Robtic-assisted radical cystectomy:Literature review
2021-03-26MrcioCovsMoschovsKultheRmeshSeethrmBhtCthyJensonVipulPtelGbrielOgyPinies
Mrcio Covs Moschovs , Kulthe Rmesh Seethrm Bht Cthy Jenson Vipul R.Ptel Gbriel Ogy-Pinies
a Department of Urology, AdventHealth Global Robotics Institute, Celebration, FL, United States
b Department of Urology, Hospital Rey Juan Carlos, Madrid, Spain
c Lyx Instituto de Urologia, Madrid, Spain
Abstract Radical cystectomy (RC) with pelvic lymph node dissection (PLND) is the standard treatment for localized muscle-invasive bladder cancer (MIBC) and non-muscle-invasive bladder cancer(NMIBC)with recurrence or high risk of progression.Also,the robotic approach to this type of surgery is well established in the literature.Our objective is to summarize in this manuscript the most relevant articles related to the robotic-assisted radical cystectomy for prostate cancer.We performed a literature review of articles describing the robotic approach to RC in patients with bladder cancer.Also, we described the procedure since the patient selection until the bladder removal.The reconstructive techniques were not included in this review.Twenty-five articles were used to divide our manuscript into key points such as preoperative patient selection and protocols, surgical technique, pathology report, oncological outcomes, complication rates, and quality of life after the procedure.Robotic-assisted radical cystectomy is feasible and safe with satisfactory oncological outcomes.The robotic approach is related to lower blood loss and fewer transfusion rates.However,when compared to open surgery, the use of this technology increases the operative time.
KEYWORDS Bladder cancer;Radical cystectomy;Robotic-assisted radical cystectomy
1.Introduction
Radical cystectomy (RC) with pelvic lymph node dissection(PLND) is the standard treatment for localized muscleinvasive bladder cancer (MIBC) and non-muscle-invasive bladder cancer (NMIBC) with recurrence or high risk of progression [1,2].
During the last 20 years,different authors described the benefits of robotic assistance during minimally invasive surgery for a variety of surgical techniques in urology [3],especially in procedures such as robotic-assisted radical prostatectomy (RARP) in terms of decreased morbidity and improved recovery time.
RC has always been associated with a high rate of perioperative complications ranging from 30%to 70%of the cases[4],and with a readmission rate up to 25%in the first 30 days after the surgery.Therefore, the natural evolution of the urologic surgery was to incorporate robotic assistance to this procedure[5].In this context,Menon et al.[6]reported the first robotic-assisted radical cystectomy (RARC) with a nerve-sparing technique in 17 patients, and described a viable option for the treatment of bladder cancer (BCa).
Since the introduction of robotic technology to treat BCa,some authors in the literature performed comparisons between open and robotic radical cystectomies.The RARC has shown to be equivalent to open radical cystectomy(ORC) in terms of oncological and functional outcomes [7].However, only a few trials have presented evidence of similar intra- and perioperative complications [8-10].
2.Material and methods
We summarized in this manuscript a literature review of the most relevant articles reporting RARC for bladder cancer.However,the different reconstruction techniques after the bladder removal will be considered in a separate article,also described by our group.
2.1.Before the surgery
2.1.1.Patient selection
The current literature does not describe absolute contraindications for RARC.The standard contraindications apply to all patients, including coagulopathy, severe ascites, or advanced disease.Careful consideration should be given to those patients with significant pulmonary disease or severe obesity (BMI>35 kg/m), which may preclude them from tolerating a steep Trendelenburg position or prolonged pneumoperitoneum [5].Moreover, high-complexity cases,such as patients with extensive intestinal adhesions, and previous pelvic radiotherapy, should be performed by an experienced surgeon [7].
2.1.2.Preoperative preparation
All patients undergoing RARC should be included in an enhanced recovery after surgery (ERAS) or a fast-track surgery program.Recognized differences to ERAS designed for RARC include a minimally invasive approach,less blood loss,avoidance of an epidural, earlier mobilization, and reduced postoperative analgesic requirements [11,12,13].Other key principles of these protocols regard the preoperative patient education,optimization of nutrition,as well as standardized anesthetic and antiemetic regimens [11].
Mechanical bowel preparation is usually omitted for patients undergoing RARC [14].A prospective randomized control trial (RCT) comparing bowel preparation versus nobowel preparation for open RC shows no difference in terms of sepsis, wound infection, postoperative ileus, and hospital stay[2].However,a fleet enema can be administered on the night before surgery because having a decompressed rectum helps create a wider space resulting in easier mobilization during the posterior dissection.Also,in case of an inadvertent rectal enterotomy, rectal decompression may help to facilitate a primary closure [3].
Vegetables should be avoided for 24 h before the surgery; otherwise, the nondigestible vegetables can be seeded into the peritoneum with spillage from the opened ileum during the intracorporeal urinary reconstruction [4].In the case of an ileal conduit, the stoma site should be marked before the surgery, preferably with the help of a stoma therapist.
2.2.Technique
2.2.1.Patient’s positioning and trocar placement
The patient is placed in steep Trendelenburg position, the legs in low lithotomy, and the arms tucked to the body.Pads protect all extremities and articulations.An anti-slip surface is recommended to avoid the use of shoulder supports.With the da Vinci Xi® (Intuitive Surgical, Sunnyvale,California, USA), side docking is preferred, especially with female patients, allowing more accessible access to the perineum during the procedure.
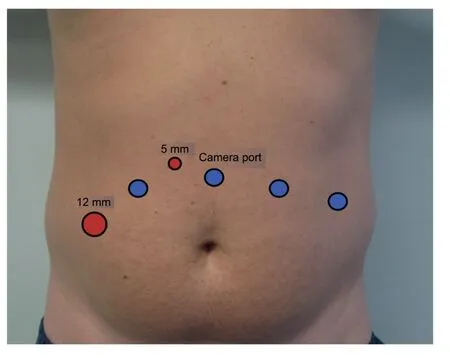
Figure 1 Trocar placement.Blue dots represent robotic ports.Red dots represent assistant ports.
The trocar placement is very similar to the roboticassisted radical prostatectomy (RARP).Four robotic trocars are used, the camera trocar is placed 4-5 cm above the umbilicus, and the remaining three are placed as in Fig.1, at least 8 cm away from each other, however,should be adapted to patient’s anatomy.Two additional trocars are placed for the bedside assistant, a 12 mm trocar in the right flank, and a 5 mm trocar between the camera trocar and the right side robotic trocar.This 5 mm trocar should be placed more cephalic to avoid clashing with the robotic arms.
The patient is prepped and draped in the usual sterilefashion, including the perineum.The Foley catheter is placed after the sterile field is set.
2.2.2.Cystoprostatectomy: Step by step
2.2.2.1.Ureteral dissection.The first step is identifying the ureters at the common iliac artery crossing.Once the ureters are identified, the caudal release is performed with caution to avoid grasping, stretching, and excessively defat of the ureter.If coagulation is needed, pinpoint bipolar energy should be used.Once the bladder insertion is reached, a distal Hem-o-lok® and a more proximal Hem-o-lok® with a pre-tied suture are applied to avoid urine spillage.The ureter is then transected,and the distal margin is sent for the frozen section (Fig.2A).
2.2.2.2.Posterior dissection.An incision is made in the peritoneum of the cul-de-sac.The fourth arm can then be used to retract the peritoneal reflection upward,resulting in better exposure.The fatty and fibrovascular tissue is dissected off the posterior peritoneal fold.When the seminal vesicles (SV) are identified, they are dissected down to their base, being careful not to perform an extensive lateral dissection of the SV if a neurovascular bundle (NVB) preservation is planned(Fig.2B).Once the Denonvillier’s fascia is reached, it is sharply dissected in the midline, and the plane between the rectum and the prostate is created (Fig.2C).At this point, using the toggling feature of the da Vinci Xi®endoscope to 30up will allow better exposure and dissection of the plane to the posterior prostatic apex,as well as a better view laterally to continue the NVB preservation.
2.2.2.3.Bladder pedicles.The anterior peritoneum is incised laterally to the medial umbilical ligaments,keeping the ligaments in place and taking the peritoneum down until the vas deferens.The lateral bladder space is created in the same way it is performed during a RARP until reaching the endopelvic fascia.The fascia is then opened,and the space is dissected by gently sweeping the fibers of the levator ani muscle laterally,and continuing down to the prostatic apex(Fig.2D).At this point,the bladder pedicles are transected to join the lateral bladder space with the posterior space, using either a vessel sealer or Hem-olok® clips if preservation of the NVB is intended.
2.2.2.4.NVB preservation.Patients who were potent preoperatively, with organ-confined disease, motivated to preserve their sexual function, will benefit from NVB sparing techniques.
The NVB sparing begins with a dissection of the seminal vesicles.It is crucial to avoid the use of energy at this point.In this context, we perform this step using Hem-olok® clips for dissection of the bladder pedicles.The NVB preservation is continued at the opening of the Denonvillier’s fascia, finding the right plane to begin the release of the NVB from below.The NVB release from the prostate needs to follow the same principles as in the RARP: No energy and minimal traction.An interfascial plane between the prostate and the NVB is created at the level of the mid-prostate until the previously created posterior plane.The dissection is then continued in a retrograde fashion toward the base of the prostate to completely detach the NVB from the prostatic pedicle.The plane is then continued toward the apex by detaching the prostate from the NVB.

Figure 2 Intraoperative images.(A) Right ureter before clipping.Noticed the periureteral fat preservation.(B)Dissection of the left seminal vesicle (Green Arrow) and Denonvillier’s fascia in the back (Blue Arrow).(C) Seminal Vesicles are pulled up while the free edge of the Denonvillier’s fascia is pulled down in order to create the space to dissect the posterior plane of the prostate.(D) Right lateral dissection.The endopelvic fascia is open, and the lateral aspect of the prostate (blue arrow) is gently pulled apart from the elevator ani muscle (green arrow).(E) Once the lateral plane is connected to the posterior plane by dissecting the bladder and prostatic pedicles, the urachus is divided.
2.2.2.5.Dorsal vein complex and urethra.The urachus and the median umbilical ligaments are dissected(Fig.2E),and the posterior aspect of the bladder and prostate are exposed.Once the puboprostatic ligaments are reached,they are incised, and the dorsal vein complex (DVC) is ligated using a 0 Vicryl on a CT1 needle.The plane between the DVC and the urethra is gently developed to expose the anterior urethral wall.The anterior urethra wall is sharply transected with the scissors, a Hem-olok® clip is placed on the Foley catheter, and the catheter is cut distal to the clip, keeping the balloon inflated inside the bladder to avoid spillage of urine.The Foley catheter stump can be pulled back to expose the posterior urethra wall, which is divided, released from the rectourethralis muscle and Denonvillier’s fascia, and the specimen released.
2.2.2.6.Specimen retrieval.The specimen is immediately bagged in an Endocatch bag.In a female patient,the specimen can be extracted through the opening in the anterior wall of the vagina.In male patients, after the bag is closed, the string of the Endocatch bag is transferred from the 12 mm assistant trocar to the 5 mm trocar, and pulled back through the trocar and clamped with a hemostat, preventing it from interfering with the rest of the surgery.
2.2.2.7.Considerations in female patients[11].The same surgical technique, as aforementioned, is described for female patients in terms of bladder dissection and vascular control.However, in this group, we also perform the anterior vaginal resection, salpingectomy, and hysterectomy.
2.2.2.8.Lymph node dissection.We performed the PLND after the cystoprostatectomy specimen is bagged.In our view performing the PLND first doesn’t add any technical advantages,and in fact,having the bladder in place during the PLDN may require the use of the fourth arm to retract the bladder,compromising the use of the arm during this step[15].
The standard PLND during a cystoprostatectomy involves the bilateral removal of nodal tissue cranially up to the common iliac bifurcation,with the ureter being the medial border,and including the internal iliac,obturator fossa and external iliac nodes [1].The extended PLND includes all lymph nodes in the region of the aortic bifurcation, presacral, and common iliac vessels medially to the crossing ureters.
The therapeutic value of PLND is under ongoing debate,and controversy exists concerning the optimal anatomic extent of PLND [16].In a recent RCT the extended PLND failed to show an advantage over standard PLDN regarding recurrence-free survival, cancer-specific survival, and overall survival.Lymphoceles requiring intervention within 90 days after surgery was higher in the extended PLND group (8.6%) compared with the limited PLND group (3.4%;p=0.04) [17].
2.3.Oncological outcomes
2.3.1.Positive surgical margins
In a recent meta-analysis comparing RARC vs.ORC,fivestudies with more than 540 patients showed no differences in terms of positive surgical margins (PSM) (relative risk [RR] 1.16, 95%confidence interval [CI] 0.56-2.40), between the two different approaches[1].The same conclusion was reached in a systematic review of the literature,where PSM ranged from 0 to 12% in the different studies, but no difference was observed between RARC and ORC[5].
2.3.2.Recurrence and progression
The RAZOR trial compared the 2-year progression-free survival of RARC with ORC, showing non-inferiority for the robotic approach (difference 0.7%, 95% CI 9.6%-10.9%;p=0.001) [18].Regarding the recurrence rate, in a systematic review and meta-analysis previously mentioned,three studies with 458 patients showed no difference between RARC and ORC (RR 0.94, 95% CI 0.69-1.29) [19].
In a European multi-institutional series, unusual recurrence patterns were not identified.Peritoneal carcinomatosis and metastasis at the port site represented only 0.7%and 0.3% of the recurrences, respectively.The recurrence patterns in RARC appeared similar to those in the ORC series.Distant recurrences most frequently occurred in the bones, lungs, and liver, whereas pelvic lymph nodes were the most frequent site of local recurrence [11].
2.3.3.Survival rates
In a recent study published by the Mayo Clinic,for 10 years,481 patients underwent RC (203 RARC and 278 ORC).No differences in recurrence-free survival (RFS) were observed: 5-years 70.8% vs.64.7%, and the 10-year RFS rates were 69.6% vs.62.7% for the RARC vs.ORC, respectively.No differences in the overall survival (OS) were described between the two groups: 58.9% and 39.9% at 5 and 10 years for RARC and 57.7%and 45.6%at 5 and 10 years for ORC patients (p=0.466) [20].
Similar results were published in the largest multiinstitutional study to date.The International Robotic Cystectomy Consortium (IRCC) reported 5-year recurrencefree,cancer-specific,and overall survival rates of 67%,75%,and 50%, respectively, which were comparable to ORC series [21,22].
2.4.Complications
In the CORAL RTC (three arm study that compares open,laparoscopic, and robotic cystectomy) the 30-day complication rates (Clavien-Dindo system) between the three different techniques were: Seventy percent for ORC, 55%for RARC and 26% laparascopic radical cystectomy (LRC)(p=0.024).These differences are statistically significant only when ORC was compared to LRC (p<0.01).There was no significant difference in 90 days complication rates between the three arms [1].No differences were observed between RARC and ORC when Clavien-Dindo grade ≥3 were analyzed: Twenty percent of the cases of each group presented at least one event.
In two studies analyzed in one meta-analysis, Sathianathen et al.[19] revealed that the need for perioperative transfusions was lower for RARC than for ORC (RR 0.58, 95% CI 0.43-0.80).This finding is endorsed by the RAZOR trial where RARC described a significantly lower estimated blood loss(EBL)300 mL when compared to ORC 700 mL(p<0.0001),requiring less frequent intraoperative(13% vs.34%, p<0.0001) and postoperative blood transfusions (25% vs.40%, p<0.0089).
Recently, Moschovas et al.[23] reported a multicentric study evaluating the RARC outcomes and complication rates during the learning curve of surgeons that already had expertise in robotic-assisted radical prostatectomies.Interestingly, the complication rates were similar to the reference centers of radical cystectomies.Therefore, the author concluded that the robotic prostatectomy expertise could minimize the complications during the learning curve of radical cystectomies [23].
Several studies have shown a longer operative time for the RARC,with surgical time ranging from 252 min to 456 min for RARC and 210-329 min for ORC[9,10,24].Similar results were seen in the RAZOR trial,where the median operative time for RARC was 428 min and 361 min for ORC (p=0.0005).This operative time length is related to the type of diversion performed, being the intracorporeal urinary diversion linked to higher operative time[25,26].
2.5.Quality of life (QoL)
SeveralstudieshaveevaluatedtheQoLof thepatientsafterRC using validated questionnaires, without finding differences between RARC and ORC[2,18,24].In the RAZOR trial the RARC group,the mean estimated score for emotional wellbeing was significantly higher at 3 months (p=0.0007) and 6 months(p=0.0014)than at baseline.Similarly,in the ORC group,the mean estimated emotional wellbeing score was significantly higher at 3 months(p=0.0007)and at 6 months (p=0.0007)than at baseline.Both groups had significant improvement in mean total Functional Assessment of Therapy-Vanderbilt Cystectomy Index (FACT-VCI) score, 6 months after surgery compared with baseline[18].
Similar results were found by Messer et al.[27] and in the CORAL trial, where using different models of the FACT questionnaire,no difference in the QoL were seen between ORC and RARC.
Furthermore, Stegemann et al.[28] sought to compare patient QoL outcomes at 90 days with baseline (before surgery) using the Convalescence and Recovery Evaluation(CARE).The average time it took for patients to reach 90%in the overall CARE difference index was 63 days.
2.6.Race cost-effectiveness
In a prospective randomized controlled trial with 124 patients, RARC with neobladder had an average additional cost of$3 920 compared with ORC(p<0.0001).For RC with an ileal conduit,RARC generated an average additional cost of $1 740 compared with ORC (p<0.05).Additional costs due to RARC were primarily related to operating room costs(robot, supplies, and facilities) and physician costs [10].
In a retrospective observational cohort study using the US Nationwide Inpatient Sample, with 1 444 ORCs and 224 RARCs to compare population-based perioperative outcomes and costs, patients undergoing RARC experienced fewer inpatient complications(49.1%and 63.8%,p=0.035),fewer deaths (0% and 2.5%, p<0.001), and less parenteral nutrition use (6.4% and 13.3%, p=0.046).However, there was no difference in length of stay and RARC was $3 797 more costly when compared with ORC (p=0.023) [29].
Michels et al.[30] showed lower rates of minor and major complications of RARC versus ORC were 18% vs.23%and 16% vs.25%, at 30 and 90 days respectively.However,the 30 and 90 days extra costs needed to prevent one major complication were €62 582 and €37 007,respectively[30].
2.7.RC in the elderly
A multicentric European study with prospectively collected data of patients 80 years old or older who underwent RC and ureterocutaneostomy,aimed to assess patient frailty as a risk factor for RC complications, using a simplified frailty index(sFI)with a 5-item score,including diabetes mellitus,functional status, chronic obstructive pulmonary disease(COPD), congestive cardiac failure, and hypertension.Most of the major complications (Clavien-Dindo ≥3) occurred in patients with sFI ≥3:13 (11.1%) versus 4 (3.4%; p=0.02)[31].Similar results were found by Sathianathen et al.[32]using an extended sFI patients with sFI ≥3 had a greater likelihood of experiencing a major complication(odds ratio 3.22, 95% CI 2.01-5.17), especially in the subgroup of patients aged ≥65 years.
3.Conclusion
Several studies with level-1 evidence have demonstrated that RARC is technically feasible and safe.Also, the oncological, pathological, and perioperative outcomes are equivalent to ORC.The robotic approach improves some important perioperative outcomes, such as blood loss and transfusion rates.The major drawback is the longer operative time of RARC compared to ORC.However,OT in RARC decreases significantly as the surgeon gain experience.
Author contributions
Study concept and design: Gabriel Ogaya-Pinies, Marcio Covas Moschovas.
Data acquisition: Kulthe Ramesh Seetharam Bhat.
Data analysis: Cathy Jenson.
Drafting of manuscript:Gabriel Ogaya-Pinies,Marcio Covas Moschovas, Cathy Jenson.
Critical revision of the manuscript: Vipul R.Patel.
Conflicts of interest
The authors declare no conflict of interest.
杂志排行
Asian Journal of Urology的其它文章
- Nerve-sparing robot-assisted radical prostatectomy: Current perspectives
- Robot-assisted endoscopic inguinal lymphadenectomy: A review of current outcomes
- Robot-assisted retroperitoneal lymphadenectomy: The state of art
- Robotic surgery techniques to approach benign prostatic hyperplasia disease: A comprehensive literature review and the state of art
- Robotic renal and adrenal oncologic surgery:A contemporary review
- Comparison of the oncological,perioperative and functional outcomes of partial nephrectomy versus radical nephrectomy for clinical T1b renal cell carcinoma: A systematic review and metaanalysis of retrospective studies
