Obstructive sleep apnea increases heart rhythm disorders and worsens subsequent outcomes in elderly patients with subacute myocardial infarction
2021-03-03LingJieWANGLiNaPANRenYuYANWeiWeiQUANZhiHongXU
Ling-Jie WANG, Li-Na PAN, Ren-Yu YAN, Wei-Wei QUAN✉, Zhi-Hong XU,✉
1. Department of Cardiology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China; 2. Department of Geriatrics, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China
ABSTRACT OBJECTIVE Obstructive sleep apnea (OSA) is a potential cardiovascular risk. We aimed to investigate the association of OSA with heart rhythm disorders and prognosis in elderly patients with new-onset acute myocardial infarction (AMI).METHODS We prospectively enrolled 252 AMI elderly patients (mean age, 68.5 ± 6.9 years) who were undergoing revascularization and completed a sleep study during their hospitalization. All subjects were categorized into non-OSA (apnea-hypopnea index (AHI) < 15, n = 130) and OSA (AHI ≥ 15, n = 122) groups based on the AHI. The changes in the autonomic nervous system,incidence of arrhythmia during nocturnal sleep, and major adverse cardiovascular and cerebrovascular events (MACCEs) were compared between the groups.RESULTS The mean AHI value in all AMI patients was 22.8 ± 10.9. OSA patients showed higher levels of body mass index and peak high-sensitivity C-reactive protein and lower levels of minimum nocturnal oxygen saturation (MinSaO2), as well as greater proportion of multivessel coronary artery disease (all P < 0.05). The OSA group also showed significant increases in heart rate variability and heart rate turbulence onset (both P < 0.05) and higher incidence of arrhythmia (including sinus, atrial, and ventricular in origin). At a median follow-up of 6 months (mean 0.8-1.6 years), OSA (AHI ≥ 15) combined with hypoxia (MinSaO2 ≤80%) was independently associated with the incidence of MACCEs (hazard ratio [HR]: 4.536; 95% confidence interval [CI]:1.461−14.084, P = 0.009) after adjusting for traditional risk factors.CONCLUSIONS OSA and OSA-induced hypoxia may correlate with the severity of myocardial infarction, increase the occurrence of heart rhythm disorders in elderly subacute MI patients, and worsen their short-term poor outcomes.
Obstructive sleep apnea (OSA) is an increasingly prevalent sleep disorder. A body of evidence has shown that OSA may increase the risk of hypertension,[1]ischemic heart disease,[2]heart failure,[3]cardiac arrhythmia,[4,5]and mortality for cardiovascular diseases.[6,7]Although the relationship between OSA and longterm cardiovascular disease is well established, the association between OSA and cardiovascular outcomes following acute coronary syndrome is controversial.[6-9]Recent studies have observed a potential protective effect of OSA in cases of myocardial infarction (MI), which is attributed to chronic intermittent hypoxia resulting in ischemic preconditioning of the myocardium.[10−12]Advanced old age was related to high prevalence of OSA, as well as poor in-hospital prognosis and recurrence of acute MI(AMI) in China.[13,14]However, the relationship between OSA and AMI remains unclear in elderly patients. Our study aimed to investigate the link of OSA with autonomic nervous system function changes and occurrence of heart rhythm disorders in elderly subacute MI patients, as well as the major adverse cardiac and cerebrovascular events(MACCEs) during a 6-month follow-up.
METHODS
Patients Characteristics
We prospectively enrolled 252 consecutive elderly patients (≥ 60 years, mean age: 68.5 ± 6.9 years)admitted because of AMI to the Critical Cardiac Care Unit in Ruijin hospital affiliated with Shanghai Jiaotong University School of Medicine (Shanghai,China) between March 2018 and March 2020. The diagnoses of AMI were made according to the Fourth Universal Definition of Myocardial Infarction,[15]including ST-elevation myocardial infarction (STEMI) and non-STEMI (NSTEMI).
The exclusion criteria were as follows: patients who (1) had undergone treatment for OSA; (2) had moderate or severe pulmonary diseases such as chronic obstructive pulmonary disease, bronchiectasis, or pulmonary embolism; (3) used medications affecting mental health and the nervous system within the last three months; (4) had severe maxillofacial deformities.
Basic data such as blood biochemical indicators,inflammatory markers, and echocardiography of the patients were obtained. The study was approved by the Institutional Research Ethics Committee of Ruijin hospital affiliated with Shanghai Jiaotong University School of Medicine, and written informed consent was obtained from each patient.
Clinical Procedure and Data Collection
Baseline characteristic data, including of sex, age,body mass index (BMI), smoking, history of hypertension, diabetes mellitus, ischemic stroke, renal dysfunction, and snoring, were collected. Each patient was closely managed in our cardiac intensive care unit according to the European Society of Cardiology guidelines on STEMI (2017) or NSTEMI(2015).[16,17]All patients underwent regular laboratory tests on the 2nd day and echocardiography within 3 days after admission. The following data were collected: serum or plasma levels of glucose and lipids, liver and renal function, electrolytes and peak values of high-sensitivity C-reactive protein(hs-CRP), N-terminal of the prohormone brain natriuretic peptide (NT-proBNP), and biomarkers of myocardial injury (including creatine kinase-MB[CK-MB] and troponin I).
Overnight Sleep Study
Every patient underwent an overnight sleep study within 7 days after MI (from 21: 00 to 7: 00 the next day). The sleep study was performed using portable type III sleep monitors (Philips-Respironics Stardust II Sleep Recorder, Bend, OR, USA). Patients refrained from caffeine, sedative, or hypnotic drug intake 1 day before their sleep study. The diagnoses of OSA were made according to the Guideline of the American College of Physicians (2013).[18]Oral and nasal airflow and blood oxygen saturation from nocturnal pulse oximetry were traced. The apnea-hypopnea index (AHI, number of apnea or hypopnea events per hour during sleep) was measured as the main parameter. Data obtained from monitoring were subjected to automatic computer analysis followed by manual correction. Based on the AHI values, the patients were categorized into two groups: the non-OSA group (AHI < 15/h) and OSA (AHI ≥ 15/h) group.
Heart Rate Variability (HRV) and Heart Rate Turbulence (HRT) Analysis
Twenty-four-hour (from 8:00 to 8:00 the next day)electrocardiographic (ECG) recordings were acquired with the Labtech EC-12H-channel Holter ECG system and analyzed with the Cardiospy analysis software (Labtech Kft., Debrecen, Hungary).Holter monitoring and the sleep study were carried out in each patient on the same day. The examinations were analyzed by one physician who had no insight into the patient’s clinical data and the results of sleep study.
The HRV and HRT were automatically calculated by the Labtech system. To properly prepare the ECG for subsequent analysis, the editing of the automatic record was verified visually. The HRV was assessed in frequency parameters and consisted of the following: (1) very-low-frequency power (VLF, frequency band: 0.003−0.04 Hz),serving as an indicator of sympathetic neural activity; (2) low-frequency power (LF, frequency band:0.04−0.15 Hz), reflecting sympathetic dominance,with a larger amplitude indicating higher sympathetic neural tension; (3) high-frequency power (HF,frequency band: 0.15−0.4 Hz), being related to vagal neural activity; (4) the LF/HF ratio, as a measure of the sympatho-vagal balance. HRT indicators included the turbulence onset (TO) and turbulence slope (TS). TO was defined as the percentage difference between the heart rate immediately following premature ventricular complex and the heart rate immediately preceding premature ventricular complex, while the TS was defined as the steepest slope of the linear regression line for each sequence of five consecutive normal intervals in the local tachogram. HRT analysis was considered normal when TO < 0 and TS > 2.5 ms/RR interval.
Regarding arrythmias, sinus bradycardia and tachycardia were defined as the average heart rate being lower than 60 beats/min and higher than 100 beats/min in 24 h, respectively. Atrial arrythmias included premature atrial contractions, atrial tachycardia, and atrial fibrillation or atrial flutter.Ventricular arrythmias included multifocal premature ventricular contractions and ventricular tachycardias. Atrioventricular block (AVB) included all three degrees of AVB.
Major Adverse Cardiac and Cerebrovascular Events (MACCE) and Follow-up
All patients were monitored and followed up for a minimum of 6 months. MI outpatient clinic visits were scheduled for all patients at 1, 3, 6, and 12 months. MACCEs were defined as composite events of cardiovascular death, MI, stroke, ischemiadriven revascularization, or hospitalization for unstable angina or heart failure.
Statistical Methods
Statistical analyses were performed with SPSS 22.0 (IBM Corp., Armonk, NY, USA). A P-value below 0.05 was considered statistically significant.Continuous variables are shown as means ± SD or medians (interquartile range) and categorical variables as counts and proportions (%). The variable distribution was verified using Lilliefors and WShapiro-Wilk tests. Given that the quantitative variables were not normally distributed, the Mann-Whitney U test was used for further analyses. Univariate analysis was used to determine the clinical parameters related to OSA. Outcomes of MACCEs were estimated using logistic regression for binary outcomes. Potential risk factors, including age, sex,BMI, smoking, comorbid conditions, Syntax score,left ventricular fraction, serum glucose, low-density lipoprotein (LDL)-C, peak levels of hsCRP, biomarkers of myocardial ischemia and NT-proBNP as well as OSA (AHI ≥ 15) and hypoxia (minimum nocturnal oxygen saturation (MinSaO2) ≤ 80%),were adjusted in the multivariable analysis. Adjusted hazard ratios (HR) are reported together with their 95% confidence intervals (CI). Kaplan-Meier MACCE-free survival analysis was performed using the log-rank (Manel-Cox) chi-square test.
RESULTS
Clinical Characteristics
The baseline clinical characteristics of the two OSA groups are presented in Table 1. Among the 252 patients, 130 patients had no OSA (mean age:68.9 ± 8.0 years, male/female = 85/45) and 122 had OSA (mean age: 68.4 ± 6.7 years, male/female =106/16), with AHI values of 8.6 ± 4.7 and 34.2 ± 13.6,respectively (P < 0.001). There was no significant difference in age, sex, history of hypertension, diabetes or renal dysfunction, cardiac function, and peak levels of myocardial injury biomarkers or NTproBNP between the two groups.
However, the OSA group has significantly higher BMI (25.8 ± 3.6 vs. 24.1 ± 3.1 kg/m2), peak hs-CRP levels (52.81 ± 27.0 vs. 23.99 ± 10.91 mg/L),lower MinSaO2(79.7 ± 8.7% vs. 86.0 ± 4.5%), and a higher proportion of hypoxia with MinSaO2≤ 80%(41.8% vs. 4.3%) compared to the non-OSA group(all P < 0.05). Although no difference was found in the percentage of STEMI or Killip Class between groups, OSA appeared to be associated with the severity of coronary disease, as more OSA patients had multivessel coronary artery disease and were prescribed beta-blockers (31.5% vs. 24.5% and 93.1%vs. 84.3%, both P < 0.05).
HRV and HRT with OSA
Compared to the non-OSA group, the OSA group showed significant increases in heart rate variabil-ity, including significantly higher VLF (12.7 ± 3.0vs.9.2 ± 2.5), LF (20.7 ± 7.5vs. 13.4 ± 8.5), LF/HF ratio(1.8 ± 0.7vs. 1.4 ± 0.5), and TS (0.04 ± 0.01vs. −0.04 ±0.01, allP< 0.05). Furthermore, the HF, (9.0 ± 3.5vs.12.2 ± 4.7) and TO (4.2 ± 1.5vs. 7.6 ± 3.1) were significantly decreased in the OSA group (all P<0.05, the details are shown in Table 2).

Table 1 Comparison of baseline characteristics between non-OSA and OSA group.
Comparison of the Incidence of Arrhythmia Between Two Groups
The incidence of arrhythmia is shown in Figure 1.In summary, the OSA group showed almost twofold higher incidences of sinus bradycardias, sinus tachycardias, AVB, and ventricular arrhythmia thanthe non-OSA group (allP< 0.05). In addition, the occurrence of atrial arrythmias was almost threefold higher in the OSA than in the non-OSA group(44.4%vs. 16.7%,P< 0.05).

Table 2 Comparison of the HRV and HRT data between the two groups.
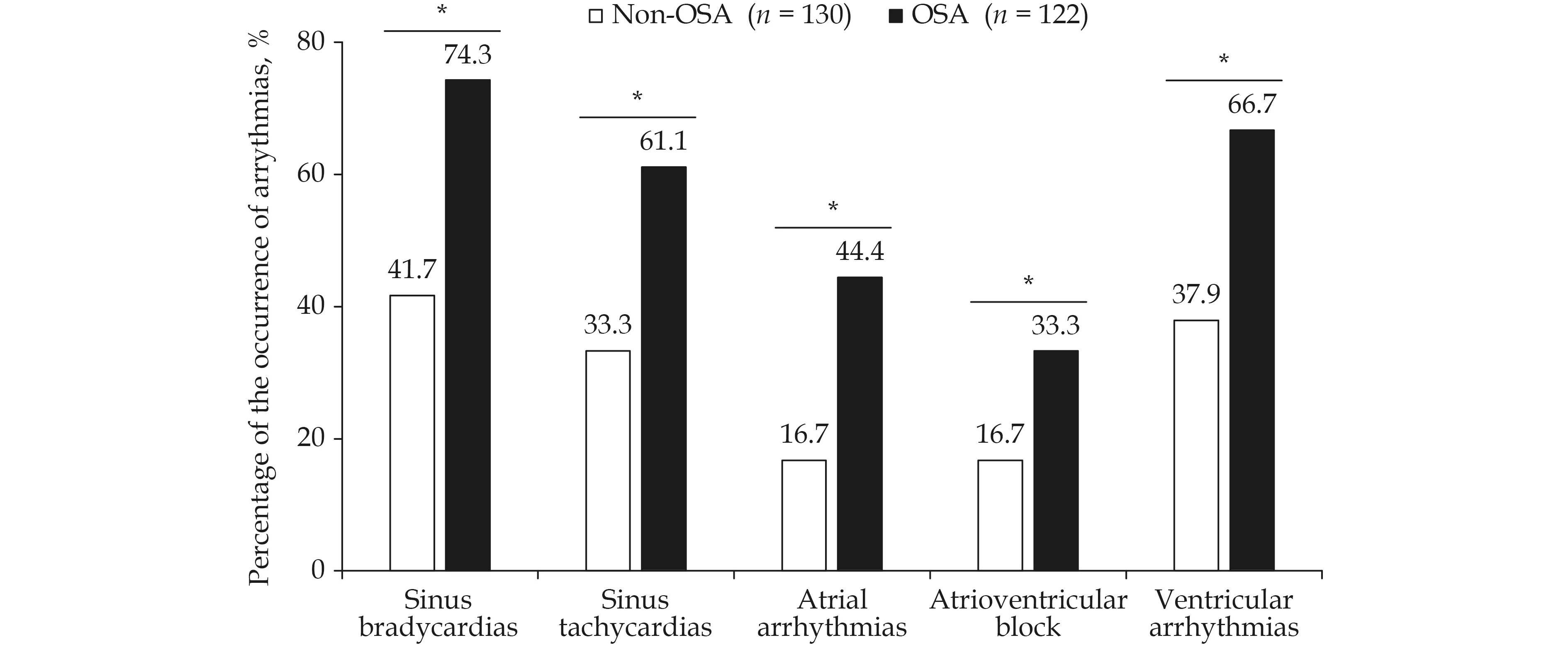
Figure 1 The difference of the proportions of arrythmias in subacute MI patients between non-OSA and OSA groups. *P < 0.05,different between OSA and Non-OSA groups. MI: Myocardial infarction; OSA: obstructive sleep apnea.
The Follow-up Prognosis and OSA-induced Hypoxia
The median follow-up time was 0.8 (0.6−1.8)years. There was a significant difference in the occurrence of MACCEs during the follow-up between the two groups (P< 0.01). In the OSA group, MACCEs occurred in 24 patients (19.7%), including 2 cardiac death (1.6%), 2 non-fatal myocardial infarction(1.6%), 2 heart failure (1.6%), 10 re-hospitalizations because of unstable angina (8.2%), 5 revascularization (4.1%), and 3 non-fatal stroke (2.5%). In the non-OSA group, MACCEs occurred in 14 patients(10.7%), including 1 cardiac death (0.8%), 1 nonfatal myocardial infarction (0.8%), 2 heart failure(1.5%), 6 re-hospitalizations because of unstable angina (4.6%), 3 re-vascularization (2.3%), and 1 non-fatal stroke (0.8%).
Multivariate Logistic Regression Analysis
Univariate analysis showed that OSA severity was correlated with age, BMI, and MinSaO2. Multivariate logistic regression analysis showed that MACCEs were independently correlated with heart failure, Syntax score, CK-MB peak levels after adjusting for age, BMI, sex, smoking, chronic kidney diseases, hypertension, diabetes, and LDL levels;furthermore, OSA (AHI ≥ 15) combined with OSAinduced hypoxia (MinSaO2≤ 80%) was independently associated with the incidence of MACCEs(HR: 4.536; 95% CI: 1.461-14.084,P= 0.009) after adjusting for heart failure, Syntax score and CK-MB peak levels (Table 3). Kaplan-Meier survival analysis showed that the MACCE-free survival in pa-tients with both AHI ≥ 15 and MinSaO2≤ 80% was significantly poorer than that in the comparative patients with AHI < 15 or MinSaO2> 80% from the 3rd month during the follow-up (Figure 2, log-rank chi-square test,P= 0.011).

Table 3 Multiple logistic regression analysis of risk factors for 6-Month MACCEs in AMI patients.
DISCUSSION
Our prospective study showed that OSA and its associated hypoxia may lead to instability of autonomic nervous system function, thus triggering the incidence of ischemia-related arrhythmia or heart rhythm disorders in elderly subacute MI patients and further worsening their poor prognosis.
OSA has been well associated with increased risk of MI, arrythmia, sudden cardiac death, and longterm outcomes of cardiovascular diseases.[3,4,6,7,10,19]However, there are limited data on the association between OSA and subacute MI in the elderly. Previous study showed that OSA and myocardial ischemia may interact as both cause and effect. The prevalence of OSA (AHI ≥ 10/h) was two to threefold higher in patients with a history of MI, and patients with nocturnal onset of MI have a six-fold higher likelihood of having OSA than those with diurnal MI.[20,21]Conversely, OSA was associated with the development and instability of atherosclerotic plaques and thus may act as a trigger for MI.[22]In our study, we found that approximately 48% of the elderly subacute MI patients were diagnosed with OSA with AHI ≥ 15. Besides the traditional risk factors for OSA such as higher BMI and hs-CRP level, a greater proportion of OSA patients had multivessel coronary artery disease, which supports the hypothesis that OSA may be significantly associated with the severity of myocardial infarction.

Figure 2 The Kaplan-Meier survival curve of OSA (AHI ≥ 15) combined with hypoxia (MinSaO2 ≤ 80%) on MACCE-free survival in elderly subacute myocardial infarction patients during the 6-month follow-up. AHI: apnea-hypopnea index; MACCEs: major adverse cardiac and cerebrovascular events; MinSaO2: minimum nocturnal oxygen saturation; OSA: obstructive sleep apnea.
OSA-induced hypoxia can lead to nocturnal dysautonomia, characterized by sympatho-adrenal over-activation. Both HRV and HRT are important prognostic indicators for the activity of the autonomic system in cardiovascular diseases, especially in myocardial infarction,[23,24]while reduced HRT has been proven to be a relatively accurate indicator of autonomic system damage in severe OSA,sudden death, and the prognosis after myocardial infarction.[25,26]Our study found that the OSA group showed significant increases in HRV frequency-domain metrics (including VLF, LF, and the LF/HF ratio) and TO, as well as significant decreases in HF and TS compared with the non-OSA group. Besides, the incidences of sinus, atrial, and ventricular arrhythmia in the OSA group were also significantly increased compared with those in the non-OSA group. These data further support the relationship of OSA with imbalanced autonomic nervous system function and heart rhythm disorders in elderly AMI patients. The detailed mechanisms may be explained by hypoxia-induced inflammatory response, oxidative stress, and hypercoagulability, as well as increased expression of ventricular calcium channels.[27,28]
The link between OSA and cardiovascular events remains controversial. Recent studies have observed a potential protective effect of OSA in cases of myocardial infarction, and the prescription of continuous positive airway pressure (CPAP) compared with usual care did not result in a positive effect on the incidence of cardiovascular events, which is attributed to chronic intermittent hypoxia resulting in ischemic preconditioning of the myocardium.[10−12,29]However, the issue remains controversial. Researchers have attempted to determine prognostic parameters of OSA for cardiovascular outcomes and showed that AHI ≥ 15, MinSaO2≤ 85%, Epworth Sleepiness Scale scores ≥ 11 and the presence of excessive daytime sleepiness were all independent risk factors of MACCEs during a follow-up of 3-5 years.[30−32]Similarly, our present study demonstrated that OSA (AHI ≥ 15) together with hypoxia(MinSaO2≤ 80%) was independently associated with the incidence of MACCEs during the 6-month follow up (HR = 4.53), and patients with both OSA and severe hypoxia showed poorer MACCE-free survival than those without OSA or hypoxia. Our results further support the hypothesis that OSA-associated apnea can aggravate the severity of myocardial ischemia in elderly subacute MI patients and thus produce adverse effects on the middleterm outcomes during the follow-up.
A prospective design and a rather homogeneous group of elderly people are the strengths of our study. However, there are some limitations in our study. First, the sleep studies were performed by using type III portable sleep monitors, which may underestimate the AHI values compared with the gold standard of polysomnography. Second, identifying the prevalence of OSA in the AMI patient population was not a goal of this study, as we only recruit stable subacute MI patients who can tolerate the sleep study, which may conceal the real influence of OSA on more severe patients complicated with heart failure, severe kidney diseases, or infection. Third, the sample size of our prospective, crosssectional study conducted at a single medical center was relatively small. Large-scale multicenter double-blind prospective clinical studies may be required for further confirmation of the influence of OSA on AMI patients.
In conclusion, OSA-induced hypoxia may correlate with the severity of myocardial infarction, increase the occurrence of heart rhythm disorders in elderly subacute MI patients, and worsen their poor outcomes in the short term.
ACKNOWLEDGEMENTS
The authors are grateful to Dr. Feng-Ru ZHANG and Dr. Jia-An HU for their skilled technical assistance. This work was supported by National Natural Science Youth Fund of China (81100098), Shanghai Municipal Commission of Health and Family Planning for Key Discipline Establishment (2015ZB0503& 201840083), and Production, Teaching and Research Program for University Teachers in Shanghai (RC20190079). The authors declare that they have no conflict of interest.
杂志排行
Journal of Geriatric Cardiology的其它文章
- Journal of Geriatric Cardiology
- Potassium variability during hospitalization and outcomes after discharge in patients with acute myocardial infarction
- Beta-blocker therapy in elderly patients with renal dysfunction and heart failure
- Sex modification of the association of the radial augmentation index and incident hypertension in a Chinese communitybased population
- Neurohumoral, cardiac and inflammatory markers in the evaluation of heart failure severity and progression
- Patent foramen ovale closure in non-lacunar cryptogenic ischemic stroke: where are we now?
