Potassium variability during hospitalization and outcomes after discharge in patients with acute myocardial infarction
2021-03-03XiLingZHANGHengXuanCAIShanJieWANGXiaoYuanZHANGXinRanHAOShaoHongFANGXueQinGAOBoYU
Xi-Ling ZHANG, Heng-Xuan CAI, Shan-Jie WANG, Xiao-Yuan ZHANG,Xin-Ran HAO, Shao-Hong FANG, Xue-Qin GAO,✉, Bo YU,✉
1. Department of Cardiology, Second Affiliated Hospital of Harbin Medical University, Harbin, China; 2. The Key Laboratory of Myocardial Ischemia, Chinese Ministry of Education, 246 Xuefu Road, Nangang District, Harbin, China;3. Department of Neurobiology, Neurobiology Key Laboratory, Harbin Medical University, Education Department of Heilongjiang Province, Harbin, China
ABSTRACT BACKGROUND The variability of metabolic biomarkers has been determined to provide incremental prognosis information,but the implications of electrolyte variability remained unclear.METHODS We investigate the relationships between electrolyte fluctuation and outcomes in survivors of acute myocardial infarction (n = 4 386). Ion variability was calculated as the coefficient of variation, standard deviation, variability independent of the mean (VIM) and range. Hazard ratios (HR) were estimated using the multivariable-adjusted Cox proportional regression method.RESULTS During a median follow-up of 12 months, 161 (3.7%) patients died, and heart failure occurred in 550 (12.5%) participants after discharge, respectively. Compared with the bottom quartile, the highest quartile potassium VIM was associated with increased risks of all-cause mortality (HR = 2.35, 95% CI: 1.36-4.06) and heart failure (HR = 1.32, 95% CI: 1.01-1.72) independent of cardiac troponin I (cTnI), N terminal pro B type natriuretic peptide (NT-proBNP), infarction site, mean potassium and other traditional factors, while those associations across sodium VIM quartiles were insignificant. Similar trend remains across the strata of variability by other three indices. These associations were consistent after excluding patients with any extreme electrolyte value and diuretic use.CONCLUSIONS Higher potassium variability but not sodium variability was associated with adverse outcomes post-infarction. Our findings highlight that potassium variability remains a robust risk factor for mortality regardless of clinical dysnatraemia and dyskalaemia.
Potassium and sodium have essential roles in maintaining myocardial physiological activity.[1-4]Electrolyte disturbances, such as dysnatraemia and dyskalaemia (hyper- or hypo-),are common in cardiovascular disease and associated with adverse outcomes.[5-9]Especially in the setting of acute myocardial infarction (AMI), circulating potassium or sodium abnormalities are significantly associated with risk of mortality.[7,10−12]Retrospective analysis based on the Cerner Health Facts database demonstrated a U-shaped relationship between the mean serum potassium level and in-hospital mortality in patients with AMI.[7]Sodium level on admission was reversely associated with long-term risk of mortality after AMI hospitalization.[10]However, the clinical implication of electrolyte variability is unclear.
The variability of metabolic biomarkers has emerged as a novel risk factor to provide incremental information. Variability in blood pressure (BP),[13,14]heart rate[15]and blood gamma-glutamyl transferase[16]and glycosylated hemoglobin[17]has been authenticated to provide additional information for adverse outcomes independent of each biomarker level. It has been demonstrated the distinctive value of blood pressure variability for directing therapy and risk stratification in cardiovascular disease.[13,14,18]Given the pivotal effect of potassium and sodium homeostasis, it can be hypothesized that electrolyte fluctuation extends the clinical implications of electrolyte level. This study determines the relationship between baseline potassium or sodium fluctuation and adverse outcomes in survivors of myocardial infarction.
METHODS
Study Population
Supported by China’s national key program for health, the prospective, hospital-based AMI cohort was established in 2017 in the Second Affiliated Hospital of Harbin Medical University. Consecutive patients with AMI, aged 18 years or older were enrolled on admission to the cardiac care unit. AMI was defined as chest pain ≥ 20 min or dynamic electrocardiogram variations, and cTnI exceeding the 99thpercentile of the healthy population. All patients underwent optimized medical treatment and revascularization of the culprit vessel was given if appropriate. Dual antiplatelet therapy (loading dose of 300 mg of aspirin, 180 mg of ticlopidine, or 600 mg of clopidogrel), beta-blockers, and statins were performed if appropriate. In this study,among 4 909 survivors after AMI hospitalization between February 2017 and April 2019, excluding prior heart failure (n = 110), renal failure with replacement therapy (n = 13), tumour diseases (n =21) according to self-reports and records, blood electrolyte test < 3 times (n = 324), and loss to follow-up after discharge (n = 55). Finally, 4 386 individuals were available for analysis (Figure S1). The study was conducted in compliance with the principles of the Declaration of Helsinki. The research protocols were approved by the Ethics Committee of Harbin Medical University, and the written informed consent was obtained from all participants.
Definition of Electrolyte Variability
Potassium or sodium variability was defined as the fluctuation of three or more serum ion levels measured during AMI admission. Four indices of variability were applied: (1) Variability independent of the mean (VIM), (2) coefficient of variation(CV), (3) standard deviation (SD), and (4) range.VIM was calculated as the SD/mean.[13]The power β was derived from curve fitting of the natural logarithm of SD over the natural logarithm of the mean. CV was calculated as the SD/mean. Range was calculated as maximum minus minimum.Quartiles of VIM were used for primary analysis,and electrolyte range as continuous variable was also applied for visualization because of its simple method and easy explanation.
Covariates
Information on age, sex, smoking status, prior diseases, examination, laboratory data, and medication was collected from medical records and self-report. Blood sample collection and blood tests were based on a predetermined uniform protocol.Routine blood tests, myocardial injury assessment,as well as electrolyte, liver, and renal function tests were measured on admission to hospital and reviewed when needed or scheduled. Diabetes mellitus was identified by anti-hyperglycemic therapy or HbA1c ≥ 6.5%. Hypertension was defined as prior antihypertensive treatment, the average systolic BP ≥ 140 mmHg or the diastolic BP ≥ 90 mmHg at baseline. Acute heart failure was defined as clinical features (Killip class ≥ 2) and intravenous diuretics during admission. The estimated glomerular filtration rate (eGFR) was calculated using baseline serum creatinine based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.
Assessment of Clinical Outcomes
All patients were recorded for all-cause death,heart failure, recurrence of MI, and stroke after hospitalization, with a median follow-up of 12 months(1-24 months). The regular follow-up points were 1 month, 3 months, 6 months, 12 months, 18 months,and 24 months. The management of all data and quality control were performed with electronic data capture system. The primary endpoint was allcause mortality and heart failure after leaving hospital. The association with each HF subtype was also assessed. Heart failure post-discharge was defined as dyspnea, diuretics medication, and pulmonary rales, third heart sound, persistent sinus tachycardia, edema of lower limbs or pneumonemia sign.[19]HF events included death or rehospitalization due to HF and outpatient diagnosis and treatment. The classification of HF subtypes was assessed using echocardiography within 4 weeks before or after HF diagnosis. HF with left ventricular ejection fraction (LVEF) ≤ 40%, 40% < LVEF < 50%,or LVEF ≥ 50% was categorized as HF with reduced ejection fraction (HFrEF), HF with mid-range ejection fraction (HFmrEF), and HFpEF, respectively. Patients without LVEF data were individually defined as having unclassified HF. The secondary endpoints included recurrence of MI and stroke post-AMI. To ensure data quality, two cardiac doctors reviewed the medical records of all inpatient and outpatient diagnosis for self-report.
Statistical Analysis
Variables were described as mean ± SD, medians(interquartile ranges, IQR), and counts (proportions).[20]Restricted cubic spline curves were used to visualize the non-linear trends between the electrolytes and endpoints.[21]The associations between ion variability and risks of mortality, heart failure, reinfarction or stroke were estimated using Cox proportional hazards regression. Crude and multivariable adjusted models were adopted. The final model was fully adjusted for age, sex, BMI, smoking, hypertension, diabetes, prior MI, cTnI, NTpro-BNP, C reactive protein (CRP), eGFR, triglyceride (TG),total cholesterol (TC), High-density lipoprotein cholesterol (HDL-C), MI type, anterior MI, percutaneous interventional therapy (PCI), ACEI or ARB,spironolactone, acute heart failure and mean potassium level. The Fine-Gray sub-distribution hazards method was further used to repeat the association of HF post-discharge with electrolyte fluctuation, treating mortality without HF as a competing factor. Other HF subtypes and unclassified HF were also considered competitive factors when examined the association between electrolyte variability and each HF subtype.[19]After excluding patients with any extreme potassium or sodium value,repeated analysis was conducted in patients with potassium level 3.0-5.5 mmol/L and sodium 130−150 mmol/L during admission. Stratified analysis was presented with a fully adjusted model (except for subgroup factors). Interaction tests between electrolytes and interaction terms were performed using the Wald test. P ≤ 0.05 with a two-tailed test were considered statistically significant. Statistical analyses were conducted with R version 3.6 and STATA SE 15.
RESULTS
Baseline Characteristics
The baseline characteristics of all survivors after AMI hospitalization are presented in Table 1. The mean age was 60.6 ± 11.6 years, and most patients were males or smokers, had ST-elevation myocardial infarction (MI), complicated with hypertension,and were treated with percutaneous coronary intervention (83.9%). The participants with higher variability of potassium or sodium were more likely to have higher cTnI, NTpro-BNP, and C-reactive protein (CRP) levels as well as a higher proportion of anterior infarction and acute myocardial infarction.While the average values of electrolyte across quartiles of potassium or sodium variability VIM had minor differences. The median ranges of potassium and sodium were 0.8 (IQR, 0.5-1.1) mmol/L and 6.1(IQR, 4.1-8.4) mmol/L, respectively (Figure 1).
Of the 4 386 survivors with AMI, 161 (3.7%) patients died, and re-MI and stroke occurred in 96(2.2%) and 53 (1.2%) participants during a median follow-up of 1 year, respectively. There had 550(12.5%) experienced HF after discharge. The proportions of HFrEF, HFmrEF, HFpEF and uncertain subtype were 26.0%, 20.6%, 37.8%, and 15.6%, respectively.
Associations with All-cause Mortality, Recurrence of MI and Stroke Post-infarction
Elevated potassium or sodium variability was strongly associated with mortality in the crude and sex- and age-adjusted models (Table 2 and Figure 1).After full adjustment as before mentioned, the association still complied with a dose-response pattern with an elevated risk of all-cause mortality by 68%per each 1 mmol/L increase of potassium range(HR = 1.68, 95%CI: 1.29-2.19, P < 0.001). Similar results were observed when analyzing cross strata of baseline potassium variability by the 4 indices (each P for trend ≤ 0.003). Compared with the bottom quartile of potassium VIM, more than a 2-fold increased risk for patients with potassium VIM in thehighest quartile (HR = 2.35, 95% CI: 1.36-4.06). By contrast, though a dose-response trend was observed for the association of sodium fluctuation with mortality after AMI hospitalization in univariate regression model, the significance almost vanished in fully adjusted model (Table 2 and Table S2).However, no significant association between potassium or sodium variability by all four indices and risk of re-MI or stroke was found in fully adjusted model (Table S2).
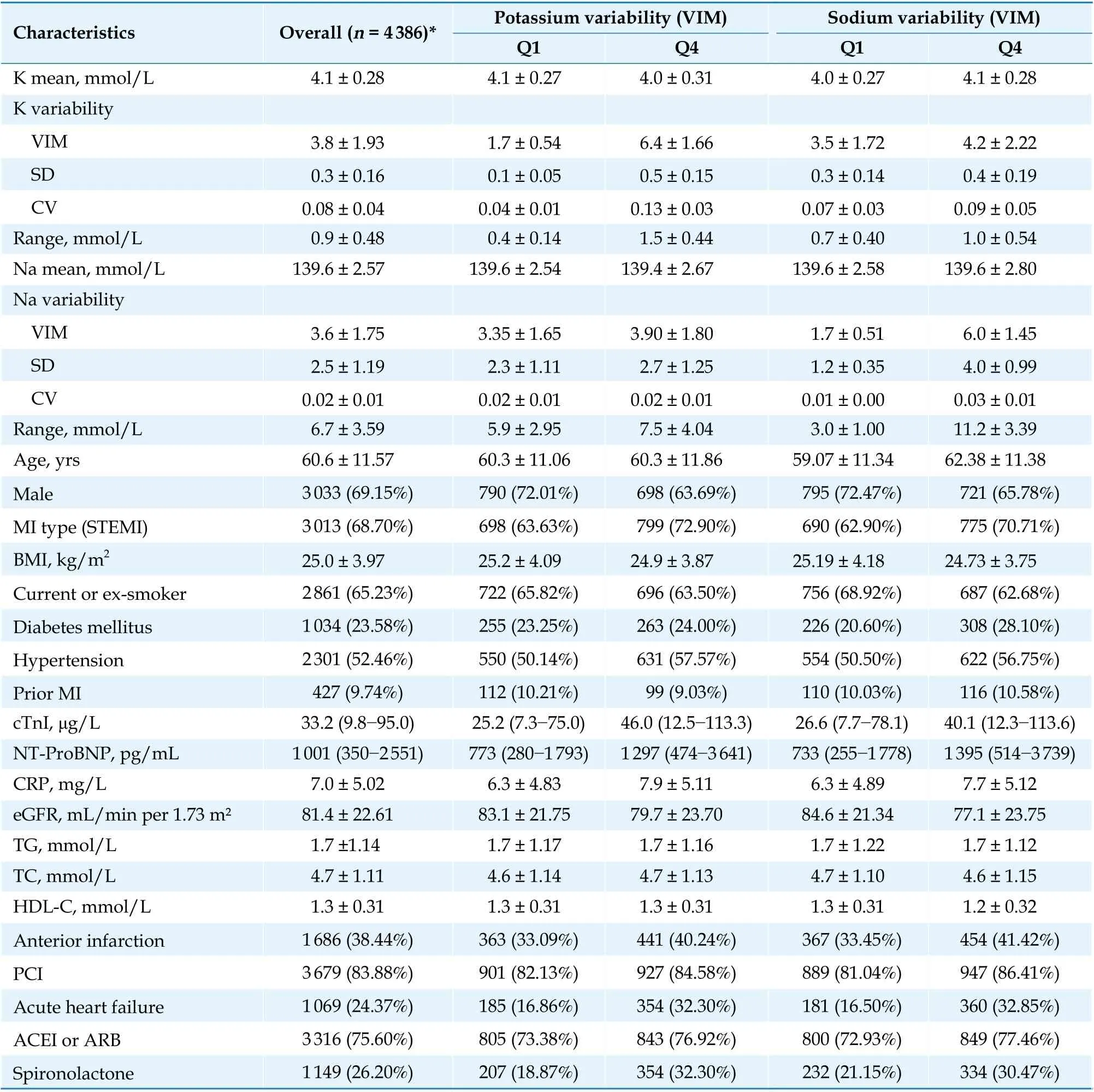
Table 1 Baseline characteristics of AMI patients according to the electrolyte variability assessed by variability independent of the mean.

Figure 1 Restricted cubic spline fitting for the association between ion variability and the risk of all-cause death after AMI discharge. (A): Potassium; (B): Sodium. Restricted cubic spline curve indicates the age- and sex-adjusted HRs (95%CI) estimated by Cox proportional remodel, setting the median value (0.8 mmol/L for potassium range and 6.0 mmol/L for sodium range) as reference.Knots include the 5th, 27.5th, 50th, 72.5th, and 95th percentiles of potassium or sodium range. Solid line represents point estimates. Dashed line represents 95%CIs. AMI: acute myocardial infarction; CI: confidence interval; HR: hazards ratio.
Associations with HF Risk after AMI Discharge
The potassium and sodium ranges had dose-response trends with HF post-discharge (Figure S2).Using the bottom quartile (Q1) as a reference, higher baseline potassium VMI was strongly associated with increased risk of HF after-discharge (Q2-4 versus Q1, hazards ratio (HR) 1.48, 1.73 and 1.99, respectively, Ptrend< 0.001) according to univariate analyses. In multiple Cox regression model with full adjustment including maximum cTnI, maximum NT-ProBNP, CRP, MI location, acute HF, mean potassium, and other traditional factors, all four potassium variability indices were moderately associated with risk of HF post-discharge (each P trend =0.005-0.051). The association followed a dose-response pattern with a 27% increased risk of chronic HF for each 1 mmol/L increase in potassium range(HR = 1.27; 95% confidence interval [CI]: 1.08-1.50,P = 0.004). After adjusting for identical confounders, elevating sodium fluctuation was associated with risk of HF after AMI hospitalization, with 16% increased HF risk per 5 mmol/L increment in sodium range (Table 2). However, compared with Q1 reference, only the top quartile of potassium variability, but not sodium’s was related to increased risk of HF post-discharge (Table 2 and S3).
Sensitivity and Subgroup Analysis
Competing risk model was applied to investigate the relationship between potassium variability and HF during follow-up again. There was also a marginal significance between all indices of potassium variability and HF post-discharge (Ptrend= 0.009 to 0.077, Table S4). The associations of potassium range with HFrEF, HFpEF and HFmrEF post-discharge are presented in Figure S3. After fully adjusting for the above potential confounders, the higher potassium range was an independent predictor of HFrEF risk post-discharge, with sub-distribution HR (sHR = 1.40, 95%CI: 1.02-1.91; P = 0.038)per 1 mmol/L potassium range increment. However,the association of potassium variability with HFmrEF or HFpEF was insignificant. It is generally known that extremely low or high ion concentrations impaired cellular physiological activity and elevated risk of adverse events. Thus, additional analysis for AMI without any extreme values (potassium level 3.0-5.5 mmol/L and sodium 130-150 mmol/L)was conducted. The highest quartile of potassium variability was also significantly associated with higher mortality compared with those in the lower quartiles (Table S5). According to stratified analyses, the association between potassium variability and increased mortality or HF post-discharge was largely consistent across MI type, age subgroup,sex, BMI, smoker, hypertension, heart failure on admission, prior MI, and renal function. However, we observed a significant interaction between potassium variability and diabetes mellitus for mortality and HF post-discharge (Figure 2).
DISCUSSION
In this hospital-based cohort of AMI survivors,VIM, CV, SD, and range during hospitalization were analyzed as indices of electrolyte variability.We found that potassium variability was associatedwith a higher risk of mortality and heart failure risk after AMI discharge. In particular, the association between potassium fluctuation and death post-infarction remained significant after adjustment for mean potassium, peak values of cTnI and NTpro-BNP during AMI admission, as well as excluding patients with any extreme electrolyte level, while sodium variability was insignificantly or marginally associated with poor prognosis. No relationship between electrolyte variability and myocardial re-infarction or stroke events was observed.

Table 2 Crude and adjusted associations with all-cause mortality and HF after-discharge according to quartiles of electrolyte variability independent of the mean.
Numerous studies investigated the association between serum potassium or sodium levels and mortality in patients with AMI, while few studied the risk of developing HF after AMI. To the best of our knowledge, no prior study reported the prognostic value of potassium or sodium variability.The lack of attention to electrolyte fluctuation may attribute to the established conventional clinical implications of dysnatraemias and dyskalaemias. Our research provides new insights into the implications of electrolyte variability. Indeed, it should not deny that extremely low or extremely high ion concentrations were dangerous and associated with an increased death risk. Diuretics medication as one indicator of acute heart failure resulted in greater ion variability. However, after adjusting for acute HF and excluding patients with any extreme potassium and sodium values during hospitalization, potassium fluctuation was also robustly linked to poor prognosis in survivors of AMI independent of potassium concentration.
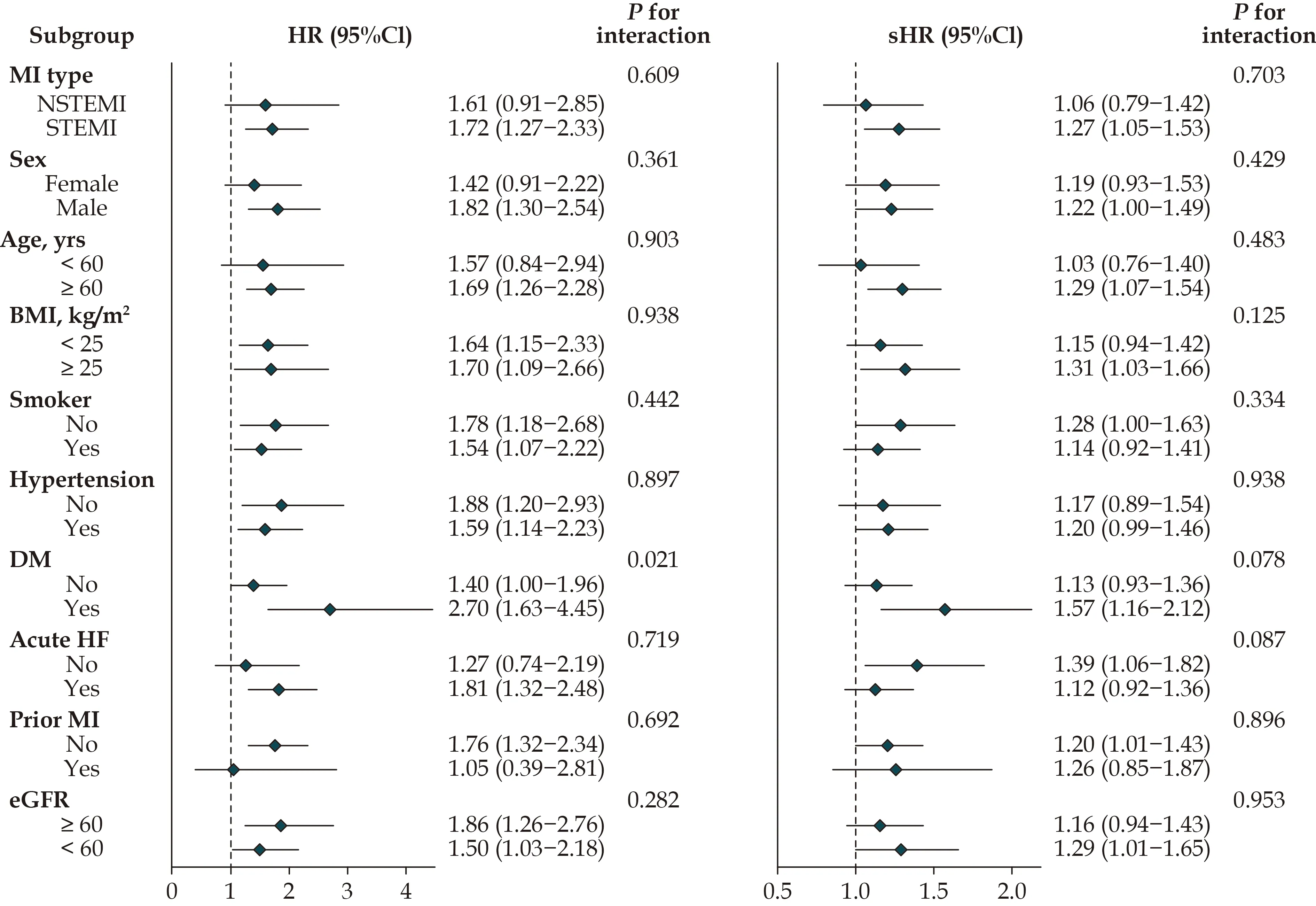
Figure 2 Subgroup analyses of baseline potassium variability and mortality or HF risk after discharge. Stratified analysis was conducted with Cox or competing risk model, which was fully adjusted for age, sex, BMI, smoking, hypertension, diabetes, piror MI, cTnI,NT-proBNP, CRP, eGFR, TG, TC, HDL, MI type, anterior MI, PCI, ACEI or ARB, spironolactone, acute heart failure and mean potassium except for subgroup factors. ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; BMI: body mass index; CI: confidence interval; CRP: C-reactive protein; cTnI: cardiac troponin I; eGFR: estimated glomerular filtration rate; HDL: highdensity lipoprotein; HF: heart failure; HR: hazards ratio; MI: myocardial infarction; NT-proBNP: N terminal pro B type natriuretic peptide; PCI: percutaneous coronary intervention; TC: total cholesterol; TG: triglyceride.
Prior studies demonstrated that baseline hyponatraemia was significantly associated with longterm death risk in patients who experienced AMI,with all-cause mortality increasing by 17%−61%during several years of follow-up after adjusting for multiple confounders but without cTn.[10,22]Interestingly, hypernatraemia increased mortality in patients with AMI,[12]but had no obvious effect on long-term mortality in patients with chronic HF.[23]Similarly, we also observed that increased sodium fluctuation was related to higher total mortality in univariate analysis, but that effect disappeared after full adjustment. Currently, whether serum sodium was associated with incident HF post-MI still lacks strong evidence. Herein, we observed that the association of sodium fluctuation with HF post-discharge also became insignificant in the adjusted model. Thus, our study and prior reports suggest that sodium concentrations or variability may have a limited prognostic value based on current risk factors in patients with AMI.
Clinical guidelines recommend the range of serum potassium levels between 4.0 and 5.0 mEq/L or even 4.5 to 5.5 mEq/L in patients with AMI.[24]Potassium levels between 3.5 and 4.5 mmol/L were associated with the lowest short-[24]and long-term[5,25-27]mortality in hospitalized patients with AMI. Our observations further supported the finding that potassium disturbance increases mortality risk post-MI. even during normal electrolyte range of potassium level 3.5−5.5 mmol/L and sodium level 130−150 mmol/L, we still observed more than a 2-fold increased risk of mortality for patients with potassium variability in the highest quartile, compared with those patients in the bottom quartile. Results from the SWEDEHEART cohort demonstrated that the association of potassium level at discharge with HF post-infarction was insignificant.[27]Our data provided moderate evidence of the dose-response pattern between potassium variability and HF postdischarge, independent of maximum cTnI, NTproBNP, heart failure on admission, and mean potassium. The association was still observed in patients without acute HF (sHR = 1.39, 95%CI:1.06−1.82). Thus, potassium variability might reflect better the severity of impaired homeostasis and provide incremental prognostic information independent of potassium concentration for patients with AMI.
Current practice tended to consider HF as a complicated syndrome and study for the comprehensive mechanisms and phenotypes of HF is a great challenge. Previous studies demonstrated that HFmrEF was difficult to characterize with clinic indicators and circulating biomarkers, suggesting that patients with moderate LVEF had obvious heterogeneity.[28]Consistently, both potassium and sodium fluctuations showed no significant association with non-HFrEF in our study. Indeed, the pathophysiological evidence for classifying HF post-MI according to LVEF is unclear.[29]The description of HFpEF or HFmrEF post-infarction was just presented in a few studies and the potential mechanisms in AMI were still unclear.[29]Furthermore, LVEF is dynamic during the chronic phase of AMI and relapsing HF can be characterized as different subtypes. The heterogeneous effects to identify HF and classify HF subtypes tend to bias the difference in hazards among those HF sub-phenotypes toward the null, so the analysis regarding HF needs further investigation with larger-scale cohort. It is worth noting that, potassium fluctuation is more pronounced in the high-risk subgroup of patients. A previous study by Kraft et al. has shown that potassium homeostasis is often disturbed in critically ill patients and high-risk patients with more comorbidities.[30]This may be due to the patient's underlying disease and treatment methods that affect the Na+/K+-ATP pump. The pump is affected by many factors such as insulin level and acid-base status,and thus regulate the fluctuation of blood potassium.
Mechanisms regarding the association of ion fluctuation with adverse prognosis in patients with AMI remain unclear. Increased variability in blood pressure was linked to an increased 10%-18% risk of cardiovascular and mortality events over the effect of mean blood pressure.[14]And the potential interpretation was that continuous blood pressure monitoring provides extra information about the stability of blood pressure compared with a single or mean value. Likewise, circulating electrolytes oscillate over time will be especially amplified in the setting of AMI. Theoretically, ion variability may more effectively indicate internal environmental conditions than a single electrolyte value, even the electrolyte concentrations were in normal range post-admission. In addition, atherosclerosis progress and plaque instability were associated with recurrence of MI or stroke, which may explain the insignificant relationship between ion variability or abnormal level and angiemphraxis events in our and other reports.[12]However, subsequent exploration is required to ascertain whether and how electrolyte fluctuation participates in the early pathophysiological mechanisms of poor prognosis post-MI.
Our findings should be interpreted within the context of its strengths and limitations. The electrolyte variability was estimated with various methods. Study staffs were especially responsible for this prospective hospital-based cohort that facilitated relatively complete data with less misclassification bias. Moreover, patients with AMI were admitted from 2017 to 2019, baseline characteristics and therapeutic strategies reflected contemporary epidemiology. This study has some limitations. First,participants in this study were primarily residents in Northeast China where they are accustomed to a high-salt diet. Thus, our findings should be further explored in populations from other regions and backgrounds. Second, heart failure is characterized by highly heterogeneous manifestations and mechanisms, so a larger scale cohort may need to further investigate the association between ion variability and HF risk. Third, we did not acquire the information about the Charlson Index in the present study.Even though the potential confounders were adjusted to the extent possible, the residual confounding could not be completely ruled out. Fourth, the causality could not be concluded for the observational study design.
In conclusion, our study shows that potassium variability during AMI hospitalization is associated with an increased risk for all-cause mortality and heart failure post discharge independent of its concentration. By contrast, sodium variability had a limited association with poor prognosis post-infarction. Attention should be given to longitudinal electrolyte fluctuation more than single ion measurement, even in those patients without extreme value.
ACKNOWLEDGEMENTS
We would like to thank the staff of cardiac followup team for raw data collecting.
AUTHOR CONTRIBUTIONS
XLZ, SJW and BY conceived and designed the study. HXC and XQG developed the protocols.XRH and XYZ collected and assembled all data.SHF analysed and visualized the results. All authors contributed to the manuscript and approved to submit. Reviewed and edited the manuscript, BY and XQG take the responsibility for the integrity and accuracy of this analysis.
FUNDING
This work was supported by the National Key R&D Program of China (No. 2016YFC1301100), National Natural Science Foundation of China (No. 81 827 806,81 870 353, 31 771 241), and Key Laboratory of Myocardial Ischemia, Ministry of Education (No.KF201903).
CONFLICTS OF INTEREST
None.
杂志排行
Journal of Geriatric Cardiology的其它文章
- Journal of Geriatric Cardiology
- Beta-blocker therapy in elderly patients with renal dysfunction and heart failure
- Obstructive sleep apnea increases heart rhythm disorders and worsens subsequent outcomes in elderly patients with subacute myocardial infarction
- Sex modification of the association of the radial augmentation index and incident hypertension in a Chinese communitybased population
- Neurohumoral, cardiac and inflammatory markers in the evaluation of heart failure severity and progression
- Patent foramen ovale closure in non-lacunar cryptogenic ischemic stroke: where are we now?
