in the MEGA 5.05 software package (https://www.megasoftwa re.net). DNA sequences ofandgenes were obtained from GenBank.andMSAs were concatenated to form a singleMSA. A neighbor-joining phylogenetic tree was constructed based ongene sequences using MEGA 5.05.
3 Results and discussion
3.1 General description of the genome
The genome sequence ofZS2-28was obtained from a total of 3.0 Gb of high-quality data, which comprised 1.653 Gb Illumina HiSeq X data and 1.396 Gb PacBio RSII data. These data respectively represented 391- and 331-fold coverage of the genome. The genome ofZS2-28consists of one chromosome (3537072 bp, 63.45 mol% G + C), and four plasmids, named as pRA01 (425208 bp, 62.70 mol% G + C), pRA02 (131922 bp, 59.56 mol% G + C), pRA03 (103727 bp, 60.17 mol% G + C) and pRA04 (55166 bp, 58.51 mol% G + C), respectively (Table 2). Graphic circular maps ofZS2-28are shown in Figure 1.
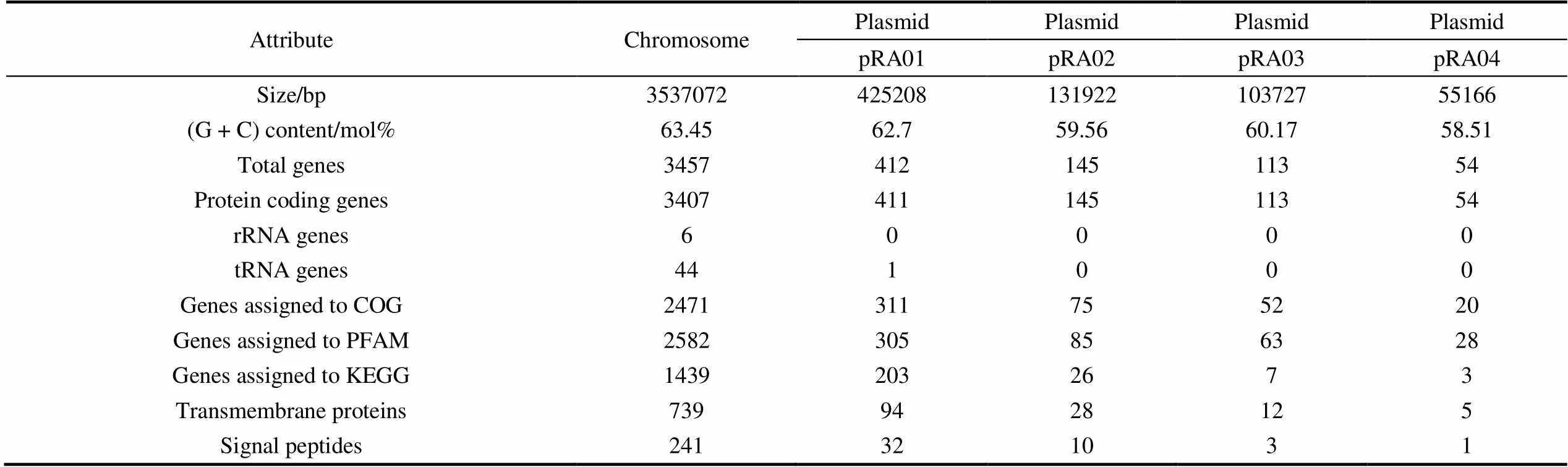
Table 2 Genomic features of Roseicitreum antarcticum ZS2-28T
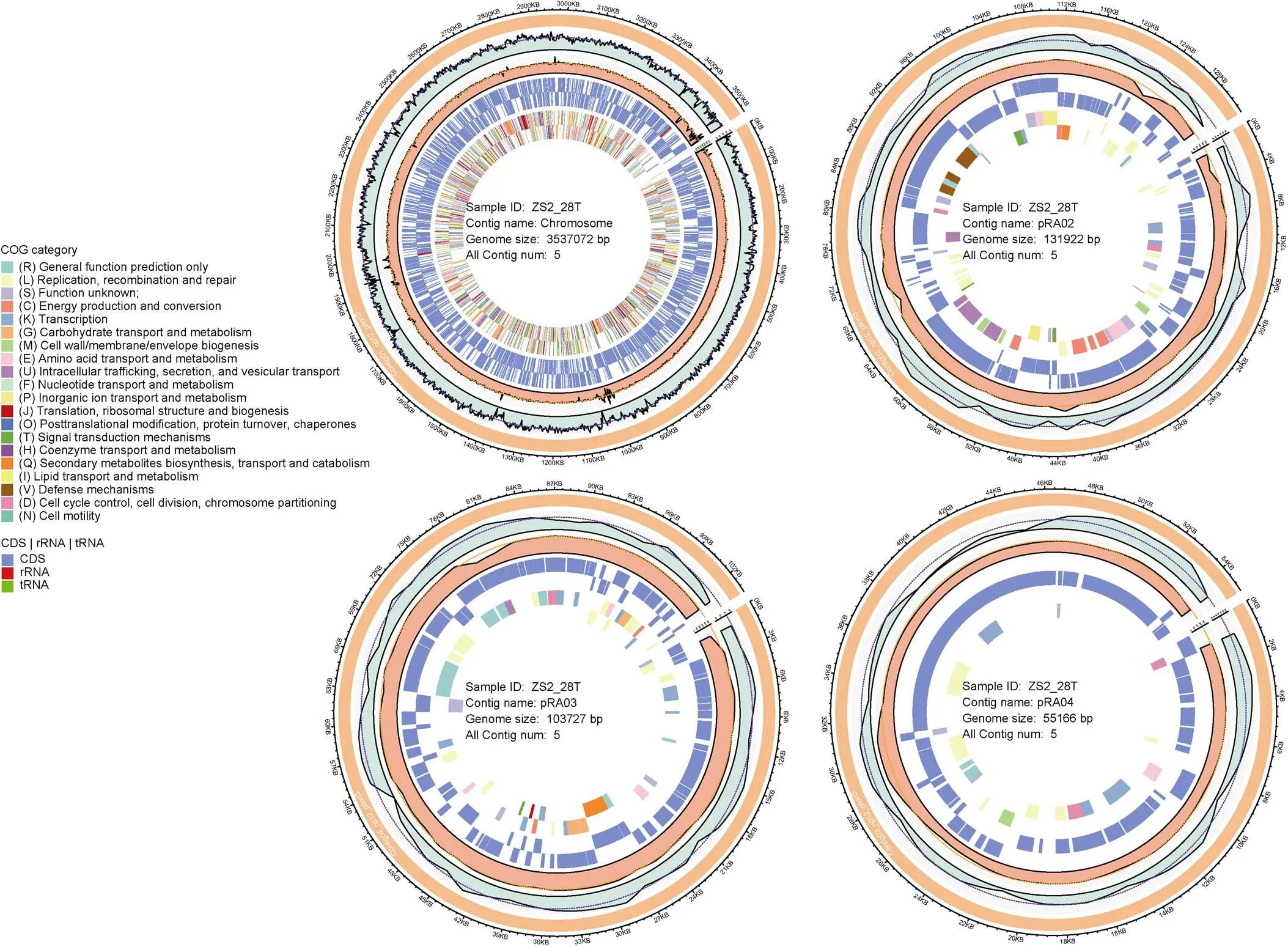
Figure 1 Circular map of one chromosome and three plasmids in the genome ofZS2-28. From outside to the center: the number of bases, chromosome or plasmid, G+C content, coverage, CDS/rRNA/tRNA on the forward strand, CDS/rRNA/tRNA on the reverse strand, COG on the forward strand, and COG on the reverse strand (colored by COG categories).
The genome contains 45 tRNA genes and 2 rRNA operons. The numbers of 5S, 16S and 23S rRNA genes were all two. Among the 4181 predicted protein-coding genes, 3251 (78.93%), 2929 (71.11%), 3918 (95.12%), 3063 (74.36%), 2773 (67.32%), 3904 (94.78%), 2692 (65.36%), and 1856 (45.06%) were annotated by querying the CDD, COG, NR, PFAM, Swiss-Prot, TrEMBL, GO, and KEGG databases, respectively. There were 257 (6.14%) genes that failed to annotate in at least one database. Based on KEGG pathway classification (Figure 2), 74.77% of the annotated genes were found to be involved in metabolisms, including amino acid metabolism (17.75%), carbohydrate metabolism (14.11%), overview (11.61%), energy metabolism (6.76%), metabolism of cofactors and vitamins (6.75%), nucleotide metabolism (5.14%), xenobiotics biodegradation and metabolism (4.26%), lipid metabolism (4.26%), biosynthesis of other secondary metabolites (1.68%), metabolism of terpenoids and polyketides (1.58%), and glycan biosynthesis and metabolism (0.99%).
3.2 Genes related to photoheterotrophic lifestyle
Strain ZS2-28is obligately heterotrophic (Yu et al., 2011), utilizing L-arabinose, cellobiose, D-galactose, gentiobiose, D-glucose, maltose, D-mannose, L-rhamnose, D-ribose, sucrose, trehalose, turanose, D-xylose, D-mannitol, adipic acid, gluconate, malic acid, glycerin, amygdalin, pyruvate, casein hydrolysate and yeast extract as sole carbon and energy sources. This strain is also positive for arginine dihydrolase, lysine decarboxylase, ornithine decarboxylase, urease, β-galactosidase and β-glucosidase activities (Yu et al., 2011). The major COG categories of the genome were amino acid transport and metabolism (10.17%), carbohydrate transport and metabolism (8.71%), transcription (7.17%), replication, recombination and repair (7.10%), energy production and conversion (6.21%), inorganic ion transport and metabolism (5.84%), and translation, ribosomal structure and biogenesis (5.71%). Annotation based on the CAZy database indicates that strain ZS2-28genome contains a large number of carbohydrate-active genes, including 18 auxiliary activities (AA), 6 carbohydrate-binding modules (CBM), 36 carbohydrate esterases (CE), 50 glycoside hydrolases (GH), 36 glycosyl transferases (GT), and 2 polysaccharide lyase (PL). In addition, annotation based on the PFAM database reveals a total of 93 peptidases and 23 proteases in the genome.
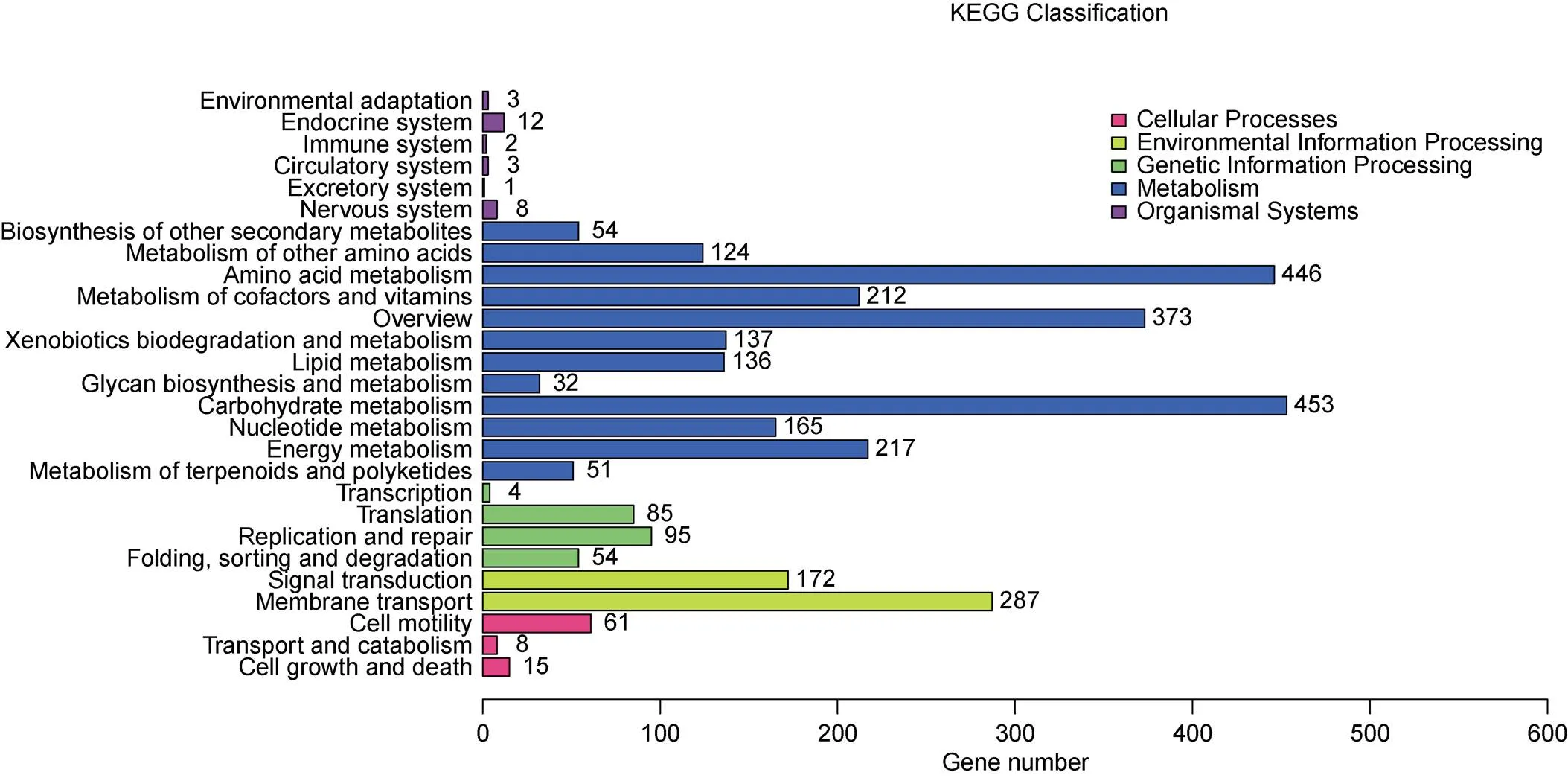
Figure 2 KEGG pathway classification of the annotated genes.
The strain ZS2-28can produce(Yu et al., 2011)In the biosynthesis of BChl, conversion of chlorin to bacteriochlorin ring is known to be catalyzed by chlorophyllideoxidoreductase (COR), which is a nitrogenase-like enzyme and a three-subunit complex consisting of BchX, BchY and BchZ (Tsukatani et al., 2013). Product of this reaction is further catalyzed by BchF and BchC (Tsukatani et al., 2013). According to genome information, thegene set, andandgenes were present in strain ZS2-28(Figure 3a), and showed more than 72% sequence similarities to those ofbased on BlastX searching in NCBI database. There were 23 genes related to the formation of Bchl found in the chromosome based on GO annotation. Two proteins (Pfam05398 and Pfam07284) involved in Bchl biosynthesis pathway were observed. At the same time, similar to the oxygen regulatedoperonof purple photosynthetic bacterium(Chidgey et al., 2017),,andgenes encoding the light-harvesting (LH)1 α and β polypeptides, and,andgenes encoding the type-II photosynthetic reaction center (RC) L, M and H submits were observed in the genome of strain ZS2-28(Lee et al., 1989; Hunter et al., 1991; Imhoff et al., 2019). In addition, a photosynthetic reaction center cytochrome C encoding genewas found in the genome. The cytochrome associated with the photosynthetic reaction center is an important component in many of the PS-II type photosynthetic bacteria (Imhoff et al., 2019). However, different from,gene encoding the PufX polypeptide was absent from the genome of strain ZS2-28. Three genes encoding proteins (Pfam02276 for photosynthetic reaction center cytochrome C subunit, Pfam03073 for sensory protein and Pfam04940 for blue light sensor protein) involved in photosynthesis were found in the chromosome. Phylogenetic analysis (Figure 3b) based onandgenes indicated that strain ZS2-28fell into theclade containing the genera,,,and. Theandgenes of strain ZS2-28showed close relationship (77.6% sequence similarity) tosp. CCS1.
Thegene encoding Mg-protoporphyrin IX monomethyl ester cyclase was observed in strain ZS2-28, showing more than 80% sequence similarities to those ofbased on BlastX searching in NCBI database. AcsF activity exists only under aerobic growth conditions in a(Pinta et al., 2002). As an analogue of the oxygen-dependant cyclase encoded bygene,gene encoding the oxygen-independent Mg-protoporphyrin monomethylester cyclase was not detected in the genome of strain ZS2-28. Thegene seems to be unessential for phototrophy inspecies (Koblížek et al., 2013). Results support that strain ZS2-28is an aerobic photosynthetic purple bacterium, and are consistent with previous finding that strain ZS2-28is photoheterotrophic (Yu et al., 2011).
3.3 Genes related to genetic exchange
Horizontal gene transfer (HGT) has been regarded to play an important role in the adaptation of the microbes to environment by providing the tools that are necessary to face the adversity and survive in a harsh environment (Springael and Top, 2003; Paquola et al., 2018). Consistent with the finding of a gene transfer agent (GTA) capsid protein gene (5) in strain ZS2-28(Zeng, 2019), a total of six genes related to GTA were observed in the chromosome based on NR annotation. GTAs have evolved from prophages that have lost the ability to target their own DNA for packaging (Lang et al., 2012). However, no prophage region was found in the genome of strain ZS2-28. Neither repeated regions nor the CRISPR-Cas system were detected in the genome.
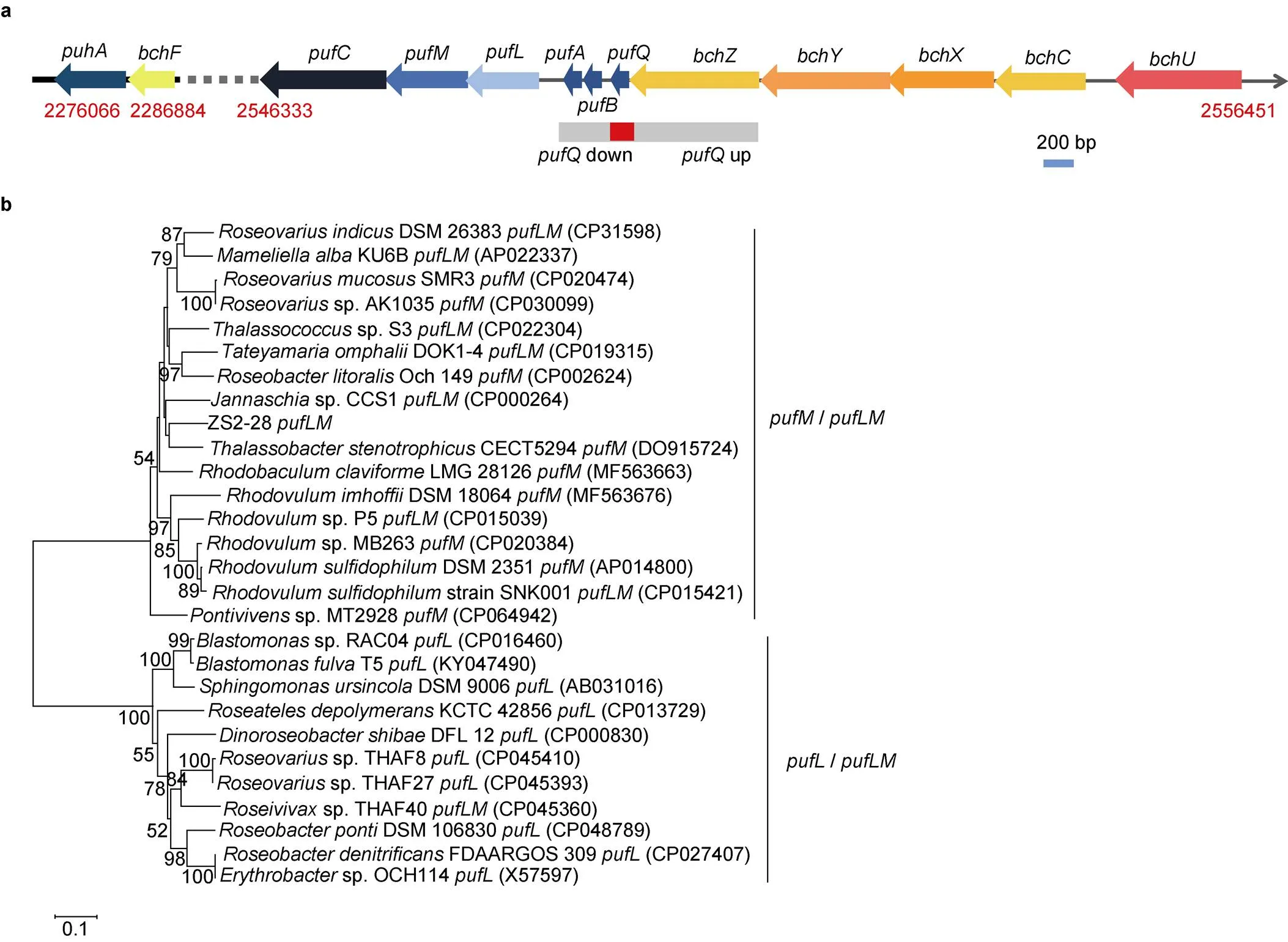
Figure 3 Photosynthesis genes in the genome ofZS2-28. a, Genes in the potential photosynthesis gene cluster ofZS2-28; b, Neighbor-joining phylogenetic tree based onandgene sequences showing the relationship between strain ZS2-28and representatives of some other related taxa. Numbers at nodes indicate percentages of 1000 bootstrap re-samplings, only values above 50% are shown. Bar, 0.1 substitutions per site.
A total of 16 genomic islands were predicted in the chromosome. At the same time, five type IV secretion system genes were observed in the genome based on Swiss-Prot annotation. Type IV secretion systems and genomic islands-mediated horizontal gene transfer have been reported inand(Juhas, 2015). Transposases and integrases can mediate the movement of DNA sequences within or between genomes (Rice and Baker, 2001). Strain ZS2-28contains 74 transposase and 42 integrase genes, and annotation based on the Pfam database indicated that diverse transposases (Pfam00872, Pfam01527, Pfam01548, Pfam01695, Pfam02371, Pfam03050, Pfam03400, Pfam04986, Pfam05598, Pfam13005, Pfam13340, Pfam13586, Pfam13610, Pfam13737, and Pfam13751) and integrases (Pfam00589, Pfam00665, and Pfam13683) are present in the genome. These results suggest thatZS2-28may adapt to the environment by acquiring exogenous nucleic acids.
3.4 Genes related to adaptation to Antarctic intertidal environment
The combination of seasonal scouring and encasement in ice, high UV irradiation, and high levels of salinity and temperature fluctuations make the Antarctic intertidal zone possibly the world’s most physically disturbed environment (Peck et al., 2006). Cells of strain ZS2-28were surrounded with slime (Yu et al., 2011). Slime is actually a kind of exopolysaccharides (EPS) produced by bacteria, and is helpful for bacteria surviving in extreme environments (Flemming, 2016). Four capsule polysaccharide biosynthesis proteins (Pfam05159) and 21 glycosyl transferases (Pfam00534, Pfam00535, Pfam00591, Pfam00953, Pfam00982, Pfam03808, Pfam04101, Pfam13632, Pfam13641 and Pfam13704) were detected in the genome.
One ultra-violet resistance protein (Pfam12344), showing 85% similarity to excinuclease ABC subunit UvrB ofstrain MAFF303099 based on Swiss-Prot database, was detected in the chromosome. At the same time, KEGG pathway annotation reveals that 8 genes encoding single-strand DNA-binding protein Ssb and ATP-dependent DNA helicase RecG are present in the genome. Those proteins may play role in repairing of DNA damage caused by ultra-violet irradiation (Trgovcević et al., 1989; Xu et al., 2020). It is interesting to find that all three genes assigned to KEGG in plasmid pRA04 (Table 2) are associated with single-strand DNA-binding protein Ssb, ATP-dependent DNA helicase RecG and ATP-dependent RNA helicase DeaD, suggesting that the smallest plasmid may play a role in genetic information processing in strain ZS2-28.
RNA helicase controls RNA folding and degradation in bacteria under low temperatures (Médigue et al., 2005). Annotation based on the Pfam database indicates that diverse RNA helicases, including 4 DEAD/DEAH box helicases (Pfam00270), 1 DEAD/H associated (Pfam08494), 1 SecA DEAD-like domain (Pfam07517), and 3 helicase conserved C-terminal domains (Pfam00271) are present in the genome of strain ZS2-28. Four genes related to cold-shock DNA-binding domain (Pfam00313) were also observed in the genome. At the same time, five salt adaptation-related genes, including,and(Kraegeloh et al., 2005; Sadeghi et al., 2014), were detected in the genome based on Pfam annotation. These results suggest that strain ZS2-28is adapted to cold intertidal environment.
In conclusion, the genomic analysis ofZS2-28isolated from Antarctic intertidal sediment has revealed that its genome contains various genes involved in the bacterium’s, photosynthetic reaction, cold adaptation, salt adaptation, ultra-violet resistance, and horizontal gene transfer events. The genome sequence will improve our understanding of the environmental adaptations and ecological functions of AAPB in the Antarctic marine environment.
Acknowledgments This work was supported by the National Key R & D Program of China (Grant no. 2018YFC1406903) and the National Natural Science Foundation of China (Grant nos. 91851201 and 41476171). We would like to thank two anonymous reviewers and Associate Editor, Dr Steve Coulson for their valuable suggestions and comments on further improvement of this article.
Akhter S, Aziz R K, Edwards R A. 2012. PhiSpy: a novel algorithm for finding prophages in bacterial genomes that combines similarity- and composition-based strategies. Nucleic Acids Res, 40(16): e126, doi: 10.1093/nar/gks406.
Auladell A, Sánchez P, Sánchez O, et al. 2019. Long-term seasonal and interannual variability of marine aerobic anoxygenic photoheterotrophic bacteria. ISME J, 13(8): 1975-1987, doi: 10.1038/s41396-019-0401-4.
Beatty J T. 2002. On the natural selection and evolution of the aerobic phototrophic bacteria. Photosynth Res, 73(1-3): 109-114, doi: 10.1023/A:1020493518379.
Bertelli C, Brinkman F S L. 2018. Improved genomic island predictions with IslandPath-DIMOB. Bioinformatics, 34(13): 2161-2167, doi: 10.1093/bioinformatics/bty095.
Boeuf D, Cottrell M T, Kirchman D L, et al. 2013. Summer community structure of aerobic anoxygenic phototrophic bacteria in the western Arctic Ocean. FEMS Microbiol Ecol, 85(3): 417-432, doi: 10.1111/1574-6941.12130.
Chidgey J W, Jackson P J, Dickman M J, et al. 2017.regulates porphyrin flux at the haem/bacteriochlorophyll branchpoint of tetrapyrrole biosynthesis via interactions with ferrochelatase. Mol Microbiol, 106(6): 961-975, doi: 10.1111/mmi.13861.
Ferrera I, Sánchez O, Kolářová E, et al. 2017. Light enhances the growth rates of natural populations of aerobic anoxygenic phototrophic bacteria. ISME J, 11(10): 2391-2393, doi: 10.1038/ismej.2017.79.
Flemming H C. 2016. EPS-then and now. Microorganisms, 4(4): E41, doi: 10.3390/microorganisms4040041.
Graham E D, Heidelberg J F, Tully B J. 2018. Potential for primary productivity in a globally-distributed bacterial phototroph. ISME J, 12(7): 1861-1866, doi: 10.1038/s41396-018-0091-3.
Hunter C N, McGlynn P, Ashby M K, et al. 1991. DNA sequencing and complementation/deletion analysis of the‐operon region of:mapping of the oxygen-regulatedpromoter. Mol Microbiol, 5(11): 2649-2661, doi: 10.1111/j.1365-2958.1991.tb01974.x.
Imhoff J F, Rahn T, Künzel S, et al. 2017. Photosynthesis is widely distributed among Proteobacteria as demonstrated by the phylogeny of PufLM reaction center proteins. Front Microbiol, 8: 2679, doi: 10.3389/fmicb.2017.02679.
Imhoff J F, Rahn T, Künzel S, et al. 2019. Phylogeny of anoxygenic photosynthesis based on sequences of photosynthetic reaction center proteins and a key enzyme in bacteriochlorophyll biosynthesis, the chlorophyllide reductase. Microorganisms, 7(11): 576, doi: 10.3390/microorganisms7110576.
Jiao N, Zhang Y, Zeng Y, et al. 2007. Distinct distribution pattern of abundance and diversity of aerobic anoxygenic phototrophic bacteria in the global ocean. Environ Microbiol, 9(12): 3091-3099, doi: 10.1111/j.1462-2920.2007.01419.x.
Juhas M. 2015. Type IV secretion systems and genomic islands-mediated horizontal gene transfer inand. Microbiol Res, 170: 10-17, doi: 10.1016/j.micres.2014.06.007.
Juncker A S, Willenbrock H, von Heijne G, et al. 2003. Prediction of lipoprotein signal peptides in Gram-negative bacteria. Protein Sci, 12(8): 1652-1662, doi: 10.1110/ps.0303703.
Koblížek M, Zeng Y, Horák A, et al. 2013. Regressive evolution of photosynthesis in theclade//Beatty J T. Advances in botanical research, Volume 66. Amsterdam: Elsevier, Academic Press, 385-405, doi: 10.1016/b978-0-12-397923-0.00013-8.
Koh E Y, Phua W, Ryan K G, 2011. Aerobic anoxygenic phototrophic bacteria in Antarctic sea ice and seawater. Environ Microbiol Rep, 3(6): 710-716, doi: 10.1111/j.1758-2229.2011.00286.x.
Koren S, Walenz B P, Berlin K, et al. 2017. Canu: scalable and accurate long-read assembly via adaptive-mer weighting and repeat separation. Genome Res, 27(5): 722-736, doi: 10.1101/gr.215087.116.
Kraegeloh A, Amendt B, Kunte H J. 2005. Potassium transport in a halophilic member of the bacteria domain: identification and characterization of the Kuptake systems TrkH and TrkI fromDSM 2581. J Bacteriol, 187(3): 1036-1043, doi: 10.1128/jb.187.3.1036-1043.2005.
Lang A S, Zhaxybayeva O, Beatty J T. 2012. Gene transfer agents: phage-like elements of genetic exchange. Nat Rev Microbiol, 10(7): 472-482, doi: 10.1038/nrmicro2802.
Lee J K, DeHoff B S, Donohue T J, et al. 1989. Transcriptional analysis ofoperon expression in2.4.1 and an intercistronic transcription terminator mutant. J Biol Chem, 264(32): 19354-19365, doi: 10.1016/S0021-9258(19)47309-2.
Lehours A C, Enault F, Boeuf D, et al. 2018. Biogeographic patterns of aerobic anoxygenic phototrophic bacteria reveal an ecological consistency of phylogenetic clades in different oceanic biomes. Sci Rep, 8(1): 4105, doi: 10.1038/s41598-018-22413-7.
Lehours A C, Jeanthon C. 2015. The hydrological context determines the beta-diversity of aerobic anoxygenic phototrophic bacteria in European Arctic seas but does not favor endemism. Front Microbiol, 6: 638, doi: 10.3389/fmicb.2015.00638.
Médigue C, Krin E, Pascal G, et al. 2005. Coping with cold: the genome of the versatile marine Antarctica bacteriumTAC125. Genome Res, 15(10): 1325-1335, doi: 10.1101/gr.4126905.
Möller S, Croning M D R, Apweiler R. 2001. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics, 17(7): 646-653, doi: 10.1093/bioinformatics/17.7.646.
Paquola A C M, Asif H, Pereira C A D B, et al. 2018. Horizontal gene transfer building prokaryote genomes: genes related to exchange between cell and environment are frequently transferred. J Mol Evol, 86(3-4): 190-203, doi: 10.1007/s00239-018-9836-x.
Peck L S, Convey P, Barnes D K A. 2006. Environmental constraints on life histories in Antarctic ecosystems: tempos, timings and predictability. Biol Rev, 81(1): 75-109, doi: 10.1017/S146479310 5006871.
Petersen T N, Brunak S, von Heijne G, et al. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods, 8(10): 785-786, doi: 10.1038/nmeth.1701.
Pinta V, Picaud M, Reiss-Husson F, et al. 2002. Rubrivivax gelatinosus acsF (previously orf358) codes for a conserved, putative binuclear- iron-cluster-containing protein involved in aerobic oxidative cyclization of Mg-protoporphyrin IX monomethylester. J Bacteriol, 184(3): 746-753, doi: 10.1128/jb.184.3.746-753.2002.
Piwosz K, Kaftan D, Dean J, et al. 2018. Nonlinear effect of irradiance on photoheterotrophic activity and growth of the aerobic anoxygenic phototrophic bacterium. Environ Microbiol, 20(2): 724-733, doi: 10.1111/1462-2920.14003.
Rice P A, Baker T A. 2001. Comparative architecture of transposase and integrase complexes. Nat Struct Biol, 8(4): 302-307, doi: 10.1038/ 86166.
Sadeghi A, Soltani B M, Nekouei M K, et al. 2014. Diversity of the ectoines biosynthesis genes in the salt tolerantand evidence for inductive effect of ectoines on their accumulation. Microbiol Res, 169(9-10): 699-708, doi: 10.1016/j.micres.2014.02. 005.
Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics, 30(14): 2068-2069, doi: 10.1093/bioinformatics/ btu153.
Springael D, Top E M. 2003. Horizontal gene transfer and microbial adaptation to xenobiotics: new types of mobile genetic elements and lessons from ecological studies. Trends Microbiol, 12(2): 53-58, doi: 10.1016/j.tim.2003.12.010.
Swingley W D, Sadekar S, Mastrian S D, et al. 2007. The complete genome sequence ofreveals a mixotrophic rather than photosynthetic metabolism. J Bacteriol, 189(3): 683-690, doi: 10.1128/jb.01390-06.
Tang K, Zong R, Zhang F, et al. 2010. Characterization of the photosynthetic apparatus and proteome of. Curr Microbiol, 60(2): 124-133, doi: 10.1007/s00284-009-9515-7.
Trgovcević Z, Lers N, Brcić-Kostić K, et al. 1989. Post-ultraviolet DNA synthesis in the absence of repair: role of the single-strand DNA-binding protein. Int J Radiat Biol, 55(5): 739-745, doi: 10.1080/09553008914550791.
Tsukatani Y, Yamamoto H, Harada J, et al. 2013. An unexpectedly branched biosynthetic pathway for bacteriochlorophyllcapable of absorbing near-infrared light. Sci Rep, 3(1): 1-7, doi: 10.1038/srep 01217.
Walker B J, Abeel T, Shea T, et al. 2014. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One, 9(11): e112963, doi: 10.1371/journal. pone.0112963.
Xu J, Wu C X, Yang Z H, et al. 2020. For: Pesticide biochemistry and physiology recG is involved with the resistance of Bt to UV. Pestic Biochem Physiol, 167: 104599, doi: 10.1016/j.pestbp.2020.104599.
Yu Y, Yan S L, Li H R, et al. 2011.gen. nov., sp. nov., an aerobic bacteriochlorophyll-containing alphaproteobacterium isolated from Antarctic sandy intertidal sediment. Int J Syst Evol Microbiol, 61: 2173-2179, doi: 10.1099/ijs.0.024885-0.
Zeng Y X, Dong P Y, Qiao Z Y, et al. 2016. Diversity of the aerobic anoxygenic phototrophy genein Arctic and Antarctic coastal seawaters. Acta Oceanol Sin, 35(6): 68-77, doi: 10.1007/s13131- 016-0877-y.
Zeng Y X. 2019. Phylogenetic diversity of dimethylsulfoniopropionate- dependent demethylase genein distantly related bacteria isolated from Arctic and Antarctic marine environments. Acta Oceanol Sin, 38(8): 64-71, doi: 10.1007/s13131-019-1393-7.
12 November 2020;
8 March 2021;
18 March 2021
, ORCID: 0000-0002-3689-7855, E-mail: yxzeng@yahoo.com
10.13679/j.advps.2020.0034
: Zeng Y X, Yu Y, Li H R, et al. Complete genome analysis of bacteriochlorophyll-containingZS2-28reveals its adaptation to Antarctic intertidal environment. Adv Polar Sci, 2021, 32(1): 20-27,
10.13679/j.advps.2020.0034
杂志排行
Advances in Polar Science的其它文章
- Information for authors
- Editorial Note
- Assessment on India’s involvement and capacity-building in Arctic Science
- Leveraging the UAV to support Chinese Antarctic expeditions: a new perspective
- Seabird and marine mammal at-sea distribution in the western Bering Sea and along the eastern Kamtchatka Peninsula
- Biomarker records of D5-6 columns in the eastern Antarctic Peninsula waters: responses of planktonic communities and bio-pump structures to sea ice global warming in the past centenary
