Sustainable functional urethral reconstruction:Maximizing early continence recovery in robotic-assisted radical prostatectomy
2021-03-26ZepengJiaYifanChangYanWangJingLiMinQuFengZhuHuanChenBijunLianMeimianHuaYinghaoSunXuGao
Zepeng Jia , Yifan Chang , Yan Wang , Jing Li, Min Qu,Feng Zhu, Huan Chen, Bijun Lian, Meimian Hua, Yinghao Sun,Xu Gao
Department of Urology, Changhai Hospital, Second Military Medical University, Shanghai, China
Abstract Objective: To evaluate the safety profile and short-term functional outcome of sustainable functional urethral reconstruction (SFUR) in robotic-assisted radical prostatectomy(RARP).Methods: One hundred and sixty-two consecutive prostate cancer patients who underwent RARP were retrospectively analyzed, in which 53 had undergone SFUR while the other 109 had undergone conventional RARP procedures.Immediate, 2-week, 1-month and 3-month continence recovery and other perioperative data were compared to evaluate short-term surgical and functional outcome.Results: The median age was 68 and 67 years in the experimental group and control group,respectively (p=0.206), with a median prostate-specific antigen (PSA) of 13.6 ng/mL (interquartile range [IQR], 8.46-27.32 ng/mL) in the experimental group and 13.84 ng/mL (IQR,9.12-26.80 ng/mL) in control group (p=0.846).Immediate, 2-week, 1-month and 3-month continence recovery rates between the groups were 34.0% vs.3.7%, 50.9% vs.14.7%, 62.3%vs.27.5%, and 79.2% vs.63.3% (all p<0.05).The morphological changes made by the new reconstruction technique were maintained on magnetic resonance imaging (MRI) 3 months postoperatively.Nerve-sparing procedures and adoption of the new reconstruction technique were significantly relevant to continence recovery on logistics regression model (p<0.001).Conclusions: SFUR is a safe and easy-to-handle modification that may contribute to early continence return for RARP.Long-term follow-up and prospective studies are required to further evaluate its value in postoperative quality-of-life improvement.
KEYWORDS Prostate cancer;Prostatectomy;Robotic surgery;Urinary incontinence;Sustainable functional urethral reconstruction
1.Introduction
Radical prostatectomy(RP)provides excellent tumor control for localized prostate cancer (PCa) [1,2], and its role in locally-advanced PCa as part of a multimodality treatment regimen has been extensively discussed in recent years[3,4].In terms of postoperative functional recovery, although postoperative continence after 12 months can be achieved in over 90%of patients in many large-volume centers nowadays[5], short-term and especially immediate continence recovery remains an unsolved issue,even with the ever-increasing popularity of robotic-assisted RP(RARP)worldwide,which is especially problematic for patients with large prostate volume, or those who are not eligible for bladder neck-sparing or nerve-sparing procedures [6].This unmet need leaves substantial room for technical refinement, and for better understanding of local anatomy and functional mechanism of urine control.
Current critical analyses have indicated that the causes to post-RP urinary incontinence may fall in three major aspects: 1) Damage to the external sphincter; 2) disturbance of the surrounding anatomical structures; and 3)pressure and tension on vesicourethral anastomosis [7].Technical modifications aiming to improve early continence recovery have been discussed in a plethora of researches[8].These studies share common strategies in highlighting the importance of better preservation of periurethral structure, by means of either minimizing neurovascular injury with more precise nerve-sparing procedures [9],better preserving a length of functional urethra (e.g.,bladder-neck preservation) [10,11], or reducing anastomotic tension with posterior strengthening(e.g.,the Rocco stitch)[12]or anterior suspension[13].Nevertheless,these studies are heterogeneous in nature, with ununified definition of continence recovery,and no technique has proven its superiority; more importantly, whether these surgical modifications can result in any morphological or urodynamic changes, and how long may these changes last thereafter, remain largely unknown.
The current study proposes a novel technique for RARP in providing adequate urethral length with bladder neck tubularization and sustainable periurethral support with peritoneal flap,with the objective of providing satisfactory early continence recovery that seems to remain functional during follow-up.
2.Patients and methods
2.1.Study design
From June 16, 2018 to February 28, 2019, 162 consecutive patients with confirmed diagnosis of PCa who underwent RARP in a single center were retrospectively analyzed after granted approval by the Changhai Hospital Ethics Committee (CHEC2019-102), in which 53 had undergone RARP with SFUR, i.e., the experimental group, and 109 had undergone conventional RARP, i.e.,the control group.The console surgeon works in a highvolume tertiary hospital with a personal RARP caseload of over 800.Informed consent was signed by each individual before surgery.
2.2.Surgical technique
Patient position and trocar placement were described as previously [14].RARP was performed using the da Vinci Si HD surgical system (Intuitive Surgical, Sunnyvale, CA,USA).A transperitoneal anterior approach with use of four robotic arms was adopted.The Retzius space was first developed by incising the lateral umbilical ligament(LUL) at the level of the umbilicus for later-on periurethral reinforcement.Ligation of the deep dorsal venous complex (DVC), transection of the bladder neck,development of the retroprostatic space, and dissection of the neurovascular bundles were performed following conventional procedures [15].The decision of pelvic lymph node dissection was determined according to preoperative risk stratification, and nerve-sparing procedures by means of bilateral intrafascial dissection were performed for preoperatively potent and eligible patients.Before vesicourethral anastomosis (Fig.1A),two incisions of approximately 3 cm in length were made vertically at the margin of the bladder neck, at 7-8 o’clock and at 4-5 o’clock, respectively, to create a muscular flap that is approximately 3 cm in length and 2.5 cm in width (Fig.1B).Then, the posterior bladder lip was closed at midline with running suture, forming a tubularized new bladder opening (Fig.1C), which was then reapproximated with the urethral stump with 3-0 two-way barbed suture (Fig.1D).Foley catheter entered and exited repeatedly during anastomosis for better mucosal apposition.Finally, the supravesical peritoneal flap, which was created after incision of the lateral umbilical ligaments when developing the Retzius Space,was incised longitudinally by approximately 2 cm at midline in two halves, and was folded over and stretched to the distal end of the retropubic space to be pleated and anchored to the fibromuscular tissue around the urethral stump, forming a cuff-like supporting structure (Fig.1E).Key surgical techniques of SFUR was narrated in Video 1.Posterior reconstruction and anterior suspension were not performed in both groups in the current study.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ajur.2020.01.003
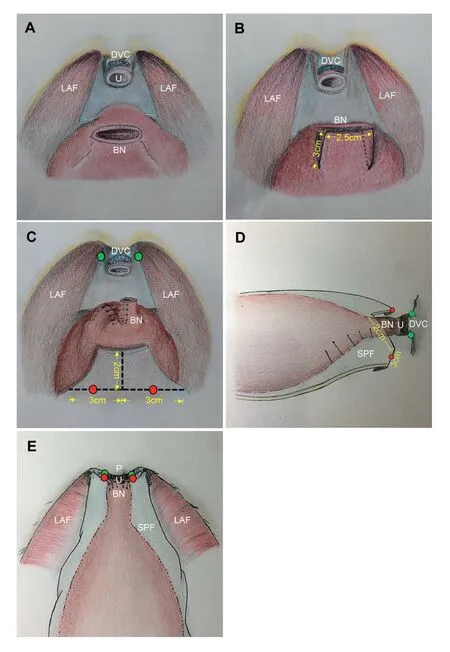
Figure 1 Illustration of the surgical technique.(A) Intraabdominal view before vesicourethral anastomosis.(B) A muscular flap sized approximately 3 cm×2.5 cm being formed before creating a new bladder opening.(C) Tubularized bladder neck before anastomosis, with design of lateral umbilical ligament reshaping for periurethral reinforcement.Red and green dots represent the location where the lateral umbilical ligament and the periurethral tissue were approximated on both sides.(D)the anastomosed bladder neck before lateral umbilical ligament reinforcement, lateral view.(E) Intraabdominal view after periurethral reinforcement with lateral umbilical ligament.BN, bladder neck; DVC, dorsal venous complex; LAF, levator ani fascia; P, pubic symphysis; SPF,supravesical peritoneal flap; U, urethra.
2.3.Measurement of perioperative parameters
Operative time was defined as skin to skin time.Extended pelvic lymph node dissection (ePLND) was defined as dissection of the external iliac,internal iliac,and obturator lymph nodes; standard PLND was defined as dissection of bilateral obturator lymph nodes only.Postoperative continence was defined as daily usage of 0-1 pad, with a pad weight gain of less than 50 g per day.
2.4.Patient follow-up
Patients were routinely followed at clinic monthly after discharge witha minimum follow-up time of3 months.Patient voiding diary, daily pad usage and pad weight gain, time to continence recovery,time to potency recovery,serum prostate-specific antigen (PSA) and testosterone level, time to biochemical recurrence(BCR),and adjuvant treatment were recorded in each follow-up sessions.Additional follow-up for continence on 1 week,2 weeks,1 month and 3 months postoperatively, including 24-h pad usage and pad weight gain were also documented.All follow-up data were uploaded to PC-Follow version 5.0,a nation-wide online PCa database for data documentation and analyses.
2.5.Statistical analyses
Statistical analyses were performed using SPSS v22.0 (IBM,Armonk, NY, USA).Age, body mass index (BMI), and PSA level were described as median with interquartile range(IQR).A p-value <0.05 is considered statistically significant.Nonparametric Mann-Whitney U tests were performed to compare numerical data between the groups and for tiered variables, e.g., clinical stages and biopsy Gleason scores.Chi-square tests were performed to compare categorical data between the groups.Logistics regression analysis was performed that included patient age, BMI, preoperative PSA, National Comprehensive Cancer Network (NCCN) risk stratification, adoption of nerve-sparing procedures, and adoption of the new technique.
3.Results
3.1.Demographics
Patient demographics and preoperative variables were summarized in Table 1.The median age of the experimental group and control group was 68 and 67 years, respectively(p=0.206).The median PSA was 13.6 ng/mL (IQR,8.46-27.32 ng/mL)in the experimental group and 13.84 ng/mL(IQR,9.12-26.80 ng/mL)in the control group(p=0.846).For the experimental group,75.5%were organ-confined,and 66% were high-risk, while 81.7% were organ-confined with 63.3%high-risk patients in the control group(66.0%vs.63.3%,p=0.733).Differences on other baseline data, including clinical stage, biopsy Gleason scores were also statistically insignificant,except for BMI(p=0.037).
3.2.Intraoperative outcomes
Operative time between the two groups had no significant difference (150 min vs.150 min, p=0.514), nor did intraoperative blood loss (100 mL vs.100 mL, p=0.125) (Table 2).The majority of patients had not undergone nervesparing procedures (81.1% vs.79.8%, p=0.844).Bilateral intrafascial dissection was performed for those eligible for nerve-sparing approaches.In the experimental group, 21(39.6%) had undergone extended PLND, and 7 (13.2%) had standard PLND, while 39 (35.8%) and 13 (11.9%) patients in the control group had undergone extended or standard PLND, respectively (p=0.559).
3.3.Complications
Overall complication rate was 6.2% (10/162) for the two cohorts combined.Table 3 listed complications occurred in the SFUR group.Five patients carried pelvic drainage output at home on discharge, and had them removed at clinic on follow-up when daily drainage was ≤50 mL.Two patients who had undergone unilateral lower limb paresthesia recovered within a week after being discharged.One patient recatheterized after removal of the catheter due to postoperative urinary retention.The Foley catheter was retained for another 4 weeks before removal, and he was completely continent and resumed normal urine outflow thereafter.No patients recorded ureteral orifice obstruction or anastomotic leak after surgery.In control group,two patients reported prolonged drainage indwelling for 3 and 7 days.
3.4.Pathological findings
Histopathological data were reported in Table 4.The overall positive surgical margin (PSM) rate was 11/53(20.8%)in the experimental group and 28/109(25.7%)in the control group, and PSM rates of pT2 and pT3 patients were 16.7% vs.10.2% and 26.1% vs.38.3%, respectively.The difference of overall PSM and PSM in pT2 and pT3 stages was not statistically significant (p=0.35).Approximately half of the patients were organ-confined on postoperative pathology (56.6% vs.45.0%, p=0.35), and patient distribution in different Gleason grade groups in the experimental group and control group appeared to have borderline significance (p=0.045).
3.5.Follow-up outcomes
Foley catheter was removed 14 days after the surgery on a routine basis.The median follow-up of the patients was 7.0 months (range, 3.5-12 months).Of the 53 men who had undergone the new technique, 34.0% (18/53),50.9% (27/53), 62.3% (33/53), and 79.2% (42/53) were continent immediately, 2 weeks, 1 month, and 3 months after catheter removal, respectively, compared with a total of 3.7% (4/109), 14.7% (16/109), 27.5% (30/109),and 63.3% (69/109) in the control group, respectively.Postoperative pelvic MRI on 3 months after surgery(Fig.2A and 2B, Supplementary Figure 2) indicated a sustainable morphological remodeling of the tubularized bladder neck, which was attempted to restore local anatomy (blue arrow).Moreover, the pleated peritoneal flap served as a periurethral structural reinforcement(asterisk).Postoperative MRI images in the control group did not show such anatomical remodeling(Supplementary Fig.1A and 1B).Potency was not followed due to limited sample of preoperatively potent patients.
3.6.Logistics regression analysis
Logistics regression model indicated that nerve-sparing procedures and adoption of the sustainable functional urethral reconstruction technique were significantly relevant to continence recovery (Table 5, p<0.001).Also, patients who had undergone nerve-sparing procedures, were statistically relevant with better continence recovery.Patient age,BMI,preoperative PSA level,or risk stratification,seemed irrelevant in our equation.
4.Discussion

Table 1 Preoperative variables.
Current modified techniques of RP that aim to improve early postoperative continence recovery beyond the current practice can be categorized into two aspects: 1) To reduce damage to periprostatic structures that are responsible for urine control either anatomically or physiologically, and 2) to restore the default anatomical configuration with various reconstruction or reinforcement approaches.Kadono et al.[16]suggests that keeping an adequate length of functional urethra significantly improves continence recovery throughout the entire recovery timeline.Anatomical preservation of periprostatic tissue, as reported by de Carvalho et al.[17] that keeps puboprostatic ligament, detrusor apron and endopelvic fascia intact, has an immediate continence recovery of 85.9% and 1-month recovery of 98.4%, with comparable overall PSM rate of 13.3%.This theory is further supported by the fact that preservation of the Retzius space, which keeps the entire anterior anatomy intact with a posterior access, has a median time to continence recovery of only 2 days after catheter removal [18].Nerve-sparing dissection is also related to improved continence return in a number of studies [9,19,20], but whether this effect is achieved by preserving the neural or vascular network responsible for external sphincter control, or simply by reducing periurethral structural damage, remains inconclusive [21].Furthermore, different reconstruction techniques after prostate removal, including posterior musculofascial reconstruction [12], anterior urethral suspension [22-24], as well as periurethral fixation to help increase anastomosis stability [25], have also been explored.

Table 2 Intraoperative variables.
Based on preexisting knowledge [26,27], our retrospective controlled study proposes a new technique for early continence improvement.The advantages of such technique are that it provides not only an adequate length of functional urethra,but also increases periurethral stability;furthermore,with postoperative radiological examinations,we have proven such modification a safe and long-lasting improvement.In the following content we try to further explain the improvement in detail.

Table 3 The Clavien-Dindo classification system for patients undergoing RALP with tubularized bladder neck reconstruction.
Before anastomosis, the bladder neck was remodeled by severing the bladder neck in two halves, the anterior lip being reshaped as a tube for the new bladder opening,and the posterior lip being closed.Similar to the bladder neck-sparing techniques proposed in a number of literatures [10,28], such manner also attempts to create an adequate length of the functional urethra, and reduces tension to the anastomosis, but may serve as a better option for those who are not candidate for bladder neck sparing, such as those with a large prostate volume, or those classified as high-risk who are prone to having a positive surgical margin after the resection [6].According to Tan et al.[29], the detrusor muscle generates a downward contractile force that presses directly onto the anastomosis during micturition, which may delay healing of the anastomosis and increase the risk of anastomotic leakage and bladder neck stricture.This funnel-like reconstruction of the bladder neck described in our study is an attempt to restore natural anatomy when the prostate is still present.This structural remodeling appears to maintain its shape and function 3 months postoperatively,if not longer.The new bladder opening can be easily reapproximated with the urethral stump, with the help of the Foley catheter that allows better mucosal apposition.Such attempt is also believed to disperse the contractile force away from the anastomosis.Whether such method of reconstruction will increase the risk of internal urethral sphincter injury remains to be further clarified; nevertheless, the internal urethral sphincter itself plays only a minor role in postoperative urine control[30], and emphasis should be made on the protection of the external urethral sphincter.

Figure 2 Postoperative pelvic MRI 3 months after RARP.(A)Sagittal T2 image;(B)The same patient on axial T2 image.Blue arrows indicate the tubular bladder neck that mimics natural anatomy of the bladder neck.A steris indicates the supporting structure around the periurethral muscular complex created by the supravesical peritoneal flap.U, urethra; R, rectum.
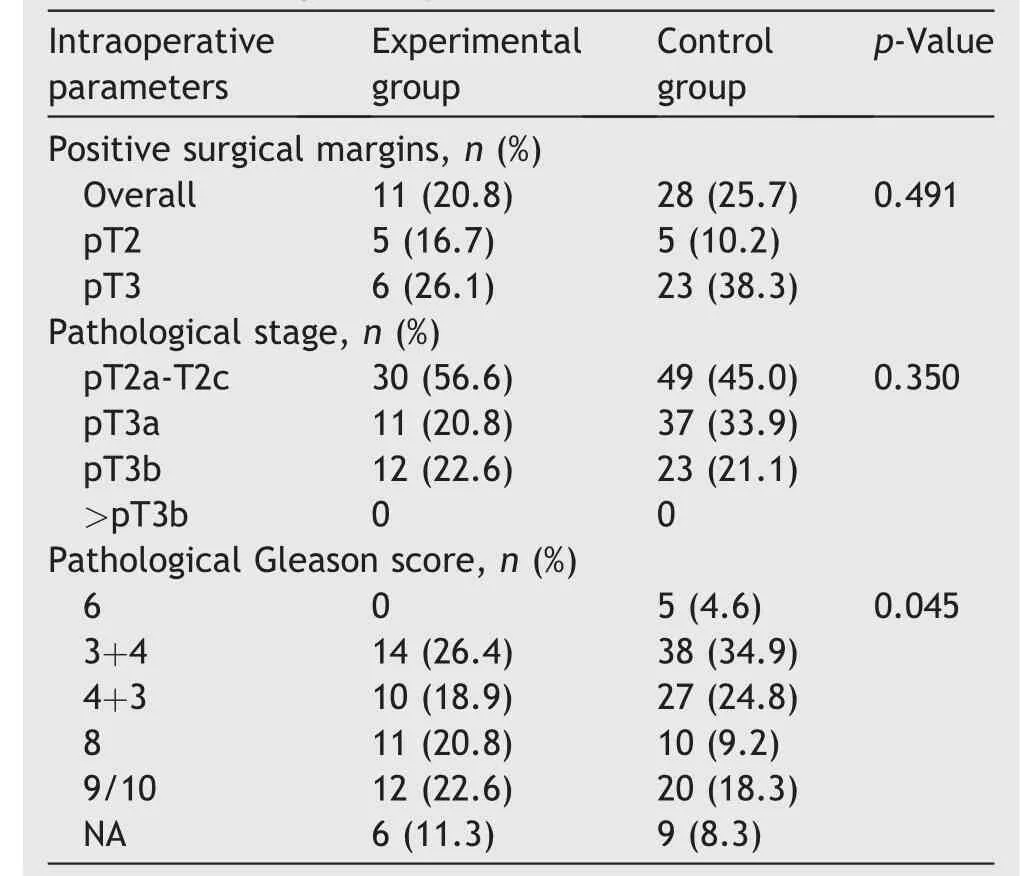
Table 4 Histopathological data.
Another prominent feature of our technique is the periurethral support with use of peritoneal flap.The peritoneal flap was separated in two halves in the midline,which was then pleated and fixated by anchoring the base of the flap to the periurethral stump and fibromuscular tissue of the urogenital diaphragm.We believe that such maneuver can help create a downward force against the urogenital diaphragm that prevents vertical repositioning of the membranous urethra which, as suggested by Kadono et al.[16] appears to be a crucial factor inducing post-RP urinary incontinence; it also creates a circumferential force to the distal urethra with a cuff-like reinforcement to give additional fixation.Together, the two maneuvers synergistically contribute to providing a sustainable support to the sphincteric muscular complex,which is more prominent than those who had adopted either bladder neck tubularization or periurethral reinforcement alone (data not shown), which is similar to the urethral fixation technique proposed by Ficarra et al.[25];more importantly, the remodeling of the reconstructed urethra is still maintained,at least for 3 months under MRI scans(Fig.2A and 2B).One patient experienced prolonged Foley catheter indwelling due to urethral stricture,which we believe, was due to the patient’s tendency to have proliferative scars, and should not be interpreted as a generalized factor to affect patient safety.Five patients had prolonged drainage output and two had transient lower limb paresthesia,which we believed were all due to neurapraxia following pelvic lymph node dissection, and were not markedly different compared with control group.Longer follow-up is still required for long-term continence outcome and tumor control effects compared to conventional techniques.

Table 5 Logistic regression model.
Several limitations should be noted before interpreting these results into clinical practice.The retrospective nature of the study still needs further verification with prospective and randomized studies.This study is conducted in a single center with one console surgeon, in which selection bias may occur.Also, potency was not evaluated due to the low proportion of patients who had undergone nerve-sparing procedures.Also, it was not evaluated to what extent SFUR is superior to either tubularization or periurethral reinforcement alone, since the sample sizes of the two subgroups were not sufficient to perform comparative analysis.Further prospective studies comparing SFUR with bladder neck sparing procedures will be conducted.
5.Conclusion
In conclusion, this study proposes a new technique of sustainable functional urethral reconstruction in RARP that aims to improve early continence recovery, and is proven safe,feasible and easy to handle.Long-term follow-up and prospective studies are required to further evaluate its value in postoperative quality-of-life improvement.
Author contributions
Study design: Zepeng Jia, Yinghao Sun, Xu Gao.
Data acquisition:Yan Wang,Min Qu,Feng Zhu,Huan Chen.
Data analysis: Zepeng Jia, Yifan Chang, Bijun Lian.
Drafting of manuscript: Zepeng Jia, Yifan Chang, Meimian Hua.
Critical revision of the manuscript: Yan Wang, Jing Li, Xu Gao.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
The work was supported by the “Excellent Academic Leadership” Project (2018BR18) of Shanghai Municipal Commission of Health and Family Planning.
Appendix A.Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ajur.2020.01.003.
杂志排行
Asian Journal of Urology的其它文章
- Nerve-sparing robot-assisted radical prostatectomy: Current perspectives
- Robtic-assisted radical cystectomy:Literature review
- Robot-assisted endoscopic inguinal lymphadenectomy: A review of current outcomes
- Robot-assisted retroperitoneal lymphadenectomy: The state of art
- Robotic surgery techniques to approach benign prostatic hyperplasia disease: A comprehensive literature review and the state of art
- Robotic renal and adrenal oncologic surgery:A contemporary review
