MIF蛋白、EMT标记蛋白在肝癌组织中的表达及与临床病理特征、预后的相关性
2021-03-22梁辉
梁辉
【摘要】 目的:探究巨噬细胞移动抑制因子(MIF)、上皮间质转化(EMT)标记蛋白钙黏附蛋白E(E-cadherin)和波形纤维蛋白(Vimentin)在肝癌組织中的表达及与临床病理特征和预后的相关性。方法:选取2016年1月-2018年1月于本院就诊的45例肝癌患者,收集患者肝癌组织和癌旁组织,采用免疫组织化学法检测组织标本中MIF、E-cadherin和Vimentin的表达,对比肿瘤组织和癌旁组织中各分子表达差异,并分析其与临床病理特征和预后的关系。结果:肝癌组织中MIF的阳性率为71.11%高于癌旁正常组织的33.33%,E-cadherin阳性率为24.44%低于癌旁正常组织的62.22%,Vimentin阳性率为64.44%高于癌旁正常组织的31.11%,差异均有统计学意义(P<0.05)。肿瘤分期Ⅲ~Ⅳ期、肿瘤直径≥5 cm、有淋巴结转移和远处转移的肝癌组织中MIF和Vimentin阳性率均较高,E-cadherin阳性率均较低,差异均有统计学意义(P<0.05)。不同性别、年龄、AFP水平和Edmondson分级肝癌患者的MIF、E-cadherin和Vimentin蛋白水平比较,差异均无统计学意义(P>0.05)。MIF阳性患者中位生存期为20.01个月,95%CI为(17.11,23.82)个月,MIF阴性患者中位生存期为25.50个月,95%CI为(17.87,25.50)个月,MIF表达为阴性的肝癌患者预后更好(P=0.028)。E-cadherin阳性患者中位生存期为22.73个月,95%CI为(19.62,22.73)个月,E-cadherin阴性患者中位生存期为16.66个月,95%CI为(13.89,25.50)个月,E-cadherin表达为阳性的肝癌患者预后更好(P=0.040)。Vimentin阳性患者中位生存期为17.11个月,95%CI为(15.55,17.87)个月,Vimentin阴性患者中位生存期为23.88个月,95%CI为(19.62,23.88)个月,Vimentin表达为阴性的肝癌患者预后更好(P=0.032)。结论:MIF蛋白、EMT标记蛋白与肝癌的病情进展有关,可作为预测肝癌预后的重要生物标志物。
【关键词】 巨噬细胞移动抑制因子 上皮间质转化 钙黏附蛋白E 波形纤维蛋白 肝癌
Expressions of MIF Protein and EMT Marker Proteins in Liver Cancer Tissues and Their Correlation with Clinicopathological Characteristics and Prognosis/LIANG Hui. //Medical Innovation of China, 2021, 18(29): 00-006
[Abstract] Objective: To explore the expressions of macrophage migration inhibitory factor (MIF) and epithelial-mesenchymal transition (EMT) marker proteins E-cadherin and Vimentin in liver cancer tissues and their correlation with clinicopathological characteristics and prognosis. Method: A total of 45 patients with liver cancer admitted to our hospital from January 2016 to January 2018 were selected, and the liver cancer tissues and paracancer tissues of the patients were collected. Immunohistochemistry was used to detect the expressions of MIF, E-cadherin and Vimentin in tissue specimens, and the differences of molecular expressions in tumor tissues and paracancer tissues were compared, and their relationship with clinicopathological features and prognosis were analyzed. Result: The positive rate of MIF in liver cancer tissues was 71.11%, which was higher than 33.33% in adjacent normal tissues, the positive rate of E-cadherin in liver cancer tissues was 24.44%, which was lower than 62.22% in normal adjacent tissues, the positive rate of Vimentin in liver cancer tissues was 64.44%, which was higher than 31.11% in normal adjacent tissues (P<0.05). The positive rates of MIF and Vimentin were higher and the positive rate of E-cadherin were lower in liver cancer tissues with stage Ⅲ-Ⅳ, tumor diameter ≥5 cm, lymph node metastasis and distant metastasis, the differences were statistically significant (P<0.05). There were no significant differences in MIF, E-cadherin and Vimentin protein levels in patients with liver cancer with different genders, ages, AFP levels and Edmondson grade (P>0.05). The median survival time of MIF positive patients was 20.01 months, 95%CI (17.11, 23.82) months, while MIF negative patients had a median survival of 25.50 months, 95%CI (17.87, 25.50) months. Patients with negative MIF expression had better prognosis (P=0.028). The median survival time of E-cadherin positive patients was 22.73 months, 95%CI (19.62, 22.73) months, while E-Cadherin negative patients was 16.66 months, 95%CI (13.89, 25.50) months. Patients with positive E-cadherin expression had better prognosis (P=0.040). The median survival time of Vimentin positive patients was 17.11 months, 95%CI (15.55, 17.87) months, while Vimentin negative patients was 23.88 months 95%CI (19.62, 23.88) months. Patients with negative Vimentin expression had better prognosis (P=0.032). Conclusion: MIF protein and EMT marker proteins are related to the progression of liver cancer and can be used as important biomarkers to predict the prognosis of liver cancer.
[Key words] Macrophage migration inhibitory factor Epithelial-mesenchymal transition E-cadherin Vimentin Liver cancer
First-authors address: The Second Affiliated Hospital of Nanchang University, Nanchang 330006, China
doi:10.3969/j.issn.1674-4985.2021.29.001
肝癌是我国常见的消化道恶性肿瘤疾病之一,属于全球第六大恶性肿瘤[1]。依据起源方式的不同,可将肝癌分为原发性和继发性两大类,原发性肝癌起源于肝脏上皮细胞或间叶组织,而继发性肝癌则是由胃癌、结直肠癌等恶性肿瘤肝转移引起的[2]。肝癌的肿瘤细胞生长发育极为迅速,具有较高的侵袭性和转移能力,恶性程度高,发病率和死亡率逐年上升[3]。早期肝癌无特异性临床症状,60%~80%的患者确诊时处于晚期,错过了最佳手术治疗时间[4]。因此,寻找诊断肝癌的生物标志物以制定新的治疗策略对肝癌患者而言意义重大。有研究发现,多种分子参与了肝癌的发生发展过程,其中上皮间质转化(epithelial-mesenchymal transition,EMT)在肝癌细胞表型转化中发挥重要作用,调控肝癌细胞的转移[5]。巨噬细胞移动抑制因子(macrophage migration inhibitory factor,MIF)是调控机体内巨噬细胞活动的细胞因子,被先前的研究证实在多种慢性炎症性疾病、自身免疫性疾病和恶性肿瘤疾病中异常表达,影响疾病进展[6-7]。然而,MIF蛋白和EMT标记基因钙黏附蛋白E(E-cadherin)、波形纤维蛋白(Vimentin)在肝癌中的相关研究较少,目前尚不清楚其与临床病理特征和预后的关系。基于此,本次实验探究MIF、E-cadherin和Vimentin在肝癌组织中的表达水平,分析其与临床病理特征及预后的相关性,现报道如下。
1 资料与方法
1.1 一般资料 选取2016年1月-2018年1月来本院就诊的45例肝癌患者。纳入标准:(1)均为肝胆外科收治的需进行手术切除患者,且术后经病理学检测确诊为肝细胞癌;(2)年龄≥18岁;(3)预期生存期≥3个月;(4)依从性较好,能够配合完成术后随访。排除标准:(1)合并其他恶性肿瘤疾病;(2)术前1个月内接受过放化疗或抗肿瘤免疫治疗;(3)哺乳期或妊娠期妇女;(4)临床资料不全。男32例,女13例,年龄18~69岁,平均(46.38±11.37)岁;Edmondson分级:Ⅰ、Ⅱ级16例,Ⅲ、Ⅳ级29例;TNM分期:Ⅰ~Ⅱ期18例,Ⅲ~Ⅳ期27例。收集所有患者肿瘤组织和癌旁约3 cm处的正常组织,脱水后制成石蜡标本待用。本研究经医院医学伦理委员会批准且所有入组患者均对本次实验知情,同意参与本次研究并签署同意书。
1.2 方法 取患者肿瘤组织和癌旁正常组织石蜡切片,采用免疫组织化学法检测组织中MIF、E-cadherin和Vimentin的表达。首先将组织切片脱蜡至水,0.01 mmol/L枸橼酸缓冲液微波炉加热15 min以修复抗原,室温下冷却后以3%H2O2灭活内源性过氧化物酶,山羊血清室温下封闭30 min后,加入MIF、E-cadherin和Vimentin一抗(均购自上海艾博抗公司),4 ℃下孵育过夜,PBS洗去浮色后与辣根过氧化物酶标记的山羊抗兔二抗室温下再次孵育30 min,DBA染色液染色后冲洗,苏木素复染,梯度乙醇脱水后,二甲苯透明,中性树胶封片,生物显微镜(生产厂家:上海普赫光电科技有限公司,型号:CX43)下拍照观察颜色情况。
1.3 观察指标与判定标准 (1)免疫组化染色结果观察:参考《免疫组化结果的图像分析與人工计数方法的对比研究》对患者免疫组化阳性和阴性进行界定,具体根据免疫组化染色强度和阳性细胞占比来进行。染色结果中胞质、胞核着色为棕黄色记3分,黄色记2分,浅黄色记1分,胞质、胞核着色程度较差,无明显染色记0分。染色结果中阳性细胞占比>75%记3分,≥50%但≤75%记2分,≥25%但<50%记1分,<25%记0分。总评分=染色强度得分×阳性细胞占比得分,当总评分<3分视为阴性,≥3分视为阳性[8]。(2)收集患者临床病理信息,分析MIF、E-cadherin和Vimentin表达水平与临床病理特征之间的关系。(3)对患者进行3年的随访,记录其生存情况,并分析MIF、E-cadherin和Vimentin表达水平与患者预后之间的关系。
1.4 统计学处理 采用SPSS 22.0软件对所得数据进行统计分析,计量资料用(x±s)表示,比较采用t检验;计数资料以率(%)表示,比较采用字2检验;采用Kaplan-Meier分析患者预后情况。以P<0.05为差异有统计学意义。
2 结果
2.1 MIF和EMT标记物在肝癌组织及癌旁正常组织中的表达 肝癌组织中MIF的阳性率为71.11%高于癌旁正常组织的33.33%,E-cadherin阳性率为24.44%低于癌旁正常组织的62.22%,Vimentin阳性率为64.44%高于癌旁正常组织的31.11%,差异均有统计学意义(P<0.05)。见表1。
2.2 MIF和EMT标记物与临床病理特征的关系 肿瘤分期Ⅲ~Ⅳ期、肿瘤直径≥5 cm、有淋巴结转移和远处转移的肝癌组织中MIF和Vimentin阳性率均较高,E-cadherin阳性率均较低,差异均有统计学意义(P<0.05)。不同性别、年龄、AFP水平和Edmondson分级肝癌患者的MIF、E-cadherin和Vimentin阳性率比较,差异均无统计学意义(P>0.05)。见表2。
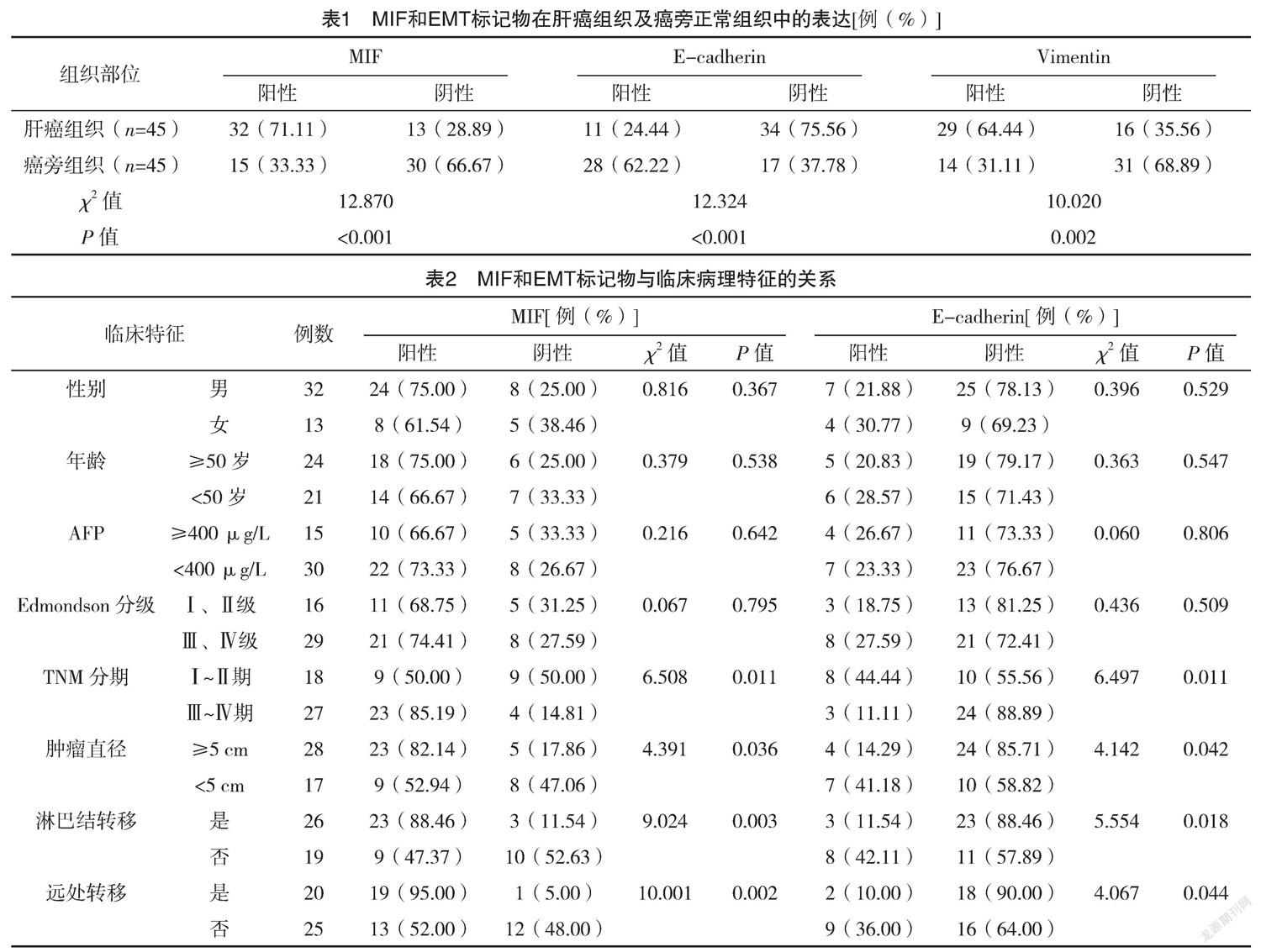
2.3 MIF表达与预后的关系 MIF阳性患者中位生存期为20.01个月,95%CI为(17.11,23.82)个月,MIF阴性患者中位生存期为25.50个月,95%CI为(17.87,25.50)个月,MIF表达为阴性的肝癌患者预后更好(P=0.028)。见图1。
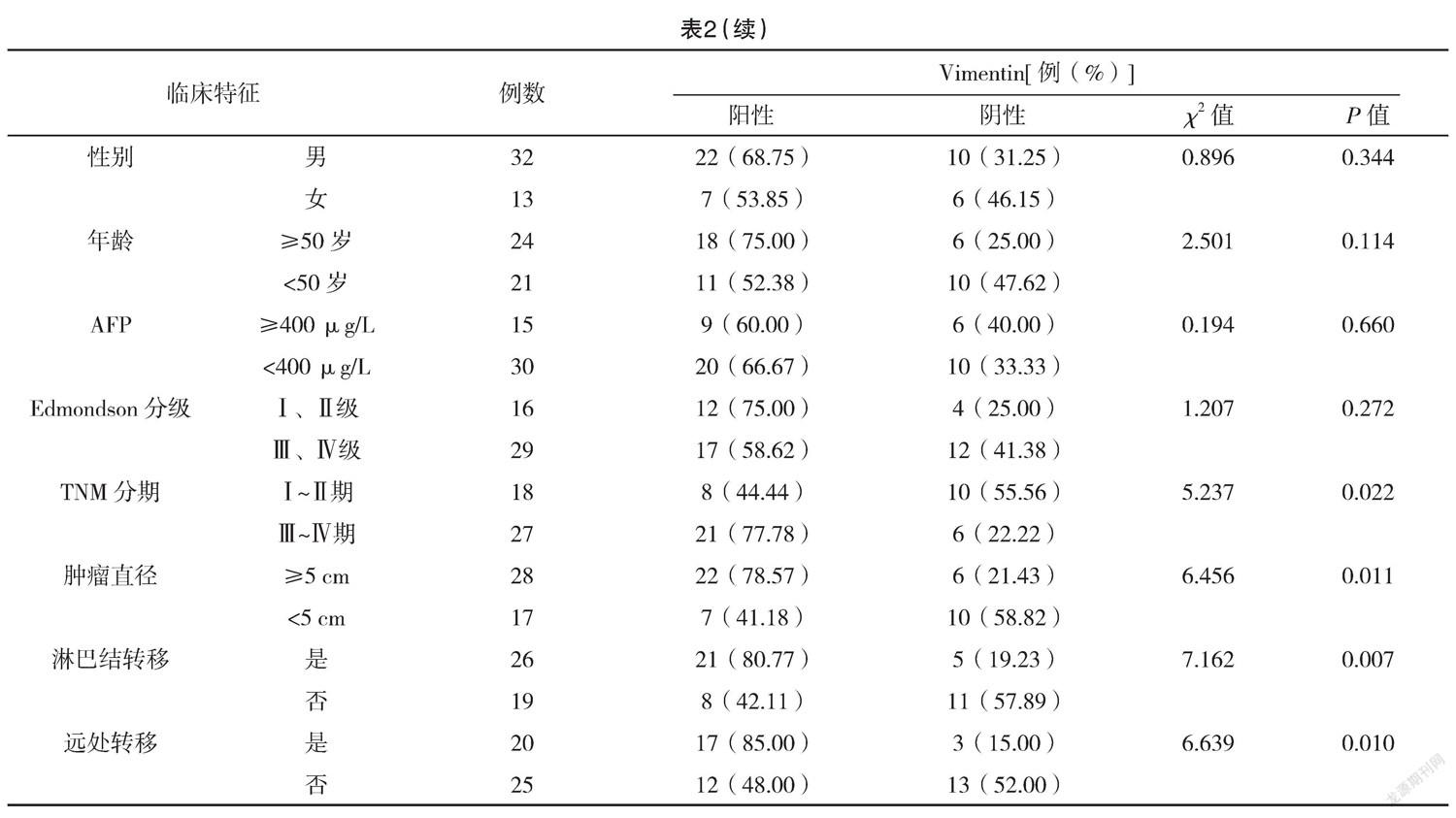
2.4 E-cadherin表达与预后的关系 E-cadherin阳性患者中位生存期为22.73个月,95%CI(19.62,22.73)个月,E-cadherin阴性患者中位生存期为16.66个月,95%CI(13.89,25.50)个月,E-cadherin表达为阳性的肝癌患者预后更好(P=0.040)。见图2。
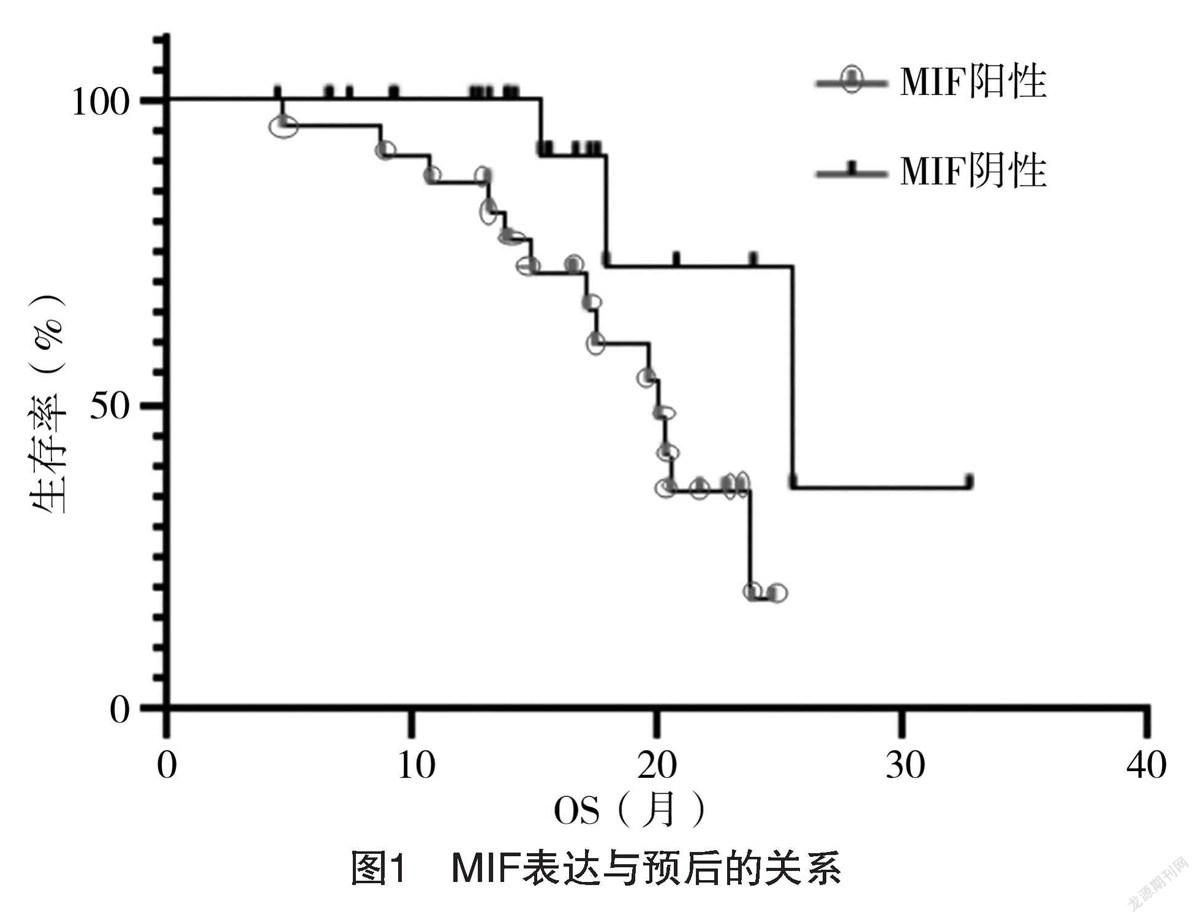
2.5 Vimentin表达与预后的关系 Vimentin阳性患者中位生存期为17.11个月,95%CI为(15.55,17.87)个月,Vimentin阴性患者中位生存期为23.88个月,95%CI为(19.62,23.88)个月,Vimentin表达为阴性的肝癌患者预后更好(P=0.032)。见图3。
3 讨论
肝癌是现阶段发病率、致死率和恶性程度极高的恶性肿瘤之一,临床表现为包膜侵犯、血管浸润和远处器官转移,手术切除、经皮穿刺肝动脉栓塞化疗等是治疗肝癌的常用方案,但因肝癌具有高侵袭性和转移性特点,治疗效果不理想[9-11]。因此,探究肝癌转移复发的相关机制,寻找新的分子标志物,揭示其与临床病理特征及预后之间的关系,对于延长肝癌患者的生命周期而言意义重大。
既往研究表明,癌症的侵袭和转移与肿瘤细胞的EMT有关,EMT标记蛋白E-cadherin、N-cadherin和Vimentin等分子的改变,导致上皮细胞失去黏附能力,由上皮细胞转化为间质细胞,并获得细胞迁移和侵袭的能力,诱导肿瘤细胞的转移[12]。T细胞分泌的多效趋化因子MIF是EMT的关键调控因子之一,并与脓毒癥、骨关节炎、急性胰腺炎等炎症性疾病的发生发展有关[13]。Cho等[14]研究发现,MIF同样可参与介导乳腺癌、肝癌等恶性肿瘤的疾病进展,影响患者预后。本次研究发现肝癌组织中MIF的阳性率为71.11%高于癌旁正常组织的33.33%,E-cadherin阳性率为24.44%低于癌旁正常组织的62.22%,Vimentin阳性率为64.44%高于癌旁正常组织的31.11%,差异均有统计学意义(P<0.05)。肿瘤分期Ⅲ~Ⅳ期、肿瘤直径≥5 cm、有淋巴结转移和远处转移的肝癌组织中MIF和Vimentin阳性率均较高,E-cadherin阳性率均较低,差异均有统计学意义(P<0.05)。不同性别、年龄、AFP水平和Edmondson分级肝癌患者的MIF、E-cadherin和Vimentin表达情况比较,差异均无统计学意义(P>0.05)。这表明,MIF和Vimentin表达的增加和E-cadherin表达的减少与肝癌患者肿瘤血管侵袭、浸润、新生血管生成、淋巴结转移和远处器官转移高度相关,推测原因,可能与MIF蛋白对EMT的调控作用有关。文献[15-17]研究指出,MIF可通过调节血管内皮生长因子的表达,促进肿瘤新生血管生成,上调N-cadherin和Vimentin的表达,下调E-cadherin的表达,激活细胞内生长因子受体信号通路的Wnt通路,影响肿瘤细胞黏附、分化、细胞骨架重塑以及外基质降解,进而诱导细胞侵袭和转移。因此,MIF和Vimentin阳性率越高,E-cadherin阳性率越低,肝癌恶性程度越高,迁移侵袭能力越强。
此外,本次研究结果还显示,MIF、E-cadherin和Vimentin的表达与患者预后有关,MIF和Vimentin阳性率越高,E-cadherin阳性率越低,肝癌患者的总生存期越短,预后越差。也这与QIN等[18]研究结果相符。肿瘤恶性程度越高,侵袭性越强,出现淋巴结转移和远处器官转移的概率越大,手术治疗无法完全切除病灶,且极易复发,严重影响患者的生存质量和生命周期[17,19]。MIF和Vimentin高表达和E-cadherin低表达与肝癌侵袭和转移能力和肿瘤进展密切相关,不利于患者预后。
综上所述,MIF蛋白、EMT标记蛋白Vimentin在肝癌组织中高表达,E-cadherin在肝癌组织中低表达,MIF、Vimentin表达上调和E-cadherin表达下调与患者肿瘤TNM分期、肿瘤直径、淋巴结转移和远处转移有关,并影响患者的疾病进展,提示MIF蛋白和EMT标记蛋白可作为预测肝癌预后的重要生物标志物。
参考文献
[1] LI L,WANG H.Heterogeneity of liver cancer and personalized therapy[J].Cancer Lett,2016,379(2):191-197.
[2] Yamashita T,Kaneko S.Liver Cancer[J].Rinsho Byori,2016,64(7):787-796.
[3] Kim B H,Park J W.Epidemiology of liver cancer in South Korea[J].Clin Mol Hepatol,2018,24(1):1-9.
[4] Shiani A,Narayanan S,Pena L,et al.The Role of Diagnosis and Treatment of Underlying Liver Disease for the Prognosis of Primary Liver Cancer[J].Cancer Control,2017,24(3):1073274817729240.
[5] YANG B,FENG X,LIU H,et al.High-metastatic cancer cells derived exosomal miR92a-3p promotes epithelial-mesenchymal transition and metastasis of low-metastatic cancer cells by regulating PTEN/Akt pathway in hepatocellular carcinoma[J].Oncogene,2020,39(42):6529-6543.
[6] WANG Q,ZHAO D,XIAN M,et al.MIF as a biomarker and therapeutic target for overcoming resistance to proteasome inhibitors in human myeloma[J].Blood,2020,136(22):2557-2573.
[7] Krammer C,Kontos C,Dewor M,et al.A MIF-Derived Cyclopeptide that Inhibits MIF Binding and Atherogenic Signaling via the Chemokine Receptor CXCR2[J].Chembiochem,2021,22(6):1012-1019.
[8]于萍,步宏,王華,等.免疫组化结果的图像分析与人工计数方法的对比研究[J].生物医学工程学杂志,2003,20(2):288-290.
[9] Craig A J,Felden J V,Villanueva A.Molecular profiling of liver cancer heterogeneity[J].Discov Med,2017,24(131):117-125.
[10] Villanueva A.Hepatocellular Carcinoma[J].N Engl J Med,2019,380(15):1450-1462.
[11] Orcutt S,Anaya D A.Liver Resection and Surgical Strategies for Management of Primary Liver Cancer[J].Cancer Control,2018,25(1):1073274817744621.
[12] Bakir B,Chiarella A M,Pitarresi J R,et al.EMT,MET,Plasticity,and Tumor Metastasis[J].Trends Cell Biol,2020,30(10):764-776.
[13] Staubli S M,Oertli D,Nebiker C A.Laboratory markers predicting severity of acute pancreatitis[J].Crit Rev Clin Lab Sci,2015,52(6):273-283.
[14] Cho E,Kim N H,Yun J S,et al.Breast Cancer Subtypes Underlying EMT-Mediated Catabolic Metabolism[J].Cells,2020,9(9):2064.
[15] Eguchi R,Wakabayashi I.HDGF enhances VEGF-dependent angiogenesis and FGF?2 is a VEGF?independent angiogenic factor in non?small cell lung cancer[J].Oncol Rep,2020,44(1):14-28.
[16] Parol M,Gzil A,Maciejewska J,et al.Clinicopathological significance of the EMT-related proteins and their interrelationships in prostate cancer.An immunohistochemical study[J/OL].PLoS One,2021,16(6):e0253112.
[17] ZHAO J,LI H,ZHAO S,et al.Epigenetic silencing of miR-144/451a cluster contributes to HCC progression via paracrine HGF/MIF-mediated TAM remodeling[J].Mol Cancer,2021,20(1):46.
[18] QIN L,QIN J,LV X,et al.MIF promoter polymorphism increases peripheral blood expression levels,contributing to increased susceptibility and poor prognosis in hepatocellular carcinoma[J].Oncol Lett,2021,22(1):549.
[19] ZHAO Y R,WANG J L,XU C,et al.HEG1 indicates poor prognosis and promotes hepatocellular carcinoma invasion,metastasis,and EMT by activating Wnt/β-catenin signaling[J].Clin Sci (Lond),2019,133(14):1645-1662.
(收稿日期:2021-09-03) (本文编辑:张明澜)
