Influence of normal to high stroke volume on congestive heart failure development after transcatheter aortic valve implantation: case series
2021-03-03TomokoTomiokaTomohiroItoRyokichiTakahasiShuheiTanaka
Tomoko Tomioka, Tomohiro Ito, Ryokichi Takahasi, Shuhei Tanaka
Department of Cardiology, South Miyagi Medical Center, Shibata, Japan
Aortic stenosis (AS) is the most common valvular disease in the older adults’population, and its prevalence will likely continue to increase with the aging society.[1]Surgical aortic valve replacement (SAVR) for elderly patients is known as a high-risk procedure.[2−4]Transcatheter aortic valve implantation (TAVI) is a reasonable alternative to SAVR for older and inoperable patients with AS, and TAVI significantly improves outcomes in elderly patients with severe AS and serious comorbidities,[5,6]TAVI achieves a 20%relative reduction in mortality compared with SAVR,[7]although complications, such as cardiovascular events, stroke, and other procedural complications, are possible. It is reported that low ejection fraction (EF < 40%) and low stroke volume index(SVI < 35 mL/m2) in patients with AS before TAVI are risk factors for a poor prognosis after TAVI.[8−10]
On the other hand, in real-world clinical practice,we sometimes encounter patients with chronic heart failure (CHF) after TAVI with mid-range or preserved EF and normal or high SVI. Thus, we investigated patients with CHF whose SVI was ≥ 35 mL/m2before TAVI.
In this study, two EF measurements based on echocardiography, i.e., Teichholz formula or Simpson’s rule,[11]were performed with case-by-case selections, and the measurements were sometimes inconsistency, resulting in sizable deviation of EF.While stroke volume (SV) was calculated based on Doppler velocimetry using the velocity-time integral of the left ventricular (LV) outflow tract, which followed normal standard distribution. Therefore,we selected the SVI as the indicator and enrolled patients with a SVI of ≥ 35 mL/m2.
Twenty-six patients who were diagnosed with severe AS at our facility (South Miyagi Medical Center, Miyagi, Japan) and who underwent TAVI from April 2014 to August 2018 were enrolled. Diagnosis of severe AS was defined as an aortic valve area (AVA) of < 1 m2and a mean pressure gradient(MPG) of > 40 mmHg according to European Society of Cardiology guidelines.[12]TAVI was performed at two collaborative facilities because our facility did not have certification to perform TAVI.We selected 25 patients with before-TAVI SVI of ≥35 mL/m2, and retrospectively analyzed these patients. Data were obtained from an electronic database at our hospital before and after TAVI, and the data of echocardiography immediately after TAVI were obtained from the referral documents from the two collaborative facilities at which patients underwent TAVI. This study approved by our institutional research ethics committee. All procedures were in accordance with the 1964 Helsinki Declaration. All patients gave informed consent, and all patients had available follow-up information after TAVI for at least one year.
We divided patients into CHF and non-CHF groups according to their history of CHF development within 1 year after TAVI. The CHF and non-CHF groups included 11 patients (42%) and 14 patients (58%), respectively, and were compared according to their background, TAVI procedure, and preoperative and postoperative cardiac function obtained from echocardiography. Statistical analyses were performed using the t-test and the χ2test. Multivariate Cox regression analyses were not possible because of the small sample size.
Patients’ baseline characteristics are shown in Table 1.Patients’ background information was not significantly different between groups. The mean age of patients was 85 ± 34 years, and patients were classified as elderly. We did not observe a significant difference between the groups in terms of comorbidities, such as cerebrocardiovascular disease, conventional cardiac risk factors,[6,13]and medications before TAVI, while laboratory data showed that brain natriuretic peptide concentration before TAVI tended to be higher in the CHF group compared with the non-CHF group (984 ± 774 vs. 743 ± 525 pg/mL,respectively; P = 0.3). Furthermore, a pressure study before TAVI using a Swan-Ganz catheter showed that precapillary wedge pressure was significantly higher in the CHF group compared with the non-CHF group (14 ± 8 vs. 9 ± 4 mmHg, respectively; P =0.03). LV end-diastolic pressure, which indicates LV overload, was also higher in the CHF group compared with the non-CHF group (22 ± 7 vs. 18 ± 2 mmHg,respectively), although this difference was non-significant (P = 0.1). These findings implicate LV pressure overload before TAVI, but this did not correlate with CHF development after TAVI.
As shown in Table 2, perioperative complications,such as moderate perivalvular leakage, annulus destruction, acute aortic regurgitation and/or mitral valve regurgitation, coronary events, and major vascular events, rarely occurred in either group.Chronically, three patients required pacemaker implantation (PMI) in the CHF group because of atrioventricular block, and one patient in the non-CHF group required PMI because of bradycardic atrial fibrillation (AF).
Echocardiography findings before TAVI are shown in Table 3A. Aortic valve area (AVA) was 0.51 ± 0.03 vs. 0.45 ± 0.02 cm2/m2(P = 0.2), MPG was 56 ± 21 vs. 50 ± 18 mmHg (P = 0.2), and peak aortic velocity was 5.1 ± 0.3 m/s vs. 4.8 ± 0.2 m/s (P =0.3) in the CHF and non-CHF groups, respectively.Thus, there were no significant differences between the groups in terms of AS severity. On the contrary,LVEF and SVI were significantly higher in the CHF group compared with the non-CHF group (LVEF:69% ± 8% vs. 56% ± 4%, respectively, P = 0.05; SVI:61 ± 11 mL/m2vs. 49 ± 14 mL/m2, respectively, P =0.03). The prevalence of LV hypertrophy, left atrial dimension index, early (E) to late (A) ventricular filling velocity ratio (E/A), deceleration time(DecT), tricuspid valve regurgitation pressure gradient (TRPG), and inferior vena cava (IVC) diameter, which indicate LV overload, were nearly at the upper limit in the CHF group, although no statistically significant difference was observed when compared with the non-CHF group.
As shown in Table 3B, with echocardiography immediately after TAVI, AVA was appropriate with the selected prosthesis size and was not significantly different between the two groups (1.34 ±0.55 cm2vs. 1.69 ± 0.13 cm2, respectively; P = 0.1).However, the Ratio of AVAI before and after TAVI(Ratio of AVAI) was higher in the CHF group compared with the non-CHF group (0.42 ± 0.18 vs. 0.30 ±0.10, respectively; P = 0.03), which suggests that TAVI was less effective in the CHF group compared with the non-CHF group. PV and MPG were higher in the CHF group compared with the non-CHF group (PV: 2.7 ± 0.3 vs. 2.1 ± 0.5 m/s, respectively; P = 0.02; MPG: 30 ± 8 vs. 19 ± 9 mmHg, respectively; P = 0.02). Furthermore, indicators of LV overload were significantly higher in the CHF group compared with the non-CHF group (E/e’:16.1 ± 3.4 vs. 12.3 ± 3.2, respectively, P = 0.02; E/A:0.98 ± 0.27 vs. 0.60 ± 0.13, respectively, P = 0.001). In addition, TRPG was higher (32 ± 5 vs. 24 ± 8 mmHg,respectively) and the IVC was more expanded (19 ±5 mm vs. 13 ± 4 mm, respectively; P = 0.05) in the CHF group compared with the non-CHF group after TAVI.
These results highlight an imbalance between AVAI after TAVI and LV intracardiac pressure, resulting in LV pressure overload. It is possible that aortic valve resistance (AVR)[14]is not sufficiently mitigated even after TAVI; thus, CHF developed in the chronic phase. It is reasonable to suggest that AS patients with a high SVI are at a higher risk of CHF development, because AVR may increase in parallel with SVI. In fact, after-TAVI MPG, which is a substitute for AVR, and before-TAVI SVI were positively correlated in this study (data not shown).
This speculated story is similar to prosthesis-patient mismatch (PPM), which is a potentially modifiable risk factor leading to cardiac events.[15]It is re-ported that an effective orifice area index (EOAI) of <0.64 cm2/m2suggests severe PPM, and an EOAI of 0.65−0.85 cm2/m2suggests moderate PPM.[15−16]In our study, EOAI (i.e., AVAI) after TAVI was > 0.85 cm2/m2in both groups, and AVAI after TAVI was comparable between groups (Table 3B). However,the ratio of AVAI in the CHF group was statistically higher than that in the non-CHF group (Table 3B).This result showed that AVA expansion was not sufficient in the CHF group even though obtained AVA were usually agreement after TAVI, resulting in leaving the patients in the CHF group in similar status to PPM, which consequently induced development of decompensated CHF. Additionally, interquartile calculation between the Ratio of AVAI and before-TAVI SVI demonstrated a positive correlation (data not shown), which implies a relationship between PPM and high SVI.
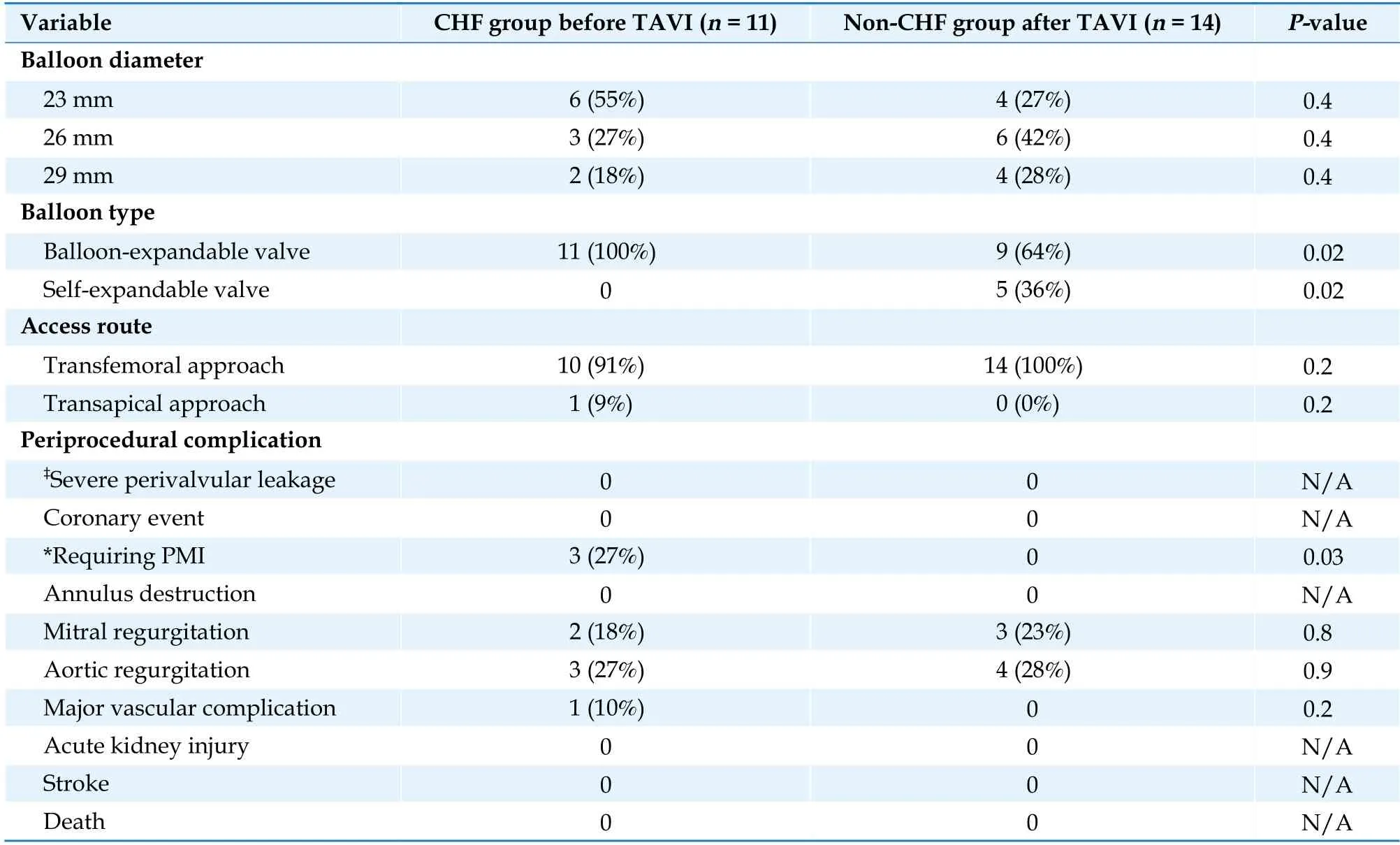
Table 2 Procedural characteristics and periprocedural outcomes in patients in the CHF and non-CHF groups after TAVI.
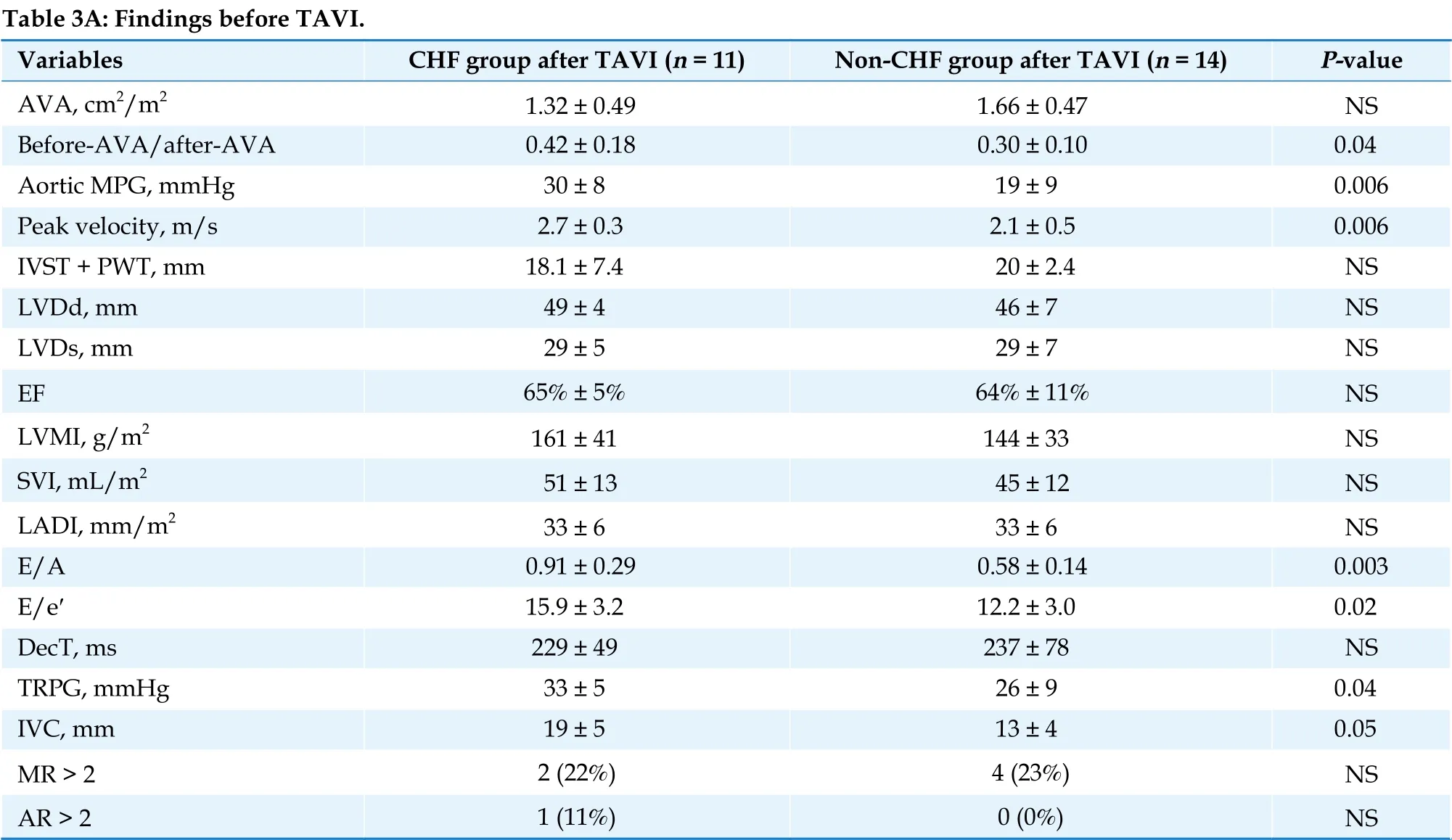
Table 3 Comparison of echocardiographic findings between the CHF group and the non-CHF after TAVI.
These results suggest that obtaining a large enough AVA both anatomically and physiologically using TAVI is desirable, especially for patients with a hyperdynamic heart[17]; that is, a high SVI, although this may be technically difficult in patients with a small body surface area and/or aortic annulus calcification.
This study included a small sample size and possibly contained selection and/or information bias.Even so, we should identify the relationship between low SV and normal to high SV to expand our perspective in the future. This understanding would lead to improvements in clinical practice for patients undergoing TAVI.
We conclude that patients with a normal to high SVI before TAVI are prone to decompensated CHF after TAVI.
ACKNOWLEDGMENTS
The authors thank the medical staff at our center for their cooperation in this study. We thank Emily Woodhouse, PhD, from Edanz Group (https://enauthor-services.edanz.com/ac) for editing a draft of this manuscript. This research did not receive any specific grant from funding agencies in the public,commercial, or not-for-profit sectors.
杂志排行
Journal of Geriatric Cardiology的其它文章
- Journal of Geriatric Cardiology
- Potassium variability during hospitalization and outcomes after discharge in patients with acute myocardial infarction
- Beta-blocker therapy in elderly patients with renal dysfunction and heart failure
- Obstructive sleep apnea increases heart rhythm disorders and worsens subsequent outcomes in elderly patients with subacute myocardial infarction
- Sex modification of the association of the radial augmentation index and incident hypertension in a Chinese communitybased population
- Neurohumoral, cardiac and inflammatory markers in the evaluation of heart failure severity and progression
