Totally intracorporeal robot-assisted urinary diversion for bladder cancer(Part 1).Review and detailed characterization of ileal conduit and modified Indiana pouch
2021-03-26HugoOtaolaAraRafaelCoelhoVipulPatelMareloOrvieto
Hugo Otaola-Ara , Rafael Coelho , Vipul R.Patel ,Marelo Orvieto ,
a Department of Urology, Clnica Alemana, Santiago, Chile
b School of Medicine, Clı´nica Alemana-Universidad del Desarrollo, Santiago, Chile
c Universidade de So Paulo Instituto do Cncer do Estado de So Paulo, Brazil
d Department of Urology, AdventHealth Global Robotics Institute, Celebration, FL, United States
Abstract Objective: To review the most used robot-assisted cutaneous urinary diversion(CUD) after radical cystectomy for bladder cancer and create a unified compendium of the different alternatives, including new consistent images Methods: A non-systematic review of the literature with the keywords “bladder cancer”,“cutaneous urinary diversion”, and “radical cystectomy” was performed.Results: Twenty-four studies of intracorporeal ileal conduit(ICIC)and two of intracorporeal Indiana pouch(ICIP)were included in the analysis.Regarding ICIC,the patients’age ranged from 60 to 76 years.The operative time to perform a urinary diversion ranged from 60 to 133 min.The total estimated blood loss ranged from 200 to 1 117 mL.The rate of positive surgical margins ranged from 0% to 14.3%.Early minor and major complication rates ranged from 0% to 71.4% and from 0% to 53.4%, respectively.Late minor and major complication rates ranged from 0% to 66% and from 0% to 32%, respectively.Totally ICIP data are limited to one case report and one clinical series.Conclusion: The most frequent type of CUD is ICIC.Randomized studies comparing the performance of the different types of CUD,the performance in an intra-or extracorporeal manner,or the performance of a CUD versus orthotopic ileal neobladder are lacking in the literature.To this day,there are not enough quality data to determine the supremacy of one technique.This manuscript represents a compendium of the most used CUD with detailed descriptions of the technical aspects,operative and perioperative outcomes,and new consistent images for each technique.
KEYWORDS Bladder cancer;Ileal conduit;Indiana pouch;Intracorporeal urinary diversion;Robot-assisted radical cystectomy;Surgical technique
1.Introduction
Bladder cancer (BC) is the 10th most commonly diagnosed cancer (3%) and the 13th highest cause of cancer mortality worldwide (2.1%) [1].Radical cystectomy (RC) with pelvic lymph node dissection(PLND)and urinary diversion(UD)is the gold-standard therapy for localized muscle-invasive bladder cancer(MIBC) [2].Moreover, it is also an alternative for the management of patients with high risk non-muscle invasive bladder cancer (NMIBC) and a palliative option for patients with locally advanced or metastatic disease[2].
The best alternative to replace the original bladder has been investigated since the 1900s.From an anatomical standpoint, the evolution of UD has followed three milestones:
- Cutaneous urinary diversions (CUDs), either incontinent(e.g., uretero-cutaneostomy and ileal or colonic conduit) or continent pouches (e.g., Indiana pouch [IP]and T-pouch).
- Urethral urinary diversions (UUDs), which includes various forms of continent gastrointestinal pouches attached to the urethra, globally called orthotopic ileal neobladder (ONB).
- Colonic urinary diversions, such as ureterorectosigmoidostomy or uretero-ileo-rectosigmoidostomy.
The uretero-sigmoidostomy reported by Simon [3], was the first form of UD and remained the standard of care until the late 1950s [4-6].However, long-term electrolyte imbalance, unacceptable high rates of upper tract obstruction and infection and secondary malignant neoplasms arising at the ureteral implantation [7-10] encouraged surgeons to develop better forms of UD.In 1950,Bricker [11] popularized the ileal conduit (IC) and it has remained the most commonly utilized form of UD worldwide up until today.
The first continent CUD was described by Gilchrist et al.[12] using a cecal reservoir.Herein, the ileocecal valve was used to achieve the mechanism of continence and the distal ileum as a catheterizable stoma.Subsequently, the importance of complete detubularization of the bowel segment was recognized and it was demonstrated that the “double-folding technique” achieved the most spherical shape [13,14].Since then, several techniques of CUD have been developed using large, small bowel and even stomach.However,all continent CUDs are associated with an increased risk of complications compared to incontinent forms of diversion, including stone formation, difficulty catheterizing and peri-stomal hernias, amongst others [15,16].
The advent of minimally invasive techniques aiming at reducing the morbidity of open surgery while maintaining comparable functional outcomes led to the description of the first laparoscopic totally intracorporeal ileal conduit(ICIC)by the end of the 20th century[17-19].In later years several series of robot-assisted ICIC, CUD and ONB have been published.
Herein, we performed a comprehensive review of the available literature on robot-assisted intracorporeal urinary diversions (ICUD).Our goal was to provide the reader with an organized compilation of the most popular reconstructive techniques described.Key technical aspects as well as operative and perioperative outcomes (including complications and functional results) were presented.Due to the extent of information available, the current manuscript was divided into Part 1 (i.e.description of most popular forms of CUD) and Part 2 (i.e.description of most popular forms of ONBs).
2.Materials and methods
A non-systematic review of the literature in English and Spanish was performed using the PubMed electronic database.Search criteria included the keywords “bladder cancer”, “cutaneous urinary diversion” and “radical cystectomy”.Additionally,a manual search of references in relevant published articles was performed.Only studies reporting robot-assisted CUD techniques in humans were included.
Data were subdivided into baseline characteristics,intraoperative, and postoperative outcomes.Baseline characteristics included age and male/female proportion.Intraoperative outcomes included operative time (OT),either total and/or for the ICUD, estimated blood loss(EBL), and the rate of positive surgical margins (PSMs).Postoperative data included both early (≤30 postoperative days) and late (31-90 postoperative days)Clavien complications, continence rates (both daytime and nighttime) and potency rates for the IP patients,when disclosed.
Firstly, we described the surgical principles of each robot-assisted CUD technique.Secondly, we assessed the results of robot-assisted ICIC, including baseline characteristics and intraoperative and postoperative data.Thirdly, we summarized the differences between intra and extracorporeal IC.Lastly, we assessed the results of robotassisted IP.
Data from all available studies were merged for combined analysis.Still, the results from large studies (i.e.greater than 30 patients) were also reported separately.Data were summarized with ranges (minimum and maximum) and when sufficient information was available,weighted means of the percentages was calculated for qualitative variables (means were weighted according to the sample size of each study).
3.Results
We included 26 studies on robot-assisted ICIC or ICIP in the treatment of BC.Outcomes were summarized in Table 1.
3.1.Preoperative considerations
The European Association of Urology Robotic Urology Section Scientific Working Group Consensus has developed a list of preoperative recommendations that should be applied to patients undergoing a robot-assisted UD [20]:
- Counsel on the surgical method, plan of hospital stay,discharge criteria and stoma nurse information preoperatively.
- Optimize comorbidities and preoperative nutrition.
- Avoid patient mechanical bowel preparation; it should be considered only in patients with a high risk of large bowel injury (e.g., pelvic radiation, and posterior bladder masses near the rectum) [21].
- Mark stoma site on the patients’ abdomen before surgery.
- Preoperative fasting: Allow to consume solid food and clear fluids until 6 h and 2 h before anesthesia,respectively.
- Encourage a low-residue diet the day before surgery
- Prescribe thromboembolic prophylaxis with low molecular weight heparin for 4 weeks after surgery.
- Administer antibiotic prophylaxis with narrow-spectrum antibiotics (Cephazolin and Gentamicin).
3.2.Surgical technique
3.2.1.Incontinent cutaneous urinary diversion: Robotassisted totally intracorporeal ileal conduit (as described by Medina et al.[22])
3.2.1.1.Patient positioning and port placement.The robotic approach replicates the principles of open surgery[11].The technical aspects of robotic RC are beyond the scope of this review.Port placement used during the RC portion can be maintained because there is no need to approximate the ileal loop to any pelvic structure.In short, the patient is placed under general anesthesia in low lithotomy position, with a 30Trendelenburg and both arms tucked.Exposed areas of the patient are entirely covered to prevent hypothermia, and the patient is securely padded and strapped.Pneumatic sequential calf compression devices are attached to the patient.A transperitoneal 6-port configuration is used: Four robotic and two assistant ports.The camera port is placed in the midline 4-5 cm above the umbilicus.Two additional robotic ports are placed in line with the umbilicus, on either side along the lateral edge of the rectus muscle(8-10 cm from the midline).The remaining robotic port is placed approximately 2 cm above and medial, to the right anterior superior iliac spine.The two assistant ports are placed in the left lower and upper quadrants (Fig.1A).The robot is docked from the foot end.Once de RC portion is completed, several authors recommend reducing the patient’s Trendelenburg position to 10to facilitate bowel work and ventilation.
After completing the RC portion, Cadiere forceps are utilized in the second and third robotic arms,while doublefenestrated forceps are applied in the first arm.
3.2.1.2.Identification of the ureters and the bowel segment.At this stage of the operation, both ureters have been isolated and clipped; they will remain clipped until they are anastomosed to favor proximal ureteral dilation, which facilitates uretero-enteric anastomosis.A sigmoid mesenteric window is created under direct vision to transpose the left ureter to the right.The left ureter should pass above the inferior mesenteric artery to avoid arterial disturbances.Once in place, it is recommended to manipulate both ureters from the previously placed clips to aid transection and spatulation.Alternatively, stay sutures can be placed on the distal ends.
A segment of 15-20 cm of ileum 20-30 cm from the ileocecal valve is now identified (Fig.1B).A 10 cm long umbilical tape can be used for more accurate bowel measurement.It is useful to approximate the distal end of the selected segment to the anterior abdominal wall to ensure the adequate length of the IC before transecting the bowel.To facilitate the remaining steps of the procedure, the IC can be held in place using the third arm or the Marionette technique described by Guru et al.[23,24].This technique consists on suspending the distal end of the bowel segment with a silk suture on a Keith needle,which is passed outside-in through the skin, transecting the ileum and coming back outside through the skin.With this maneuver the assistant moves up and down the bowel segment.
3.2.1.3.Division of the bowel segment.The distal aspect of the IC is divided with a 60 mm Endo-GIA™stapler (blue cartridge, 3.5 mm) through the lateral assistant port.If an additional mesenteric division is required, an additional vascular staple load(white cartridge,2.5 mm)can be fired.It is imperative to fire the stapler perpendicular to the bowel to prevent any devascularization.The proximal segment of the IC is then divided with another blue cartridge and depending on the mobility of the isolated segment, a white cartridge may be used for the mesentery as well.At this point some authors prefer to discard a 5 cm segment of the small bowel, proximal to the proximal end of the IC.The proximal and distal ends of the conduit are marked with non-dyed sutures, while the bowel segments that must be anastomosed side-to-side are marked with dyed sutures.
3.2.1.4.Bowel continuity restoration.An 8 mm enterotomy is made with electrocautery in the antimesenteric border.Each jaw of a 60 mm Endo-GIA™stapler (blue cartridge) is introduced through the enterotomies (using the stay sutures to slide the bowel segments over the blades), and aside-to-side anastomosis is performed above the mesentery of the IC(Fig.1C-1).Care must be taken to avoid the inclusion of mesentery into the stapled line.If a wider anastomosis is preferred, an additional blue load can be repeated to extend the depth of the side-to-side anastomosis.Finally, the upper portion of the anastomosis is stapled (Figs.1C-2).

Table 1 Study characteristics baseline and operative data,and complications after robot-assisted intracorporeal ileal conduit and Indiana Pouch.
All stapler charges are inserted through the 12 mm lateral assistant port,with the exception of the final one to close the upper portion of the anastomosis, which is introduced through the medial port (or from an additional port at the level of the suprapubic incision for the specimen extraction).
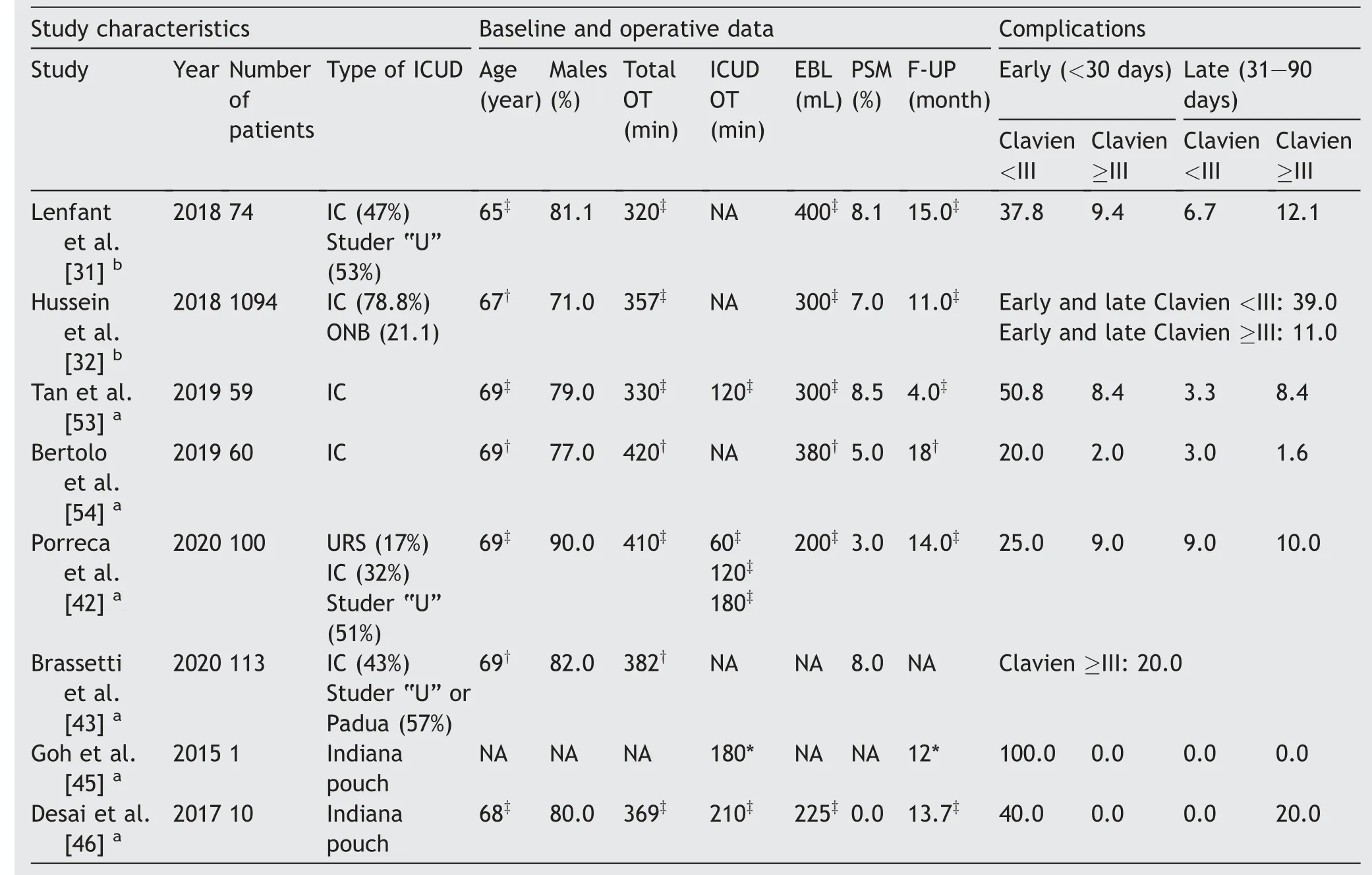
Table 1 (continued)
3.2.1.5.Uretero-ileal anastomosis and ureteric stent placement.The main objective of this step is to achieve a tension-free, watertight, well-vascularized uretero-ileal anastomosis.If there is any sign of undue tissue trauma or vascular damage to the distal ureteric end, that segment should be excised.Intravenous indocyanine green (ICG)with fluorescence imaging (i.e.Firefly™system) has been extensively described as a helpful tool to assess the vascular integrity of the ureters.
The uretero-ileal anastomosis is performed close to the proximal end of the IC.The type of anastomosis depends on surgeon’s preference.When the left ureter is too short, a transuretero-ureterostomy can be done.a.Bricker anastomosis
A small enterotomy at the proximal end of the IC is created and both ureters are spatulated 1 cm along their anterior surface.An end-to-side anastomosis is performed between the left ureter and the IC in either a continuous or interrupted fashion, starting from outside to inside the ureter at the apex of the spatulated end, and from the inside of the bowel to the outside.After completing the posterior wall of the anastomosis, a single-J stent is inserted over a wire through one of the ports or through a separate abdominal puncture: The proximal end is placed into the upper collecting system, and the distal end is placed inside the IC.Lastly, the anterior wall of the anastomosis is completed.The procedure is then repeated with the right ureter (Fig.1D-1 and Fig.1D-2).
b.Wallace anastomosis
This type of technique follows the same principles of the Bricker anastomosis.However, the uretero-ileal anastomosis is performed side-to-side.The procedure starts with side-to-side uretero-ureteral anastomosis.After the excision of the proximal staple line of the IC, a side-to-side uretero-ileal anastomosis of the right ureter is performed to create the posterior wall.Single-J stents are inserted over a wire.Finally, the anterior wall of the uretero-ileal anastomosis is completed.
3.2.1.6.Specimen removal and stoma creation.The surgical specimen is removed through a lower midline or Pfannenstiel incision.A 19 Ch Blake drain is positioned in the pelvis.
The distal end of the IC is approximated towards the anterior abdominal wall in the pre-marked site with the aid of the fourth arm.A circular incision is made on the skin, which is excised with the underlying subcutaneous fat.The fascia is now opened in a cruciate fashion and four fascial stitches of 2-0 Vicryl® are placed on each corner.The rectus muscle fibers are separated and the posterior rectus sheath is divided.The opening should allow two fingers to pass through snug.The IC is exteriorized by pulling on the previously placed stay-sutures and under robotic vision.The robotic instruments are withdrawn,and the abdomen is desufflated.The IC is anchored with the previously placed fascial sutures, and the stoma is matured (Figs.1D-3).

Figure 1 Schematic figure demonstrating the step-by-step creation of a robot-assisted ileal conduit(Adapted from Medina et al.[22]).(A)Port configuration:Four robotic and two assistant ports.(B)Identification of the bowel segment.A segment of 15-20 cm of the ileum 20-30 cm away from the ileocecal valve is identified.(C) Division of the bowel segment and bowel continuity restoration.The distal and proximal parts of the identified bowel segment are divided with a 60 mm Endo-GIA™stapler.A side-toside bowel anastomosis is performed in two steps(C-1 and C-2)above the mesentery of the IC with a 60 mm Endo-GIA™stapler to restore bowel continuity.(D) Bricker’s uretero-ileal anastomosis and stoma creation.An end-to-side anastomosis is performed between the ureters and the ileal conduit in either continuous or interrupted fashion (D-1 and D-2) [3].For the creation of the stoma (D-3), first the ileal conduit is anchored in the base by the four fascial sutures; Second, the stoma is matured.
3.2.2.Continent cutaneous urinary diversion: Robotassisted totally intracorporeal modified Indiana pouch(as described by Aron et al.[25])
3.2.2.1.Patient positioning and port placement.The robotic approach replicates the basic principles of the open counterpart [26].Patient positioning and port placement for the RC and PLND portion are the same as described previously.Before the ICIP diversion is initiated, the robot should be undocked.The patient is tilted 45to the left and a 30Trendelenburg is maintained.
A transperitoneal 7-port configuration is used.For this configuration, the previous left 8 mm and left 12 mm port sites of the robot and the assistant,respectively,are closed.A 12 mm laparoscopic port replaces the 8 mm port that was at the previous location of the third robotic arm in the right anterior axillary line.An additional 12 mm port is placed in the midline 5 cm caudal to the umbilicus,and two additional 8 mm ports are placed (one in the midline just below the xiphoid and the second one in the right midclavicular line medial to the anterior superior iliac spine) (Fig.2A).The robot is now re-docked from the right side of the patient,with two robotic arms on the left and one robotic arm on the right.
3.2.2.2.Identification of the ureters.After the extirpative portion of the procedure, the left ureter is transposed to the right side of the sigmoid colon, utilizing the same concepts as for the Bricker anastomosis.Both ureters are tagged with stay sutures, which are clipped to the parietal peritoneum of the right iliac fossa.
3.2.2.3.Identification and division of the bowel segment.The terminal ileum is divided approximately 10 cm proximal to the ileocecal valve using a 60 mm linear laparoscopic stapler with a blue load (60 mm Endo-GIA™stapler) through the assistant port in the left upper quadrant(Fig.2B).As mentioned before, it is critical to fire the stapler perpendicular to the bowel segment.An additional staple load(white vascular)may be required to deepen the mesenteric division.
The cecum and right ascending colon are now mobilized cephalad toward the transverse colon.The colon is divided approximately 25 cm distal to the ileocecal valve using a 60 mm laparoscopic stapler with a blue load through the assistant port in the midline, caudal to the umbilicus.
The proximal and distal ends of the bowel segment intended to create the pouch are marked with non-dyed sutures, while the bowel segments that will be anastomosed side-to-side are marked with dyed sutures, as previously described.
3.2.2.4.Bowel continuity restoration.Bowel continuity is restored as described previously.The 60 mm Endo-GIA™stapler(blue load)is inserted through the 12 mm port in the right anterior axillary line into the bowel lumen, ensuring that both anti-mesenteric borders are aligned using the stay sutures to slide over the blades.Care must be taken to avoid twisting the bowel segments and to avoid trapping the mesentery in the staple line.After the side-to-side portion of the anastomosis is created, a 3-0 Vicryl®seromuscular suture is placed to approximate the bowel segments at the distal limit of the staple line to prevent distraction of the bowel segments.An additional 60 mm Endo-GIA™stapler blue load is fired to close the upper opening of the anastomosis through the 12 mm assistant port located in the midline, caudal to the umbilicus.
3.2.2.5.Detubularization of the isolated colonic segment.An appendectomy is performed and a cecostomy is created through the appendiceal stump to wash out the isolated colonic segment: A 24 Ch catheter is inserted through the appendiceal stump and the segment is cleansed vigorously with a 60 mL catheter tip syringe until the return is clear.The distal staple line on the colonic segment is now excised and the bowel is opened along the antimesenteric surface, minding to preserve the cecal cap intact (Fig.2C).
3.2.2.6.Urethro-colonic anastomosis.After the posterior plate is constructed, attention is now placed on the ureteral ends previously clipped to the parietal peritoneum at the right iliac fossa.Two separate full-thickness incisions are made in the posterior wall of the ascending colon,and each ureter is brought into the lumen of the pouch through the incision.The end of each ureter is spatulated for at least 1 cm and anastomosed to the colonic wall from within the pouch,using running or interrupted 4-0 Vicryl®suture.All efforts should be made to suture the ureteral wall to the full thickness of the colonic wall to avoid any potential disruption of the ureters.The consensus is to anastomose the left ureter first and closer to the ileocecal valve followed by the right ureteral anastomosis.After completing the caudal portion of the anastomosis, a 4.8 Ch double J stent is placed through a 2 mm miniport placed in the lower right quadrant.The distal curl is placed inside the pouch, and the cephalad aspect of the anastomosis is completed.The procedure is repeated for the right ureter (Fig.2D).
3.2.2.7.Closure of the pouch.The colonic plate is now folded following an inverted U shape, and the adjacent edges sutured using a 2-0 V-Loc™suture (Fig.2E).Before the anterior wall is completely closed, a full-thickness round incision is performed at the umbilicus, and the proximal stapled end of the ileal segment is exteriorized and delivered to the skin surface using an Allis forceps.The staple line is excised, and a 12 Ch Foley catheter is inserted through the ileocecal valve into the colonic pouch and secured by inflating the balloon with 10 mL of saline.Lastly, a 24 Ch Pezzer or Malecot catheter is placed through the appendiceal stump cecostomy into the pouch, brought out through the right 12 mm anterior axillary port and secured with a 2-0 Vicryl® purse-string suture.
3.2.2.8.Tailoring of the efferent limb and stoma creation.The efferent limb is now dropped back into the peritoneal cavity with the 12 Ch Foley catheter in place(Fig.2F).A 60 mm stapler with a blue load is inserted through the umbilical incision, and the efferent limb is tapered along its antimesenteric border.The ileocecal valve is now buttressed with several interrupted 3-0 silk or Prolene® sutures.
The efferent limb is again exteriorized with an Allis forceps through the umbilical incision and the distal end sutured to the umbilical skin with 2-0 Vicryl® interrupted sutures, thus creating a catheterizable stoma.
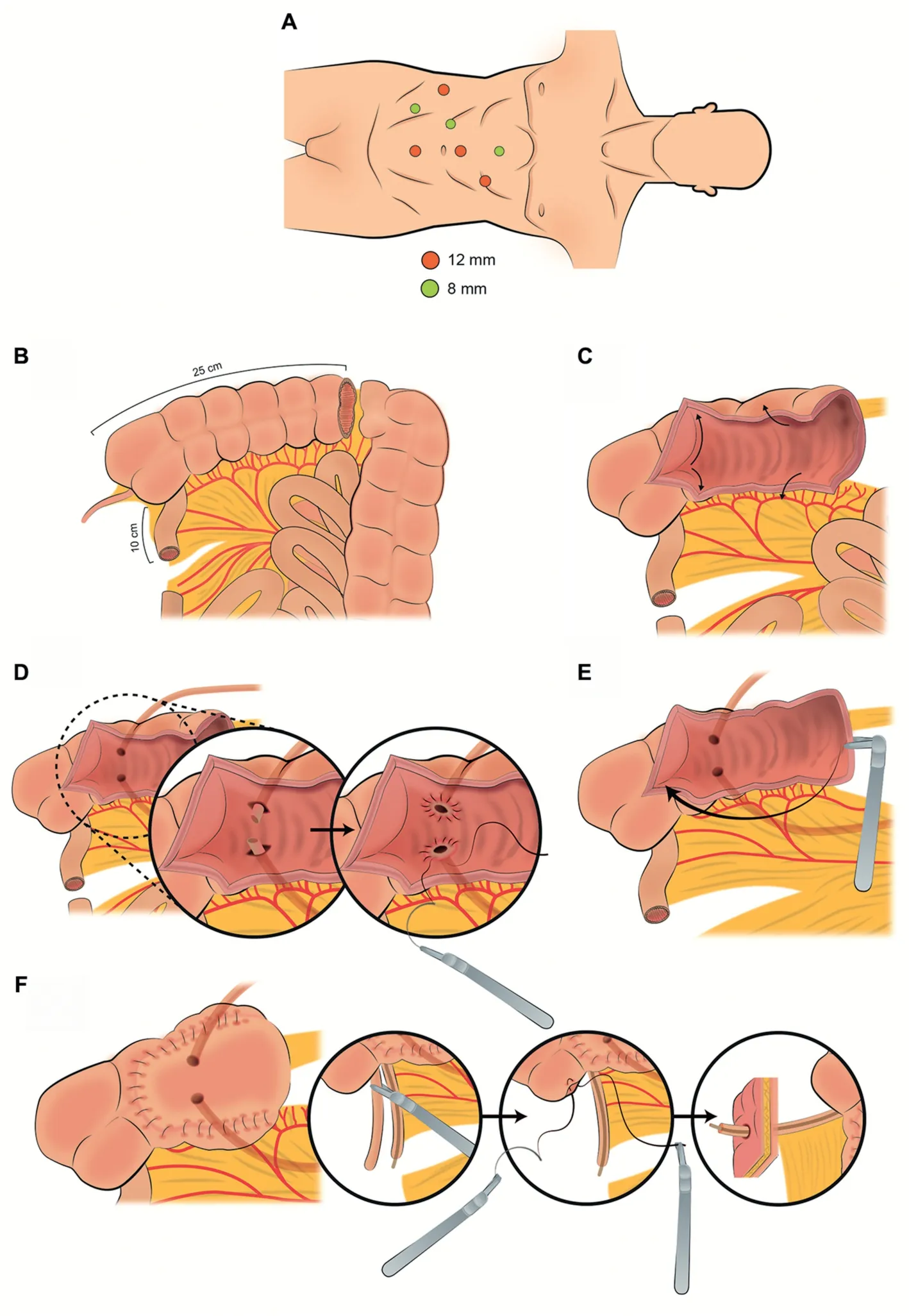
Figure 2 Schematic figure demonstrating the step-by-step creation of a robot-assisted Indiana Pouch(Adapted from Aron et al.[25]).(A)Port configuration.Seven robotic ports are used for creating a robotic Indiana pouch.(B)Identification and division of the bowel segment.A segment of 10 cm of the terminal ileum and 25 cm of the right colon are isolated and divided with a 60 mm Endo-GIA™stapler.(C) Appendectomy and detubularization of the isolated colonic segment.The cecal appendix is excised, and the colonic segment is detubularized along the antimesenteric surface, preserving the cecal cap intact.(D) Urethro-colonic anastomosis.After passing the ureters through full-thickness incisions in the posterior wall of the right colon,they are anastomosed in an end-to-side fashion.(E)Closure of the pouch.The colonic plate is folded into the shape of an inverted U,and the adjacent edges of the colon are sutured.(F)Tailoring of the efferent limb and stoma creation.The efferent limb is tapered along the antimesenteric border,and the ileocecal valve is buttressed.Finally,the efferent limb is exteriorized,and a catheterizable stoma at the umbilicus is created.
3.2.2.9.Specimen removal and drains.The surgical specimen is removed by expanding the camera port incision.When an excision of the anterior strip of the vagina is performed in female patients,the sample can be extracted alternatively through the vagina.A 19 Ch Blake drain is inserted, positioned in the pelvis, and secured to the skin.
3.3.Outcomes of robot-assisted totally intracorporeal ileal conduit
Results from all available studies (descriptive and comparative) on robot-assisted ICIC series were included.Six out of twenty-four compared performing extracorporeal with intracorporeal IC [27-32] and 5/24 compared robotassisted ICIC with totally intracorporeal ileal orthotopic ileal neobladder (ICONB) [33-39].All the others were descriptive in nature.One-third of the studies[27-32,40-43] reported intraoperative and postoperative outcomes for IC and ONB combined.
3.3.1.Baseline characteristics
In the robot-assisted ICIC group, age ranged from 60.0 to 76.1 years, and the proportion of men ranged from 55% to 100% (weighted mean 75.3%).All studies but two included females, with a proportion of females ranging from 7% to 55%.
3.3.2.Intraoperative outcomes
Among the studies reviewed, total OT (skin to skin) ranged from 292 min to 691 min, whereas ICUD OT ranged from 60 min to 133 min.One-third of the series (33%) reported an OT longer than 7 h.Total EBL(skin to skin)ranged from 200 mL to 1 117 mL,but most reported EBL rates were <500 mL(92%).The rate of PSM ranged from 0% to 14.3% (weighted mean 7%).
When focusing on the reports with larger cohorts(n>30),the maximum total OT decreased from 691 to 422 min, but the ICUD OT stayed the same.Similarly, EBL rates decreased from 1 117 mL to 550 mL.However, the rate of PSMs was comparable between smaller and larger cohort studies (weighted mean 6.8%).
3.3.3.Perioperative outcomes
3.3.3.1.Complications.Of the articles included in this review, nine (37.5%) did not include information regarding complications, did not report it as early or late, or did not report it as minor (Clavien <III) or major (Clavien ≥III).
Early minor complication rates ranged from 0% to 71.4%(weighted mean 32.3%) and early major complication rates ranged from 0%to 53.4%(weighted mean18.6%).These data were compared favorably with open IC series, in which up to 48%of patients developed early complications[44].Late minor complication rates ranged from 0% to 66% (weighted mean 14.7%)and late major complication rates ranged from 0% to 32% (weighted mean 13%).Interestingly, the rate of complications decreased in larger studies, with a weighted mean of 12.8% and 7.9% for early minor and major complications, and 5.2% and 4.8% for late minor and major complications, respectively.The main short-term complications were febrile urinary tract infections, uretero-ileal leakage, and stenosis; long-term complications were related to the stoma (stomal stenosis and parastomal hernias).
3.3.3.2.Follow-up.Follow-up duration ranged broadly amongst series, from 3.3 to 32.0 months.Fifteen out of twenty-four (62.5%) of the reviewed studies reported follow-up periods; of these, 46.6% (7/15) had a short follow-up of less than 1 year.
3.4.Results of intracorporeal versus extracorporeal ileal conduit
Whether minimally invasive approach is superior to the open approach is a matter of ongoing debate.Herein, we summarized only series comparing outcomes between intracorporeal and extracorporeal IC [27-32] (Table 2).
3.4.1.Baseline characteristics
No significant difference was found in age or male proportion amongst the studies included, except in the International Robotic Cystectomy Consortium(IRCC)study[32].The IRCC created a prospective, multi-institutional database comparing ICUD and extracorporeal urinary diversion(ECUD)and included 1 094 and 1 031 patients on each treatment arm,respectively.They found a significantly higher proportion of males in the ECUD group(71%vs.81%,p<0.001).
3.4.2.Intraoperative outcomes
When comparing OT between ICUD and ECUD, most series found significant differences between groups: Pruthi et al.[27],Kang et al.[28]and Chow et al.[30]found longer total OT in the ICUD group;conversely,the IRCC study[32]found that performing an ICUD was 47 min shorter on average than an ECUD.The ICUD OT was only reported by Kang et al.[28],and they found a significant difference in favor of the ECUD group (201 min versus 119 min (p=0.01).
EBL results are conflictive:Chow et al.[30]found higher EBL in ICUD group (300 mL vs.200 mL, p=0.001); Lenfant et al.[31] and the IRCC study [32] found lower EBL in ICUD group (400 mL vs.500 mL, p=0.04; 300 mL vs.350 mL,p<0.01, respectively); three other series did not find significant differences.PSM rates were similar in all studies.
3.4.3.Perioperative outcomes
3.4.3.1.Complications.When comparing complication rates of ICUD versus ECUD, the IRCC [32] found increased total and major complications within the ECUD group(total 58% vs.43%, p<0.001; major 13% vs.10%, p=0.02).In this working group the incidence of major complications after ICUD decreased significantly over time, whereas it remained stable for ECUD.In all other studies complication rates were comparable.
3.4.3.2.Follow-up.Only two studies reported a follow-up period.Lenfant et al.[31] found a significantly more extended follow-up period in the ECUD group (15 months versus 28 months).Conversely, the IRCC study [32] found no statistical difference in follow-up between groups (11 months vs.17 months).

Table 2 Non-randomized studies comparing intra versus extracorporeal urinary diversions.
3.5.Outcomes of robot-assisted totally intracorporeal modified Indiana pouch
Data on continent CUD following RARC are scarce and most procedures were performed extracorporeally.After an extensive review of the literature, the authors only found one case report [45] and one clinical series [46] on robotassisted ICIP.Goh et al.[45] first reported a case on a robot-assisted ICIP.OT was 180 min and no perioperative complications occurred after 12 months of follow-up(Table 1).
In a clinical series of 10 patients, Desai et al.[46] reported a median age of 68 years.Eighty percent of patients were males.Median total OT(skin to skin)was 369 min and median ICUD OT was 210 min.Median total EBL (skin to skin)was 225 mL and the rate of PSM was 0%.Early minor/major and late minor/major complication rates were 40%/0%and 0%/20%,respectively.All patients were able to selfcatheterize postoperatively.One case elected undiversion to an IC due to non-compliance with catheterization.The median follow-up period was 13.7 months.The main shortterm complications were prolonged urine leakage, alkalosis, and subcutaneous emphysema (all Clavien ≥III);long-term complications were uretero-enteric stricture(Clavien IIIb).
4.Discussion
Since the description of the first robot-assisted ICIC[23,47,48] and ICIP(45), different centers worldwide have gradually adopted this approach.However, these are technically demanding and time-consuming procedures.Thus, the number of published series worldwide has increased slowly.We were able to identify 26 studies that specifically addressed the use of robot-assisted CUD in the treatment of BC.Among these, the most frequent type of CUD was ICIC.To our knowledge, no randomized study comparing intracorporeal versus extracorporeal IC or IP has been performed.Therefore, high-quality data are lacking as studies are restricted to case reports and small clinical series.This manuscript aims to piece together the available literature, providing the reader with detailed descriptions of key technical aspects of the procedures, along with newly created consistent images.
Several factors must be considered when selecting the suitable type of UD: Physical and mental condition, age,body habitus (obesity and deformities), the extent of the disease, tumor prognosis, life expectancy, urethral involvement, cardiac, kidney and liver function, patient’s expectations, social support, surgeon’s experience and surgeon’s preference.Furthermore, distinctive bowel segments are utilized depending on the type of UD selected.As such, specific medical conditions may preclude the creation of a particular type of UD in selected patients.For example, the use of ileum is not recommended in patients with short bowel syndrome or inflammatory small bowel diseases (e.g., Chron’s disease)and in those whose ileum has received extensive radiation.Chronic kidney disease (GFR <50 mL/min/1.73 m),hepatic dysfunction, tumor infiltration of the distal prostatic urethra (in men) or the bladder neck (in women), and patients who are not candidates for a strict follow-up protocol are absolute contraindications for an orthotopic continent UD.IC is usually indicated in patients who do not qualify for a continent diversion or who are not willing to comply with the inconveniences of a continent diversion.Continent CUD could be an option when the urethra cannot be used because of previous radiation or oncologic involvement but the patient wishes a continent pouch.
While the available data on robot-assisted IP are scarce with only one case report [45] and one clinical series [46]found in the literature, data on robot-assisted IC are more abundant.Most patients included in the series are in their 7th or 8th decades of life, while 75% of the cases were performed in males.This goes in line with the increased incidence of MIBC amongst the male population.The reported total and ICUD OT ranged widely(5-10 h and 1-2 h,respectively).This wide variability is likely due to mixed levels of experience amongst surgeons included in the studies reported.Furthermore, only 2/3 of the studies reported some form of follow-up and in 50% of them this period was less than 1 year, which prevented from drawing any meaningful oncologic data.
Complications related to the uretero-ileal anastomosis were the leading cause of renal dysfunction.Several crucial tips such as avoiding excessive skeletonization of the ureters, careful transposition of the left ureter, gentle handle and wide spatulation of the ureters, and performing a tension-free watertight anastomosis may all help decrease the incidence of these undesirable outcomes.
Complications related to the stoma have been found to be the most common reason to re-operate a patient after ICUD[21].The stoma site should be marked preoperatively over the abdominal rectus muscle in a flat section of the abdomen, avoiding the beltline and abdominal folds to prevent stoma complications.It is mandatory to check the suitability of the marked site with the patient in the supine,sitting and upright positions.During the creation of the stoma, it is vital to attain adequate protrusion (i.e.,rosebud)of the conduit above the skin.In patients with BMI>30 kg/mor thick abdominal wall, a longer IC length should be used to guarantee this.The fascial opening should allow for a snug passage of two fingers while splitting the muscle, but not wider in order to prevent parastomal hernia formation.
The comparison between the performance of the IC in an intra or extracorporeal manner is not widely explored; the authors found only five studies.It stands out that none of these studies randomized the patients to the treatment group, and all of them did the analysis for the IC and the ONB together, so the conclusions must be drawn with caution.The information regarding OT and EBL offers conflicting results.One could assume that longer total OT could lead to slightly larger EBL, but that does not happen in all cases.The differences in the results could also be explained because of the difference in the surgical volume of each center.Regardless, the difference found in EBL probably lacks clinical relevance.
5.Conclusion
Robot-assisted ICIC or ICIP is feasible, yet complex and challenging procedure.The most frequent type of robotassisted CUD in the treatment of BC reported in the literature is IC.Mastering the technical aspects of these procedures is critical to achieve satisfactory outcomes and minimize unwanted complications.
Randomized studies comparing outcomes from different types of CUD (intra versus extracorporeal, or a CUD versus ICONB) are lacking.The vast majority of studies reported have been restricted to single-institution case series, with limited sample sizes.As such,to date there are not enough quality data to determine the supremacy of one technique over the other.
Author contributions
Study concept and design: Hugo Otaola-Arca, Marcelo Orvieto.
Data acquisition: Hugo Otaola-Arca.
Data analysis: Hugo Otaola-Arca.
Drafting of manuscript:Hugo Otaola-Arca,Marcelo Orvieto.Critical revision of the manuscript: Hugo Otaola-Arca,Rafael Coelho, Vipul R.Patel, Marcelo Orvieto.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
The authors express their acknowledgments to Santiago Otaola because of his invaluable support.
杂志排行
Asian Journal of Urology的其它文章
- Nerve-sparing robot-assisted radical prostatectomy: Current perspectives
- Robtic-assisted radical cystectomy:Literature review
- Robot-assisted endoscopic inguinal lymphadenectomy: A review of current outcomes
- Robot-assisted retroperitoneal lymphadenectomy: The state of art
- Robotic surgery techniques to approach benign prostatic hyperplasia disease: A comprehensive literature review and the state of art
- Robotic renal and adrenal oncologic surgery:A contemporary review
