Robotic renal and adrenal oncologic surgery:A contemporary review
2021-03-26KultheRmeshSeethrmBhtMrcioCovsMoschovsFikretFtihOnolTrvisRogersShnnonRoofVipulPtelOscrSchtloff
Kulthe Rmesh Seethrm Bht , Mrcio Covs Moschovs Fikret Ftih Onol Trvis Rogers Shnnon Roof Vipul R.Ptel Oscr Schtloff b
a Global Robotics Institute, AdventHealth Celebration Health, Celebration, FL, USA
b Sudmedica Health, Chile
Abstract Robot-assisted surgery has evolved over time.Radical nephrectomy with inferior vena cava thrombectomy is feasible and safe for level I,II and III thrombus in high volume centers.Though it is feasible for level IV thrombus,this procedure needs a multi-departmental cooperation.However, the safety of robot-assisted procedures in this subset is still unknown.Robot-assisted partial nephrectomy has been universally approved and found oncologically safe.Robotic adrenalectomy has been increasingly utilized for select cases,especially in bilateral tumors and for retroperitoneal adrenalectomy.
KEYWORDS Inferior vena cava thrombectomy;Robotic nephrectomy;Partial nephrectomy;Adrenalectomy
1.Introduction
Robot-assisted surgery is currently used widely in several urological and non-urological procedures.There is little doubt that with increasing surgical expertise, the bar has raised in robotic surgery.Besides robot-assisted laparoscopic prostatectomy (RALP) and partial nephrectomy (RAPN) for small renal tumors, which were the indications for robotic surgery at its induction,the spectrum has widened to include more complex procedures.Complex partial nephrectomies(PN) and radical nephrectomy (RN) with inferior vena cava(IVC) thrombus are now increasingly being performed robotically.Also,the use of the robot in adrenalectomy has been described.In this paper, we go beyond the initial indications for robotic kidney surgery,and explore the current status for robotic surgery in highly challenging scenarios.
2.Materials and methods
A literature search for articles in English was done using PubMed, Hinari, Web of science, Scopus, Cochrane library and Google scholar, using terms “robotic assistance”,“nephron sparing surgery”, “trifecta outcomes”, “robotic partial nephrectomy”, “renal function following partial nephrectomy”, “robotic radical nephrectomy with IVC thrombectomy”,“robotic adrenalectomy”and“minimally invasive surgery in adrenals”, among others.The search operation was performed using the operator “OR” using these key words.All relevant systematic reviews,original articles,case series,and case reports were selected for this review.
3.PN in complex scenarios
PN is the first choice for the removal of renal tumors under 5 cm.A prospective,randomised European Organisation for Research and Treatment of Cancer (EORTC 30904) intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and RN for low-stage renal cell carcinoma (RCC) up to 5 cm reported similar oncological outcomes with increased renal function (RF) preservation in PN, although with accrual limitations [1].This study also concluded that in the PN group, there was a reduced incidence of at least moderate renal dysfunction(estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m) [2].RAPN is an established treatment for kidney tumors.Together with laparoscopic PN, they have largely replaced open PN for the treatment of small renal masses.With increased expertise, the focus has now switched to whether robotic PN can achieve comparable results in more challenging cases.The concept of trifecta was developed in an effort to standardize the reporting of perioperative results in PN.In the original report, it required the simultaneous achievement of negative surgical margins, no urological complications,and a maximum decrease in RF up to 10% over the volume predicted postoperative eGFR [3].Over time, however trifecta outcomes have shown a wide range of variation not only because of normal differences in surgical expertise and volume, but also because many changes have been made to the original definition.This entirely affects the comparison of success across the contemporary series with some studies, including a warm ischemia time (WIT) ranging over 25 min or 30 min, complications over Clavien 2 or 3, or reductions up to 30% in postoperative eGFR [4,5].Another important effort in standardization is the development of nephrometry scores,which aim to objectively classify the complexity of a renal mass and to predict the possibility of postoperative complications.The most widely-used are the RENAL and the PADUA scores,coming from the US and Europe,respectively[6,7].In a recent systematic review and meta-analysis,higher scores in both classifications correlated with longer WIT and increased perioperative complications [8].Completely endophytic tumors are one of the hardest challenges in PN, and are associated with longer WIT and more frequent complications [9].Harke et al.[10]compared the outcomes of 64 RAPN vs.76 open partial nephrectomy (OPN) for completely endophytic cases.Preoperative characteristics including tumor size and complexity were comparable.RAPN resulted in increased operative time (169 min vs.140 min) and shorter WIT(13 min vs.18 min); no differences were found in positive surgical margins and complications.Intraoperative ultrasound is currently the standard to assess totally endophytic tumors, as it allows to correctly mark the perimeter of resection.However, it does not allow real time tumor identification once the resection has begun.In an attempt to overcome this limitation, Simone et al.[11] reported a novel technique consisting of preoperative tumor marking with transarterial superselective delivery of indocyanine green (ICG)-lipiodol mixture, along with the intraoperative use of near-infrared fluorescence imaging.In 10 consecutive patients operated under no ischemia,no complications or positive surgical margins were reported.Yerram et al.[12]compared the trifecta outcomes of 68 RAPN vs.42 OPN in kidneys with multiple tumors.Trifecta was defined as negative surgical margins, no urologic complications, and a glomerular filtration rate(GFR)preservation of ≥90%at last follow-up.On adjusted analyses,RAPN achieved equivalent rates of trifecta to open surgery(16.3%vs.16.5%,p=0.99),which persisted on subgroup analyses of patients with two(20.1% vs.23.7%, p=0.82) or more than two tumors (6.8%vs.7.4%, p=0.95).There were no differences between robotic and open cohorts for negative margin rates,absence of complications, or GFR≥90.A Korean study compared 64 OPN vs.85 RAPN for tumors with a RENAL nephrometry score ≥10.Although the RAPN group had significantly more stage T1b and T2, there were no significant differences in estimated blood loss (EBL), WIT, operative room(OR) time,margin status,postoperative decline in GFR, and complications [13].When RAPN was compared to OPN in hilar tumors, there were also no significant differences in outcomes.On adjusted analyses, RAPN achieved equivalent rate of trifecta to open surgery(21.1% vs.13.9%, respectively, p=0.387) [14].Malkoc et al.[15]compared the outcomes between 54 RAPN and 56 OPN for localized >7 cm tumors; WIT and transfusion rates were lower in the RAPN group (31.5 vs.35.0 min, p=0.02, and 9.4% vs.30.4%, p=0.008, respectively); complications and positive margins were similar.However, readmission rate within 30 days after discharge was higher in the OPN group(p=0.03).Simone et al.[16] reported the oncological outcomes of minimally invasive partial versus minimally invasive RN for cT1-2/N0/M0 renal cell carcinomas in a propensity score-matched analysis.The results showed comparable 5-year metastasis-free survival (88.9% vs.89.9%, p=0.811), local recurrence-free survival (94.2% vs.95.9%, p=0.283), overall survival (94.5% vs.96.8%,p=0.419) and cancer-specific survival (96% vs.98.6%,p=0.907)for PN and RN,respectively.A systematic review and meta-analysis of studies comparing PN vs.RN for T1-T2 renal tumors found that the recurrence rate (RR 0.61;p=0.004) and cancer-specific mortality (RR 0.65; p=0.03)were lower for PN, probably secondary to smaller tumor size in the PN group.Lower EBL was found for RN(weighted mean difference [WMD] 102.6 mL; p<0.001) and there was a higher likelihood of postoperative complications for PN(RR 1.74, 95% confidence interval [CI] 1.34-2.2; p<0.001)[17].PN is a complex operation with serious potential complications.RAPN has shown to be technically feasible with similar outcomes to OPN in even the most challenging scenarios.Per this review,it appears that the limit of RAPN is similar to that of OPN, however, robotic expertise and surgical volume are paramount in order to perform this operation with satisfactory outcomes.
PN is the treatment of choice for small renal masses.Studies have shown that PN achieves comparable cancer specific survival (CSS), with increased preservation of RF when compared to RN.While RN preserves overall RF in the range of 50%-70%,PN can hold preoperative function up to 85%-90% [2].Every possible effort should be made in kidney surgery to preserve RF, as there is a well-known association between chronic kidney disease (CKD) and increased risk of death and cardiovascular events, especially when the GFR goes below 45 mL/min [23].This is especially important in patients with kidney tumors, as up to 30% of these patients can have preoperative established CKD, and a higher percentage harbor risk factors such as hypertension, diabetes and dyslipidemia [24].Moreover, it was reported that after a median follow-up of 6.6 years,patients who underwent total nephrectomy and had preoperative CKD due to medical causes (CKD-M), were at a higher risk of severe CKD and death than those with newly onset CKD solely due to surgical removal of nephrons(CKD-S), emphasizing the need for maximum preservation of nephrons,especially in patients with underlying medical conditions [25].
Although PN can achieve a high rate of preserving RF,every PN entails an unavoidable loss of RF secondary to the removal of healthy nephrons or due to irreversible ischemic damage.Hypothermia has been used to protect RF from ischemic damage during open PN, and especially in transplant surgery where several hours of protection are needed, showing almost complete recovery in the vast majority of grafts.Most cases, however, are done under warm ischemia, especially for small masses using the robotic or laparoscopic approach.A number of studies have compared the degree of preservation of RF with cold vs.warm ischemia.One study including 277 patients showed that after adjusting for the loss of healthy renal tissue,complete recovery was achieved with cold ischemia and up to 91% with warm ischemia (WI) [26].Although cold ischemia seems to provide better preservation than WI,this difference appears to be minimal, at least at the standard WITs.A recent systematic review and meta-analysis of 156 studies on ischemia techniques in nephron-sparing surgery,including cold, warm, and zero-ischemia, showed no significant differences in terms of estimated blood loss and RF preservation.Postoperative complications and positive surgical margins were recorded in 14.1% (95% CI: 6.7%-27.4%), 11.1% (95% CI: 10.0%-12.3%), and 9.7% (95% CI:7.7%-12.2%), (p<0.01), and in 4.8% (95% CI: 1.9%-10.9%),4.0% (95% CI: 3.4%-4.8%), and 5.6% (95% CI: 3.1%-9.8%),(p<0.01) of patients after cold, warm, and zero ischemia respectively [27].
Traditionally, great importance has been given to the duration of WI during PN.Early studies suggested that WI was a significant predictive factor for loss of RF after PN,up to the point that ischemia time over 25 min was associated with increased risk of acute and chronic CKD.Each additional minute of warm ischemia was associated with 5% and 6% increased odds of developing ARF or a GFR<15 mL/min/1.73 min the post operative period,respectively,and was associated with a 6%increased risk of new-onset stage IV CKD during follow-up [28-30].One concern with these studies is that they were heavily biased,as they failed to control for the amount of healthy renal parenchyma removed during surgery.
However,when similar studies were performed including the amount of removed renal parenchyma as a factor, the results were surprisingly different and concluded that the amount and quality of preserved normal kidney tissue was the most determinant factor affecting RF after PN.The duration of WI was not significant, and appears to be much less determinant, unless it has significantly deviated from the traditional standard time (Table 1) [18-22].One study looking to assess the impact of WI on the operated kidney included 250 patients, 188 of them having WIT longer than 25 min[31].Renal scans were used to evaluate the pre-and postoperative RF on the operated kidney, while computer tomography (CT) volumetry was used to assess the amount of preserved renal parenchyma.On average, when 82% of the renal parenchyma was preserved after surgery, the postoperative GFR went down to 76% of the preoperative GFR,meaning that the net loss of GFR that could be related to ischemic damage was only 6% [31].The tolerance of the human kidney to ischemia was measured at the molecular level in a study where 40 kidneys undergoing open PN were biopsied before, during, and after arterial clamping; median WIT was 32 min.Electronic microscopy and markers of kidney injury were used to assess ischemia related damage.The results suggested that the human kidney might be more tolerant to ischemia than we previously thought.Structural changes in nephrons were less pronounced compared to animal models with similar duration of ischemia.Ischemia of less than 60 min was associated with reversible changes in electronic microscopy, immunofluorescence, and temporary increases in renal injury markers [32].
4.Robotic RN with IVC thrombus
Minimally invasive surgery has stood the test of time as more surgeons adopt this method for a wide range of urological procedures.However,the use of minimally invasive surgery in radical procedures like RN with IVC thrombus has always been challenging, especially using laparoscopic techniques.With increasing experience, more radical procedures were attempted.The advantages of the robot include magnification,dexterity,minimal invasiveness,and greater precision,which have allowed more surgeons to adopt robotic technology with confidence.RN with IVC thrombectomy is one of the most complex procedures described in urology,and very few cases/case series have been reported in the literature to establish its safety and feasibility.
The goal of this surgery is to completely resect the RCC with IVC thrombus.Depending upon the level of thrombus,the techniques may vary.One may have to resect the IVC and may or may not need to perform complex vascular reconstruction.In a systematic review of 14 studies of retrospective nature, Lardas et al.[33] concluded that surgical management of patients with non-metastatic RCC with IVC thrombus is complex, but potentially curative and acceptable.In a matched comparative analysis for level I-II thrombus, robotic RN with IVC thrombectomy had favourable perioperative results with similar oncological outcomes compared to open procedure [34].Davila et al.[35]reported significantly shorter length of hospital stay(p<0.05), intraoperative blood loss (p<0.01) and similar operative time for up to level III thrombus.
4.1.Planning
The key step in performing this procedure is its planning.All patients are required a contrast-enhanced CT scan of the abdomen and pelvis.Apart from determining the size,site,stage, and lymph node status of RCC, the imaging also provides the size (length/diameter) and extent of the tumor, including its distance from main hepatic veins,arterialization of the tumor,and the level of thrombus.It is also useful to assess renal vasculature and collaterals, and the hepatic anatomy,especially the number and location of hepatic veins.Three-dimensional(3D)reconstruction of the renal anatomy and thrombus might be useful in the management[36].The CT has a sensitivity of 93%and specificity of 80% in determining the extent of tumor thrombus[37-39].Chopra et al.[40] alternatively suggested the use of magnetic resonance imaging(MRI)scan in evaluating the level of thrombus and planning the surgery.MRI is known to have high accuracy in evaluating tumor volume and IVC wall involvement[41].Additional testing includes renal/hepatic function and metastatic evaluation.In large tumors with extensive thrombus, preoperative angioembolisation(PRAE) has been proposed, but its use has been controversial [42].Chan et al.[43] found that in patients with T3 RCC PRAE statistically increased the operative time, estimated blood loss and the hospital stay.There was also a higher perioperative mortality.On the other hand, Tang et al.[44] found that PRAE reduced blood loss in patients with advanced tumor thrombus, but did not have any measurable advantage in terms of long term outcomes.In a propensity score matched analysis, May et al.[45]concluded there is no survival benefit of PRAE,and the only advantage was a statistically significant reduction in blood transfusion in patients who had the embolization.In fact,89% of the patients with embolization had some form of post embolization syndrome.
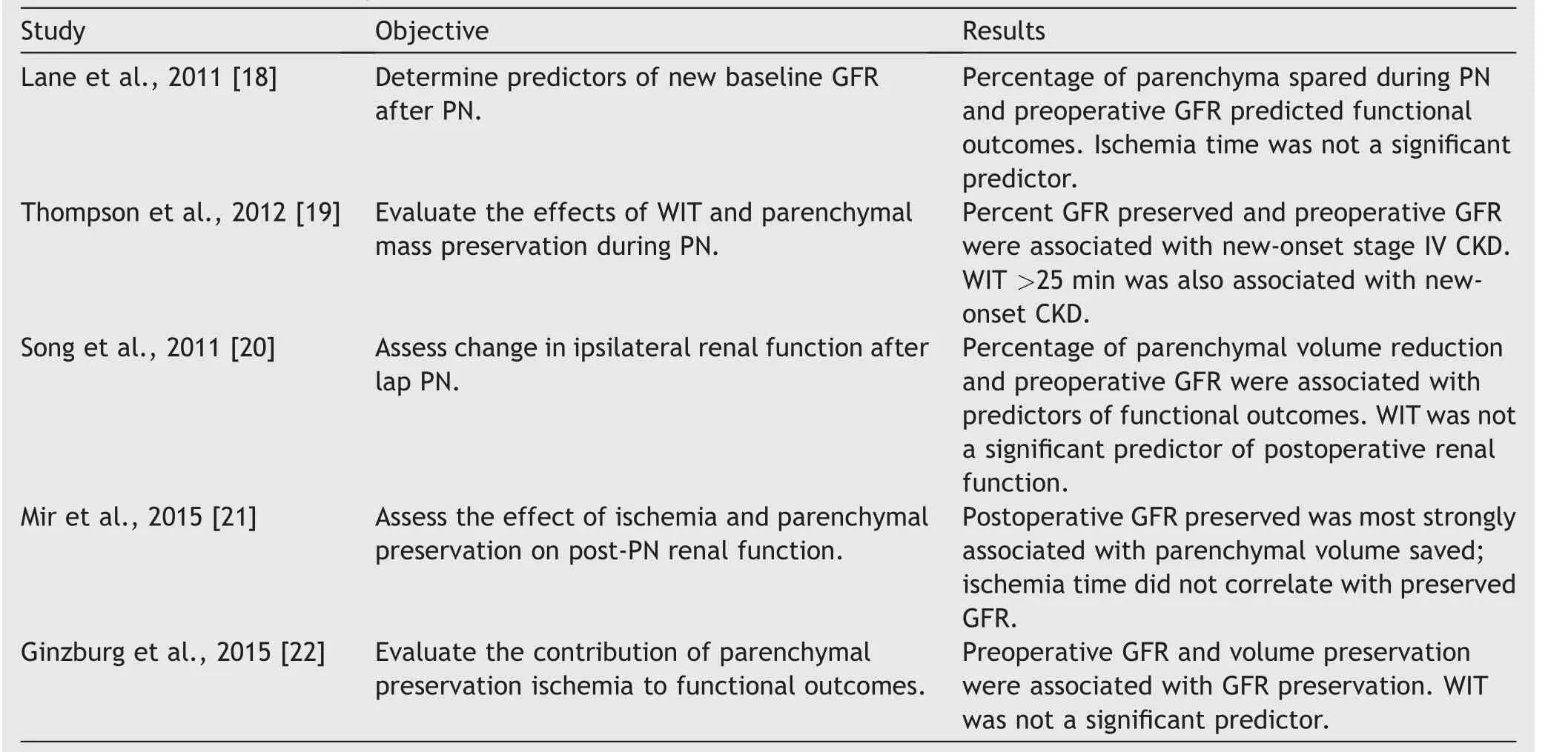
Table 1 Predictors of GFR preservation after PN.
4.2.Management of IVC thrombus
4.2.1.Level I and II thrombi
Abaza [46] described the initial series of robot-assisted RN with IVC thrombectomy in five patients.The maximum thrombus size was 5 cm, extending into the renal vein.All procedures were successful, with mean blood loss of 170 mL,OR time of 327 min,and a hospital stay of 1.2 days,with no reported complications.The author determined that to minimize the risk of tumor embolus,the renal artery control should be achieved in the interaortacaval space,thereby reducing the manipulation of the IVC.The right side RN and caval thrombectomy include temporary cessation of vascularity of the right kidney.Prior to securing the infra hepatic and supra hepatic IVC, the right renal artery and vein are controlled using individual bulldog clamps.The cavatomy is then performed to complete the thrombectomy,and following reconstruction of the IVC the right kidney is revascularized [40].Since then, many series with level II and level III thrombus have been described,reiterating the safety and feasibility of robotic surgery in this condition [36,47-50].In a series by Wang et al.[50],comparing right and left sided nephrectomy with IVC thrombectomy, the left side was technically difficult compared to the right,and the techniques varied from side to side(median OR time left vs.right:250 min vs.131 min).The median WIT during left nephrectomy for the right kidney was 18 min (14 min-22 min).Aghazadeh and Goh[48] described robot left-sided RN with level II thrombectomy in supine position with single docking.The advantage of this approach is that there is bilateral renal artery access, and one can achieve rapid caval control and avoidance of redocking/repositioning.Intraoperative ultrasound is a useful tool to assess the location and extent of the tumor thrombus.When the thrombus is located in a higher position, the use of bulldogs or Rummel tourniquets might be necessary to get proximal control during cavotomy,thrombus extraction, and resuturing.IVC is irrigated with heparinized saline prior to suturing to eliminate clots and bubbles [47].This is usually followed by retroperitoneal lymph node dissection.The use of a flexible cystoscope/ureteroscope to visualize the interior of IVC has been described [36,49].Rarely, if the tumor thrombus infiltrates the IVC wall, the excision of the IVC wall and the use of venous grafts are necessary if re-suturing occludes the lumen more than 50% [49].RN with renal vein thrombus in horseshoe kidney has been described, thus demonstrating its feasibility [51].
4.2.2.Level III thrombus
The initial case series for RN with level III thrombus was first described by Abreu et al.[52]in 2014.Adequate knowledge of anatomy, preoperative preparation, and robust surgical technique are preconditions for a successful procedure.They proposed the first IVC technique that involves adequate caval mobilization with minimal manipulation of the pericaval tissue to prevent embolization,and proximal hilar control of the infra hepatic IVC by ligating the short hepatic veins.After proximal and distal control of the IVC, the renal vein is stapled, followed by thrombectomy and nephrectomy.This is followed by IVC closure with or without venous graft,maintaining at least 50%of IVC lumen.In the series by Gill et al.[47],the reported median blood loss,OR time,and hospital stays were 375 mL,4.9 h and 4.5 days,respectively;the mean transfusion per patient was 4 units.A multi-institutional series by Abaza et al.[53] showed safety and efficacy of the robotic platform, wherein nine surgeons performed 32 surgeries, thus establishing safety and feasibility of this procedure(Table 2).
4.2.3.Level IV thrombus
Palma-Zamora et al.[54] first described the use of robotic assistance in level IV thrombus, wherein initial mobilization of the left kidney using the robot was performed, followed by open nephrectomy and thrombectomy under cardiopulmonary bypass (CPB).Wang et al.[55] described a series of 13 patients with level II-IV thrombus, and a follow-up of 1 year.Level III thrombus required extensive mobilization of liver with clamping of porta hepatis.This was followed by clamping of suprahepatic and infra-diaphragmatic IVC.CPB was needed for level IV thrombus and thrombectomy was performed thoracoscopically.They reported a median operative time of 465 min, with a median blood loss of 2 000 mL.There was one perioperative death and two patients died during the follow-up.
All of the current available literature on robotic RN and IVC thrombectomy are mainly case series.These surgeries are technically challenging and should be performed preferably by a high-volume surgical team that has other surgical and medical specialities to support in the management of these complex cases.
5.Current status of robotic adrenalectomy(RA)
The first adrenal surgery was performed in 1889 [56].Adrenalectomy was performed using the open approach for almost a century.In 1991, the period of minimally invasive adrenalectomy began, when Gagner et al.[57] performed the first transperitoneal laparoscopic adrenalectomy(TLA).However,within a decade,Piazza et al.[58]performed the first RA in 1999.
The indications for adrenalectomy include [59,60]:
·Functional adrenal masses
·Adrenal malignancy
- Primary adrenal cortical carcinoma
- Solitary metastasis from non-adrenal sources when deemed curative
- Size of incidentaloma more than 4 cm
5.1.Surgical anatomy
Adrenal glands are bilateral retroperitoneal organs that lie within the gerotas fascia on the superomedial aspect of the respective kidney.The right adrenal gland is generally more cephalad than the left, while the left is more mobile compared to the right.The superior, middle, and lower adrenal arteries from the inferior phrenic artery, the abdominal aorta, and the renal artery respectively, supply the right adrenal gland.The right adrenal vein is short,drains into the IVC posteriorly,and is exposed following the meticulous mobilization of the gland.On the left side, the arterial supply is similar.However, the vein is long and courses downward after receiving the left inferior phrenic vein to join the left renal vein.
5.2.RA
As with any surgery, the robotic system has an advantage due to its better ergonomics, magnified 3D view, tremor filtering,and movements of the Endowrist®,in comparison with laparoscopy.These properties are optimal for adrenal surgery, where one needs to handle the fragile gland in narrow spaces with many vital structures surrounding it.
5.2.1.Comparison of RA vs.laparoscopic adrenalectomy(LA)
There are several case reports and case series on the use of RA,and on the comparison of RA vs.LA(Table 3).These studies compared perioperative outcomes including the blood loss, OR time, and complications following RA.RA had lower rates of blood loss and hospital stay in most series.However, the OR times varied, with some series having better time with RA and some with LA.There was no difference in immediate postoperative complication rates between the two techniques [61-71].Of note is a large review of a national inpatient sample of 1 006 patients, in which there was no difference in complication rates and conversion rates.The length of stay was lower,and cost was higher in RA group vs.LA group.They also noted that there was a significant trend towards more RA each year[72].There are very few level 1 and 2 studies on RA vs.LA in the literature, with one randomised controlled trial, one meta-analysis, and two combined metanalysis and systematic reviews.In the RCT conducted by Morino et al.[73], there were significantly longer OR times in the RA group vs.the LA group,with no difference in hospital stay.However,the perioperative morbidity and cost were significantly higher in RA group.In Morino’s paper, 4/10 patients in RA group were converted to standard laparoscopy.This could be due to the fact that when this trial was performed, robotic surgery was new,and the team might have been confident with laparoscopy.Another metanalysis by Economopoulous et al.[74]showed no difference in intraoperative complications,postoperative complications, mortality, conversion to laparotomy, and blood loss with shorter hospital stay and longer surgical time in RA vs.LA.In a systematic review and metanalysis by Tang et al.[75], there were no significant differences in OR time, conversion rates and overall complications, but RA had an upper hand in blood loss and operative times.Brandao et al.[68], in a similar review combined with metanalysis, showed there were longer hospital stays, more postoperative complications and more blood loss in LA.Hence,there is a learning curve to perform RA irrespective of laparoscopic skills.There are no significant differences in complication and conversion rates.RA has advantages in terms of hospital stay,OR time with experience, and blood loss.However, the cost burden is enormous.
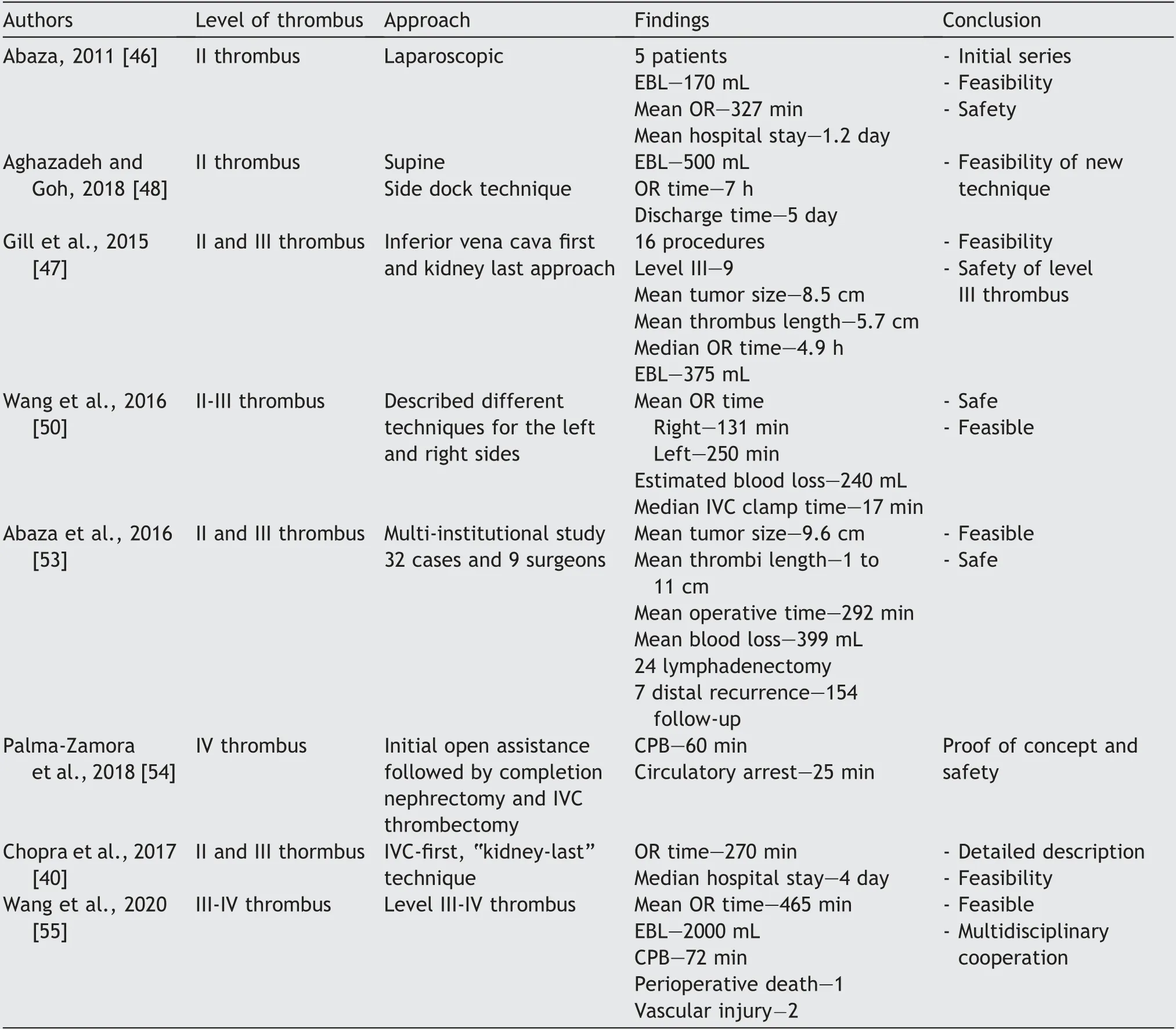
Table 2 Contemporary outcomes of robot-assisted radical nephrectomy with IVC thrombectomy (n≥5 cases).

Table 3 Comparison between laparoscopic vs.robotic adrenalectomy.
5.2.2.Robotic approaches
The optimal surgical approach for adrenalectomy is decided by many factors, such as prior abdominal surgery, body mass index (BMI), adrenal size, retroperitoneal fat, the indication of adrenalectomy, and tumor site [79].
5.2.2.1.Transperitoneal RA (TRA).This is the most common RA technique followed worldwide [80].Most surgical teams are comfortable to perform TRA as they transition from LA to RA.Once the learning curve is reached, the transition to the posterior retroperitoneal approach (PRA)is made [79].There was no difference between the Si and Xi system in performing TRA [79].The use of RA in obese patients of BMI>30 kg/mis well established [81].In 2013, the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) recommended the use of the lateral transabdominal approach in morbidly obese patients of BMI>35 kg/mand for large tumors >6 cm, in comparison with other surgical approaches [82].They also state that compared to laparoscopic techniques, RA is advantageous for large tumors and morbidly obese patients [82].
5.2.2.2.PRA.This approach is a useful tool in a patient with multiple prior abdominal surgeries, and in patients who needs bilateral adrenalectomy without changing patient position.The robot can be a useful tool when the access is difficult [83].
This approach was first used in 1994 by Walz et al.[84],who performed the procedure laparoscopically.The first robotic PRA was performed in 2010 by Ludwig et al.[85] In two different series.Berber et al.[86]and Kahramangil and Berber [87] compared transperitoneal vs.retroperitoneal approaches, and concluded that PRA was most useful in patients with kidney and adrenal glands located above the 12th rib, in patients with multiple prior abdominal surgeries, and in patients who need bilateral adrenalectomy.In fact, in their 2013 guidelines, SAGES recommended the use of retroperitoneal approaches [82].
This procedure is fundamentally different from other robotic procedures, as the ports are usually placed closer,akin to the single port technique.Usually only three trocars are used with no assistant port, and hence require experience to perform.The docking times are also reduced by experience [86].With the new DaVinci SP system, adrenalectomy could be potentially performed using a single port of 3 cm in size.
5.2.2.3.Partial adrenalectomy and cortical sparing approach.The robotic platform is best suited for performing cortical sparing adrenalectomy that is aimed at removing the medullary tissue while retaining the cortex.It is typically useful in patients with familial syndromes who develop bilateral pheochromocytomas (e.g., multiple endocrine neoplasia types 2 [MEN2], Von Hippel-Lindau [VHL], succinate dehydrogenase [SDH] gene mutations, and neurofibromatosis type 1 [NF1]).In a series of 10 patients, Boris et al.[88] successfully treated 10 patients with one open conversion and three staged procedures, and none had local recurrence at 16.2 months (range: 2-29 months).One patient needed long-term steroid replacement.It is especially useful in young patients, to prevent lifelong steroid dependence and the possibility of Addisonian crisis when bilateral adrenalectomy is performed.However, there is a risk of recurrence if medullary tissue is left behind [83].This is associated with longer operative time and higher blood loss.The cortical sparing approach can be performed both transperitoneally and retroperitoneally [88,89].The use of intraoperative ultrasound may be useful in these scenarios.Robotic-assisted partial adrenalectomy(RAPA) for renal metastatic disease was first performed by Kumar et al.[90].This patient had prior right RN and left adrenalectomy for metastatic disease and had a recurrence.Simone et al.[91] presented a detailed series of RAPA for unilateral aldosterone producing adenoma in 10 patients with perioperative and 1 year functional outcomes.They operated on a median nodule size of 18 mm, with a median hospital stay of 3 days.All patients had normal biochemical profile at 3 months and 1 year, and one required low dose amlodipine at 6 months, thus demonstrating its safety and feasibility.
5.2.2.4.RA for malignant diseases.The use of the robotic platform for adrenal malignancy is a highly debatable topic.There are no level 1 evidence or retrospective reviews supporting robot-assisted adrenalectomy for malignancy.Zafar and Abaza [92] reported the first case of robotassisted LA for adrenocortical carcinoma for an 8 cm tumor.There are a few series comparing laparoscopy with open adrenalectomy for adrenocortical malignancy, which shows some evidence supporting open surgery [93-95].Wu et al.[95] compared 23 patients undergoing open surgery vs.21 undergoing laparoscopy.The 5 year overall survival and recurrence-free survival for open vs.laparoscopic procedures were 43% vs.47% and 36% vs.39%, respectively.Similarly, Zheng et al.[94] showed overall disease-free survival duration (45 months vs.17.5 months), and the 2-year disease-free survival rates were better in open vs.laparoscopic procedures (61.1% vs.21.4%, respectively) in spite of tumors being smaller in the laparoscopic group.In the pediatric population,minimally invasive surgery (MIS) has been validated for benign tumors, but caution needs to be exercised in adrenal malignancy.In patients with image-defined risk factors, MIS may be contraindicated [96].The lack of evidence supporting performing MIS for adrenal malignancy, and the rarity of this condition could be the reason for only one case report describing the use of the robot for adrenalectomy in malignancy.
6.Conclusions
Robotic-assisted surgery is rapidly evolving to include more complex and challenging procedures.Currently, almost every urological procedure which is limited to one or two quadrants in the abdomen can be successfully performed robotically with excellent results.The development of new robotic systems and advancements in technology regarding intraoperative imaging and surgical planning, will contribute to expanding these capabilities to an even greater extent.
Author contributions
Study concept and design: Kulthe Ramesh Seetharam Bhat,Vipul R.Patel, Oscar Schatloff.
Data acquisition: Kulthe Ramesh Seetharam Bhat, Oscar Schatloff.
Data analysis: Marcio Covas Moschovas, Fikret Fatih Onol.Drafting of manuscript: Kulthe Ramesh Seetharam Bhat,Oscar Schatloff.
Critical revision of the manuscript: Shannon Roof, Travis Rogers.
Conflicts of interest
The authors declare no conflict of interest.
杂志排行
Asian Journal of Urology的其它文章
- Nerve-sparing robot-assisted radical prostatectomy: Current perspectives
- Robtic-assisted radical cystectomy:Literature review
- Robot-assisted endoscopic inguinal lymphadenectomy: A review of current outcomes
- Robot-assisted retroperitoneal lymphadenectomy: The state of art
- Robotic surgery techniques to approach benign prostatic hyperplasia disease: A comprehensive literature review and the state of art
- Comparison of the oncological,perioperative and functional outcomes of partial nephrectomy versus radical nephrectomy for clinical T1b renal cell carcinoma: A systematic review and metaanalysis of retrospective studies
