Effect of miR-200a-3p targeting transthyretin(TTR)on the biological behavior of placental trophoblasts in hypertensive disorder complicating pregnancy
2021-03-03YingLiuHaijuanLiuZhijuanHuang
Ying Liu ,Haijuan Liu* ,Zhijuan Huang
Abstract Objective:To investigate the expression of mi‐croRNA-200a-3p (miR-200a-3p) in placental tissues of pa‐tients with hypertensive disorder complicating pregnancy (HD‐CP) and its effect on the biological behavior of trophoblasts.Methods:Fifty cases of HDCP (study group) and 50 normal pregnant women (control grouo)in our hospital from January 2017 to May 2019 were collected.The expressions of miR-200a-3p and transthyretin (TTR) mRNA in placental tissues were detected by fluorescent quantitative PCR (qPCR).MiR-200a-3p mimics,inhibitor and negative control were transfect‐ed into human chorionic carcinoma JEG-3 cells by liposome.Cell proliferation,migration,invasion and apoptosis were de‐tected by CCK-8 method,Transwell test and Annexin V-FITC/PI test,respectively.Bioinformatics and dual luciferase reporter gene experiments were used to analyze the targeting relation‐ship between miR-200a-3p.Western blottingwas used to detect the TTR expression.Results:The expression level of miR-200a-3p in study group was significantly higher than that incontrol group,while the expression level of TTR mRNA in study group was significantly lower than that incontrol group (P<0.01).Transfection of miR-200a-3p mimic to overexpress miR-200a-3p in could significantly promote the proliferation,inva‐sion and migration of JEG-3 cells,and inhibit apoptosis(P<0.01).Transfection of miR-200a-3p inhibitor to inhibit the ex‐pression of miR-200a-3p could significantly inhibit the prolif‐eration,invasion and migration of JEG-3 cells,and promote apoptosis (P<0.01).MiR-200a-3p could significantly decrease the luciferase activity of TTR-WT(P<0.01).Transfection of miR-200a-3p mimic could significantly decreased the expres‐sion of TTR protein,while the result of transfection of miR-200a-3p inhibitor was on the contrary(P<0.01).Conclusion:miR-200a-3p is highly expressed in placental tissue of HDCP patients.Inhibition of miR-200a-3p expression in JEG-3 cells can inhibit cell proliferation,migration,invasion,and promote apoptosis.MiR-200a-3p may participate in the pathological mechanism of HDCP through negative regulation of TTR.
Keywords microRNA-200a-3p;transthyretin;hypertensive disorders complicating pregnancy;JEG-3 cells;biological be‐havior
Introduction
Hypertensive disorder complicating pregnancy (HD‐CP) is one of the common diseases during pregnancy.In the world,the incidence of pregnancy complica‐tions among women of childbearing age is as high as 15%.In China,compared with about 10% in China[1-2].HDCP is one of the main causes of maternal and perinatal death,which complicates pregnancy,serious‐ly endangers maternal and infant safety,and increases the risk of premature delivery and growth restriction[3].So far,the etiology and pathogenesis are still un‐clear.More and more studies have shown that HDCP is related to maternal-fetal metabolism or immune bal‐ance caused by a variety of factors,and the abnormal ability of placental trophoblasts will hinder the forma‐tion of placenta and release a large number of inflam‐matory factors,resulting in vascular endothelial dam‐age,and finally lead to the occurrence of preeclampsia[4-6].
Micro-RNA(miRNA) is a kind of non-coding singlestranded regulatory small RNA molecule,which spe‐cifically binds to the 3'UTR region of the target gene mRNA,inhibits the transcription of the target gene mRNA or promotes degradation,thus regulating the expression of the target gene[7].At present,it has been confirmed that multiple miRNA are closely relat‐ed to the pathogenesis of HDCP.For example,there is a significant difference in the expression of miR-106a-363 in placental tissues of patients with HDCP[8],and miR-19a is highly expressed in peripheral blood of patients with HDCP[9].However,the role of miR-200a-3p in HDCP has not been reported.The purpose of this study is to investigate the expression of miR-200a-3p in HDCP placenta and its effect on tropho‐blasts,and to explore the possible regulatory mecha‐nism of miR-200a-3p in HDCP.
Materials and methods
Main materials and reagents
Human chorionic carcinoma JEG-3 cell line was pur‐chased from the Institute of Basic Medical Sciences,Chinese Academy of Medical Sciences&Peking Union Medical College.Fetal bovine serum and DMEM culture medium were purchased from Gibco .Lipofectamine 2000 transfection kit and Trizol kit were purchased from Invitrogen.SYBR Green Real‐time PCR Master Mix kit was purchased from TAKA‐RA.Annexin V-FITC kit was purchased from Beijing SolarbioScience &Technology Co.,Ltd.BCA kit was purchased from Wuhan Boster Biological Engineering Co.,Ltd.CCK-8 kit and ECL chemiluminescence so‐lution were purchased from Shanghai Beyotime Bio‐technology Co.,Ltd.Plasmid pmirGLO and double lu‐ciferase detection kits were purchased from Promega.TTR antibodies were purchased from Wuhan Atage‐nix Biotechnology Co.,Ltd.Antibody GAPDH and horseradish peroxidase (HRP) labeled goat anti-rabbit IgG were purchased from Abcam.Crystal violet stain‐ing solution was purchased from Beijing Leagene Bio‐technology Co.,Ltd.Other reagents are homemade an‐alytical purity.
Clinical data
A total of 50 HDCP pregnant women (study group),aged from 22 to 36 years old,with an average age of 28.2±3.2 years,were collectedfromourhospital from January 2017 to May 2019.The average gestational age was 37.9±1.9 weeks.There were 29 cases with mild preeclampsia,systolic blood pressure ≥18.62 kPa and/or diastolic blood pressure ≥11.97 kPa after 20 weeks of gestation.24 h urine protein ≥0.3 g or random proteinuria (+).There were 21 cases of severe preeclampsia,systolic blood pressure ≥21.28 kPa and/or diastolic blood pressure ≥14.63 kPa.24 h urine pro‐tein ≥5.0 g or random proteinuria (+++).In the same period,50 healthy pregnant women (control group)aged from 23 to 35 years old,with an average age of 27.5±4.8years old,were collected in our hospital.The average gestational age was 38.3±1.1 weeks.The pro‐cess of pregnancy was successful and the blood pres‐sure <18.62/11.97 kPa.Urinary protein was nega‐tive.There was no significant difference in age and gestational age between the two groups (allP<0.05).All the subjects were singletons,had no other medical history,and had signed informed consent.This study has been approved by the Medical Ethics Committee of our hospital.After the placenta was delivered,the tissue mass of the umbilical cord root of the mother's central placenta (1 cm×1 cm×1 cm) was removed to avoid bleeding,necrosis and calcification area.The PBS solution was rinsed fully,frozen in liquid nitro‐gen and quickly stored in the refrigerator at-80℃.
Detection of miR-200a-3p and TTR expression by Real-time fluorescence quantitative PCR(qPCR)
The total RNA of placental tissue was extracted by Trizol.The purity and concentration of RNA were de‐tected by NanoDrop2000,and were reverse tran‐scribed into cDNA.The gene expression was detected by SYBR Green Realtime PCR Master Mix kit.U6 and GAPDH were used as the internal reference genes of miR-200a-3p and TTR,respectively.The program was set to 95 ℃10 min,95 ℃2 s,60 ℃20 s,72 ℃10 s,a total of 40 cycles.The sequence of the primers was as follows:miR-200a-3pupstream:5’-CAGTAACACTGTCTGGTAACG-3’,downstream:5’-ATCCAGTGCAGGGTCCGAGG-3’.U6 upstre -am:5’-CTCGCATCGGCAGCACA-3’,downstream:5’-AACGCTACTCGAATTGCGT-3’;TTR upstream:5’-TGGGAGCCATTTGCCTCTG-3’,downstream:5’-AGCCGTGGTGGAATAGGAGTA-3’;GAPDH upstream:5’-AAGGTCATCCCTGAGCTGAAC-3’,downstream:5’-ACGCCTGCTTCACCACCTTCT-3’.The relative expression of miR-200a-3p and TTR mRNA was calculated by 2-ΔΔCtmethod.
Cell culture and transfection
After resuscitation,JEG-3 cells were cultured in DMEM medium containing 10% fetal bovine serum,penicillin (100 U/mL)/streptomycin (100 μg/mL),and was placed in an incubator at 37℃with 5%CO2.
The liquid was changed every 2 days,and when the fusion degree reached 80%-90%,0.25% trypsin was digested and subcultured.The cells were divided in‐tomiR-NC group,miR-200a-3p mimic group and miR-200a-3p inhibitor group.The cells were inoculat‐ed in 6-well plate according to 1×105/well and incu‐bated overnight in a 5% CO2incubator at 37℃.Trans‐fection was carried out according to the instructions of Lipofectamine 2000 transfection reagent.MiR-NC group was transfected with miR-200a-3p negative control,miR-200a-3p mimic group was transfected with miR-200a-3p mimic,and miR-200a-3p inhibitor group was transfected with miR-200a-3p inhibitor.48 hours after transfection,the cells were digested by trypsin and collected.
Detection of cell proliferation by CCK-8 method
JEG-3 cells were inoculated in 96-well plate accord‐ing to 3×104/well,and cultured in 37 ℃,5%CO2incu‐bator for 0 h,12 h,24 h,48 h and 72 h.10 μL CCK-8 reagents were added into each well,and then incubat‐ed for 2 h.According to the instructions of CCK-8 kit,the absorbance at 450 nm wavelength of each well was determined by ELISA.
Detection of cell invasion and migration by Tran⁃swell assay
JEG-3 cells were resuscitated in serum-free medium and the density was adjusted to 4×104/mL.Upper chamber of Transwell was added with 50 μL diluted Matrigel glue and wasplaced at 37℃for 40 min.100 μL cell suspensions were added to the upper Tran‐swell chamber,and 500 μL DMEM medium contain‐ing 10%FBS fetal bovine serum was added to the low‐er chamber.The cells were cultured at 37℃for 24 h in 5% CO2incubator.Took out the chamber,discarded the culture medium,washed the chamber with PBS,the cells in the upper chamber that did not pass through the basement membrane were gently wiped off with cotton swab.The chamber was fixed with 4%paraformaldehyde for 20 min,stained with 0.1% crys‐tal violet staining solution for 15 min,washed with PBS.The thin film at the bottom of the chamber was took out,sealedwith neutral resin and observed under the electron microscope.The number of cells passing through the membrane was counted.Except that Matrigel glue was not applied in the migration experi‐ment,the other operations were the same.
Detection of apoptosis by flow cytometry
JEG-3 cells were inoculated on a 6-well plate at a rate of 2×105/well.After overnight culture,the cells were digested and collected with 0.25% trypsin without EDTA,and washed twice with precooled PBS.Cell apoptosis was detected according to Annexin V-FITC kit.500 μL 1×Binding Buffer resuscitated cells were then mixed with 5 μL Annexin V-FITC binding solu‐tion and 10 μL PI,and then incubated at room temper‐ature without light for 20 min.Flow cytometry FC500 was used to detect apoptosis.
Detection of TTR protein expression by Western blotting
RIPA lysate (containing 1% PMSF) was added to JEG-3 cells to extract total protein,and the protein concentration was determined by BCA method.10%SDS-PAGE protein was isolatedwith 30 μg protein for 90 min,then cut the gel and wet transferred to PVDF membrane.5% skimmed milk powder was used to sealed the sample at room temperature for 2 hours,rabbit anti-TTR (1∶500) was added,GAPDH(1∶1,000) was used as internal reference,the sample was placed overnight in 4℃refrigerator,washed with TBST membrane for 3 times,added withHRP to label the goat anti-rabbit IgG (1:2,000) and incubated at room temperature for 1 h.After the membrane was washed with TBST at room temperature for 3 times,the membrane was developed by dripping ECL and exposed in gel imaging system.The gray values of each band were analyzed by Image J.
Double luciferase reporter gene experiment
The binding fragment of miR-200a-3p and TTR gene was predicted by TargetScansoftware (http://www.tar‐getscan.org/).The wild type 3'UTR TTR and mutant 3'UTR TTR double luciferase reporter plasmids were designed and synthesized by Sangon Biotech (Shang‐hai) Co.,Ltd.,and the synthesized plasmid fragments were connected to the pmirGLO report vector respec‐tively.JEG-3 cells in logarithmic growth phase were inoculated on 12-well cell plate with 1×105/well.The constructed double luciferase recombinant plasmid(100 ng) combined with 100 nmol/L miR-200a-3p mimic or miR-NC was co-transfected into the cells by Lipofectamine 2000.After transfection,the cells were cultured in a 5% CO2incubator at 37 ℃for 24 h.The luciferase activity of cells in each group was detected according to the double luciferase reporter gene assay kit.
Statistical method
The data were processed by SPSS 21.0 statistical soft‐ware,and the measurement data were expressed by mean±standard deviation (±s).Single factor analysis of variance was used for comparison among groups.LSD-ttest was used for pairwise comparison between groups,P<0.05.
Results
MiR-200a-3p targeted to regulate TTR expression
TargetScan software analyzed and predicted that there was a binding fragment between miR-200a-3p and TTR 3'UTR,indicating that TTR may be the target gene of miR-200a-3p,as shown in Figure 1A.The re‐sults of luciferase report experiment showed that the luciferase activity in miR-200a-3p mimic and TTRWT co-transfection group was significantly lower than that in miR-NC and TTR-WT co-transfection group (P<0.01),but there was no significant differ‐ence between miR-200a-3p mimic and TTR-MUT cotransfection group and miR-NC and TTR-MUT cotransfection group (P>0.05),as shown in Figure 1B.The expression of TTR protein in miR-200a-3p mim‐ic group was significantly lower than that in miR-NC group (P<0.01),while the expression of TTR protein in miR-200a-3p inhibitor group was significantly higher than that in miR-NC group (P<0.01),as shown in Figure 1C.

Figure 1 Verification of targeting relationship between miR-200a-3p and TTR.A:3'UTR complementary binding sites of miR-200a-3p and TTR.B:Comparison of luciferase activity in three groups of JEG-3 cells.C:The expression of TTR protein in JEG-3 cells was detected by Western blotting.**P<0.01vs.miR-NC group.
Expression of miR-200a-3p and TTR mRNA in two groups
The expression level of miR-200a-3p in the study group was significantly higher than that in the control group (P<0.01),while the expression level of TTR mRNA in the study group was significantly lower than that in the control group (P<0.01),as shown in Figure 2.

Figure 2 Detection of miR-200a-3p and TTR expression by qPCR.**P<0.01vs.miR-NC group.
Detection of miR-200a-3p expression in JEG-3 cells of each group after transfection
Compared with miR-NC group,the expression of miR-200a-3p in JEG-3 cells in miR-200a-3p mimic group was significantly increased(P<0.01),while the expression of miR-200a-3p in miR-200a-3p inhibitor group was significantly decreased (P<0.01),as shown in Figure 3.

Figure 3 Detection of miR-200a-3p expression in JEG-3 cells by qPCR.**P<0.01vs.miR-NC group.
Effect of miR-200a-3p on the proliferation of JEG-3 cells
Compared with miR-NC,the ability of cell prolifera‐tion was significantly increased after miR-200a-3p mimic transfection into JEG-3 cells at 48 h and 72 h(P<0.01),while miR-200a-3p inhibitor significantly in‐hibited cell proliferation (P<0.01),as shown in Fig‐ure 4.
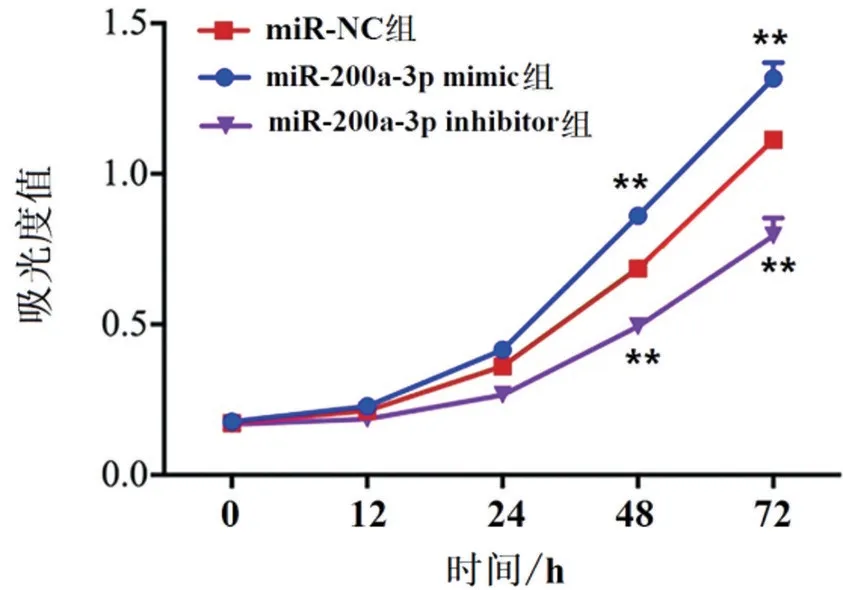
Figure 4 Detection of proliferation ability of JEG-3 cells by CCK-8.**P<0.01vs.miR-NC group.
Effect of miR-200a-3p on invasion and migration of JEG-3 cells
Compared with miR-NC group,miR-200a-3p mimic transfection could significantly promote the invasion and migration of JEG-3 cells(P<0.01).After transfec‐tion of miR-200a-3p inhibitor,the number of invasion and migration was significantly lower than that of miR-NC group(P<0.01),as shown in Figure 5.
Effect of miR-200a-3p on apoptosis of JEG-3 cells
The results of flow cytometry showed that the number of apoptosis of JEG-3 cells transfected with miR-200a-3p mimic was significantly lower than that of miR-NC group(P<0.01),and that of JEG-3 cells transfected with miR-200a-3p inhibitor was signifi‐cantly increased(P<0.01),as shown in Figure 6.
Discussion
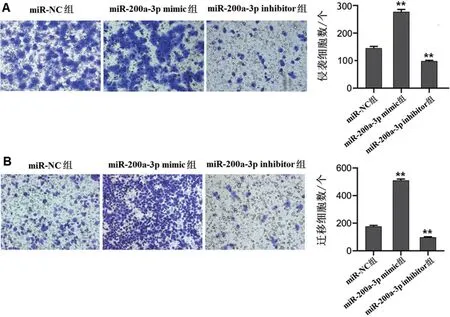
Figure 5 Detection of the invasion and migration of JEG-3 cells in each group by Transwell assay.A:Cell invasion number.B:The number of cell migration.**P<0.01vs.miR-NC group.

Figure 6 Detection of apoptosis of JEG-3 cells in each group by flow cytometry.**P<0.01vs.miR-NC group.
HDCP accounts for about 3% to 10% of pregnancies,and despite in-depth research,it is still the most im‐portant and difficult disease in obstetrics and gynaeco‐logy[2].The pathogenesis of HDCP originates from placental abnormality,followed by the secretion of placental toxic factors,which leads to extensive endo‐thelial dysfunction,and it's central factor is the placen‐tal physiological disorder caused by abnormal tropho‐blast function[10].Therefore,abnormal trophoblast function plays an important role in the pathogenesis of HDCP.
MiR-200a-3p belongs to the miR-200 family.MiR-200a-3p is a functional gene involved in many biolog‐ical processes,which plays an important role in the development of many kinds of cancers by regulating cell differentiation,proliferation,invasion and migra‐tion[11-13].Mateescu et al.[14]pointed out that miR-200a-3p responds to oxidative stress by directly tar‐geting kinase p38α,thus promoting the occurrence and development of ovarian tumors in mice.Eades et al.[15]found that the interaction between miR-200a-3p and the 3'UTR of Kelch-like ECH-associated pro‐tein 1 (Keap1) mRNA in breast cancer cells leads to the imbalance of Nrf2-mediated antioxidant defense in breast cancer cells.The results of this study showed that miR-200a-3p was highly expressed in HDCP placenta (P<0.01).The biological behavior of trophoblasts and its related regulatory mechanisms are key physiological factors to maintain placental ho‐meostasis.The imbalance of some biological process‐es (such as proliferation and apoptosis) can damage placental function[16].In this study,after transfected with miR-200a-3p mimic,the overexpression of miR-200a-3p could promote the proliferation,invasion and migration of JEG-3 cells and inhibit the apoptosis of JEG-3 cells,while the transfection of miR-200a-3p in‐hibitor could inhibit the proliferation,invasion and mi‐gration of cells and promote the apoptosis(P<0.01).TTR is a translocator,which is mainly synthesized and secreted by the liver[17].In addition,TTR is also secreted by placental trophoblast cells at the maternalfetal interface.TTR was found in the early stage of placental development,and it was expressed in villi at 6 weeks of pregnancy.The expression levels of TTR protein and mRNA were linearly correlated with the increase of gestational age from 6 to 13 weeks.The expression level of TTR reached the peak from 13 to 17 weeks of gestation and lasted to the whole gesta‐tional period[18].TTR tetramer binds to T4 to form a stable combination.TTR-T4 complex has endocytosis in placental trophoblasts.In addition to transport func‐tion,TTR is also related to the pathophysiological mechanism of several diseases.If abnormal expres‐sion of TTR was observed in patients with preeclamp‐sia[19],the expression of TTR in placenta of pregnant women with preeclampsia was lower than that in nor‐mal placenta[20].In this study,it was found that the expression of TTR was low in HDCP placenta,and miR-200a-3p could complement the 3'-UTR of TTR(P<0.01),suggesting that miR-200a-3p may regulate the biological activity of trophoblasts by negatively regulating the expression of TTR.
In conclusion,miR-200a-3p is highly expressed in the placenta of patients with HDCP.MiR-200a-3p is ex‐pected to be a new way of HDCP therapy in the future by regulating TTR in JEG-3 cells to affect the biologi‐cal behaviors of cell proliferation,invasion,migration and apoptosis.
AcknowledgmentsThis study was funded by Key Research and Development Project of Shanxi Prov‐ince(No.2019SF-127).
