Study on Low-temperature Preparation of High-purity Lycopene Isomers and Their Isomerization and Degradation in Edible Oils
2021-01-29BOLATIDinaSUNGuangyingHOUXuelingAISAHajiAkber
BOLATI Dina,SUN Guang-ying,HOU Xue-ling,2*,AISA Haji Akber*
(1.State Key Laboratory Basis of Xinjiang Indigenous Medicinal Plants Resource Utilization,and Key Laboratory of Chemistry of Plant Resources in Arid Regions,Xinjiang Technical Institute of Physics and Chemistry,Chinese Academy of Sciences,Urumqi 830011,China;2.University of Chinese Academy of Sciences,Beijing 100039,China)
Abstract:In this work,an orthogonal separation method was developed for the separation of lycopene isomers based on the combination of low temperature(L-T) C18 column and C30 column.As the temperature decreased to -20 ℃,the resolution for the separation of lycopene isomers on a normal C18 column was greatly improved.After investigating the relation of solubility and resolution,seven kinds of lycopene isomers were finally prepared by the orthogonal separation method with their purities all higher than 97%.The loading capacity and purification effect of the orthogonal method for lycopene were much higher than that of the method only based on L-T C18 or C30 column.Then,the obtained all-trans lycopene were dissolved in walnuts oil,black seed oil,almonds oil,grape seed oil,pumpkin seed oil and peach kernel oil,respectively,and heated at 120 ℃ to study their isomerization.Grape seed oil was regarded as the most valuable solvent for developing lycopene product due to its highest cis/all-trans ratio of lycopene isomers after heating.Finally,the stability of cis-isomers at 4 ℃ in edible oils was investigated by taking walnut oil as the example.Both the isomerization and degradation of these isomers happened with the increase of storage time.All-trans lycopene and(13Z)lycopene were found as the preferential conformations in walnut oil.The obtained results were expected to provide some beneficial information for the development of lycopene product.
Key words:lycopene;low temperature preparation;isomerization;degradation;edible oils

Fig.1 The structures of lycopene isomers
Lycopene,a promising natural antioxidant belonging to carotenoids,had been widely used as food additives due to its rich resources and positive bioactivities in anti-cancer[1],tumor inhibition[2],and cholesterol modulation[3].As demonstrated in
Fig.1,there are mainly two types of lycopene normally concluded:all-transisomer easily found in natural tomato[4]and many types ofcis-isomers found in processed tomato products[5].Compared to all-translycopene,the bioavailability ofcis-isomers of lycopene was higher,probably because their better solubility in micelles like human plasma[6].The stability of lycopene isomers,especially for that ofcis-isomers,was highly correlated with high temperature,illumination,oxygen,pH value,metal ion,and so on[7].The mutually isomerization and degradation between isomers of lycopene easily occurs in processing and storage,which strictly restricts the development of lycopene products[8].Consequently,the study of the transformation rules of lycopene isomers became very critical for improving the level of application in tomato industry.In an earlier study,the kinetic study of the isomerization between all-transand 13-cis-lycopene in Tributyrin Model System at Gastric pH was studied[9].Other isomers like 9-cis-isomers generally generated,which weaking the correlation of isomerization between 13-cis-lycopene and all-translycopene.To solve this issue,it is urgent to prepare the main lycopene isomers with high purity for the further study of isomerization.
There are two key factors in the preparation of high-purity isomers of lycopene.The first one is the limited resolution betweencis-isomers on chromatography.Although C30column was highly effective[10-12],somecis-isomers were still unable to be completely separated.The separation ability of C30column could be hardly improved only by optimizing mobile phase.Yield Microelectronics Corp(YMC) carotenoid column[13],high speed counter current chromatography(HSCCC)[14]and non-aqueous C18[15-16]column were also adopted to prepare lycopene,however,their separation ability towards lycopene isomers was relatively poor.Purification methods based on these techniques normally failed to meet the purity and loading capacity simultaneously.Thermal instability of lycopene was the second difficulty in the preparation and purification of lycopene,especially forcis-isomers.Transformation happens at any step during the preparation,concentration and dryness.
Low temperature technique appears to be a promising approach to offer unprecedented opportunities for solving these problems.As reported in early study,low temperature liquid chromatography had played an unique and positive role in the separation of analogues with similar structure[17].By using liquid chromatography,Yamasaki et.al[18]have successfully separated oxygenated terpenes from terpene hydrocarbons at -80 ℃.Theoretical study revealed that phase transition occurs at reduced temperature due to the increase of ordering of alkyl chains,which could dramatically enhance the shape selectivity[17].Consequently,although most of C18columns demonstrated limited separation ability for isomers of lycopene,there are enough reasons for us to believe that low temperature C18column have the potential for the separation of lycopene isomers.Moreover,low temperature was beneficial for improving the stability of lycopene.With the aid of low temperature storage,lycopene would be obtained by nitrogen gas blowing without degradation[19].
Here,the influence of low temperature(L-T) on the separation of lycopene isomers on C18column was first investigated.After comprehensive consideration of solubility,selectivity and loading capacity,an orthogonal separation method for lycopene isomers was established based on L-T C18and C30column.Seven types of lycopene isomers with high purity were finally L-T prepared.Secondly,in order to select an appropriate solvent for the isomerization,all-translycopene were heated in edible oils of walnuts,black seed,almonds,grape seed,pumpkin seed,and peach kernel to produce mixtures of lycopene.Finally,the isomerization and degradation rules ofcis-isomers were determined in edible oils to reveal their L-T stability,which would play an important role in developing the storage technique.These results were of great significance for the development of lycopene products.
1 Experimental
1.1 Materials and equipment
HPLC grade acetonitrile and methanol were purchased from Merck KGaA(Darmstadt,Germany).Analytical grade methyl tert-butyl ether(MTBE) and ethyl acetate(EtOAc) were purchased from Yongsheng Chemical Reagent Co.(Tianjin,China) and Xinbote Chemical Reagent Co.(Tianjin,China),respectively.The tomato oleoresin was purchased from Keyu Technology Co.(Xinjiang,China),and the edible oils were sponsored by Sahar Bio-technology Development Co.(Xinjiang,China).
Analytical HPLC was performed on a YMC C30column(4.6 mm×250 mm,5 μm,Kyoto,Japan) and an Xbridge BHE Shield C18column(4.6 mm×250 mm,5 μm,Waters,America) using a Dionex UltiMate 3 000 system(Thermo Fisher Scientific,Waltham,MA,USA).The scale-preparation was performed on an Xbridge BHE Shield C18column(10 mm×250 mm,5 μm,Waters,America) and a YMC C30carotenoid column(10 mm×250 mm,5 μm,Kyoto,Japan) in sequence.L-T was controlled through an Eyela PFR-1000 freezer equipment(Tokyo).
1.2 Preparation and thermal-induced isomerization of all-trans lycopene
All-translycopene was purified with the similar procedure as reported[20]:fine red powder(248.2 mg) were recrystallized from 16.67 g of tomato oleoresin.The chromatographic conditions used for the determination of lycopene were as follows:column:YMC C30column(4.6 mm×250 mm,5 μm);mobile phase:methanol-methyl tert-butyl ether(35∶65,by volume);flow rate:1.0 mL/min;temperature:30 ℃;detection wavelength:472 nm.The high-purity all-translycopene obtained(25 mg) was dissolved in 10 mL of chloroform,and refluxed at 70 ℃ for 6 h in oil bath.After the thermal isomerization of lycopene,the residue obtained was dried by nitrogen gas flowing.
1.3 Preparation of lycopene isomers
1.3.1 Solubility testThe residues(2 mg) were ultrasonically dissolved in different proportion of EtOAc-Acetonitrile(ACN) solvent for 5 min,and the supernatant was injected and determined by HPLC.Chromatographic conditions were as follows:column:YMC C30column(4.6 mm×250 mm,5 μm);mobile phase:methanol-methyl tert-butyl ether(35∶65,by volume);flow rate:1.0 mL/min;temperature:30 ℃,detection wavelength:472 nm.
1.3.2 Separation of lycopene isomers by C18column at different temperatureThe residues were ultrasonically dissolved in chloroform at a concentration of 1 mg/mL.After centrifuging,the supernatant was filtered through a 0.2 μm PTFE membrane and determined by HPLC instrument under the following conditions:column:Xbridge BHE Shield C18column(4.6 mm×250 mm,5 μm);mobile phase:acetonitrile-ethyl acetate(45∶55-55∶45,by volume);flow rate:1.0 mL/min;temperature:-20 ℃- -40 ℃;detection wavelength:472 nm.




Fig.2 Chromatograms of the separation of lycopene isomers mixture on a Waters Xbridge BHE Shield C18 column mobile phase:A.EtOAc-ACN(45∶55),B.EtOAc- ACN(50∶50),C.EtOAc-ACN(55∶45);temperature in A-C(a-f)∶30,0,-10,-20,-30,-40 ℃; D:a.mobile phase:EtOAc-ACN(45∶55),temperature: -30 ℃;b.mobile phase:EtOAc-ACN(55∶45), temperature:-40 ℃
1.3.3 Preparation of lycopene isomers by L-T C18×C30columnThe residues were ultrasonically dissolved in chloroform at a concentration of 10.0 mg/mL.After centrifuging,the supernatant was filtered through a 0.2 μm PTFE membrane and prepared by HPLC under the following conditions:column:Xbridge BHE Shield C18column(10 mm×250 mm,5 μm);mobile phase:acetonitrile-ethyl acetate(1∶1,by volume);flow rate:2.0 mL/min;temperature:-40 ℃;detection wavelength:472 nm.Three fractions of C1at a retention time of(16-52 min),C2(58-91 min),and C3(100-166 min) were collected,which was further purified by a YMC C30column(10 mm×250 mm,5 μm) under the following conditions:mobile phase:methanol-methyl tert-butyl ether(35∶65,by volume);flow rate:3.0 mL/min;temperature:(25±1)℃;detection wavelength:472 nm.
1.4Isomerizationanddegradationoflycopeneinedibleoils
The edible oils used in this study were walnuts,black seed,almonds,grape seed,pumpkin seed,and peach kernel oil.The prepared all-translycopene(2 mg) were dissolved in 500 μL of oils and heated at 120 ℃ with the protection of nitrogen gas for 5 h.During the thermal treatment,10 μL of sample was transferred into a small vial,and diluted with 1 mL of chloroform for the further HPLC analysis.
Seven kinds of lycopene monomers were obtained after the two-step separation of L-T C18×C30column,which blow-dried with nitrogen at -20 ℃.Each monomer was dissolved in 100 μL of walnut oil,and stored at 4 ℃ in refrigerator for HPLC analysis within seven days.
The conditions for HPLC analysis applied were as follows:column:YMC C30column(4.6 mm×250 mm,5 μm);mobile phase:methanol-methyl tert-butyl ether(35∶65,by volume);flow rate:1.0 mL/min;temperature:30 ℃;detection wavelength:472 nm.
2 Results and discussion
2.1InfluenceoftemperatureonselectivityoflycopeneonL-TC18column
Fig.2 showed the separation of lycopene isomers on C18column at the temperature ranging from 30 ℃ to -40 ℃.Lycopene isomers failed to be separated when the temperature was higher than 0 ℃,but good resolution began to appear as the temperature decreased to -20 ℃.Moreover,the resolution generally increased with the decrease of temperature.However,the resolution of lycopene would not be dependent on the temperature as decreasing to a certain degree,which was confirmed by the similar resolution of lycopene shown in
Fig.2D.The retention time of lycopene in two methods was almost the same by controlling the low temperature and mobile phase at the same time.In
Table 1,the intervals(δt) of the two main peaks on L-T C18column were presented to further confirm this conclusion.This could be interrupted by the fact that high ordering of the bonded solid phase was formed when the temperature decreased to a certain level(for example,-30 ℃ here)[17].Continuous decrease of the temperature may cause higher back pressure,stronger mass transfer resistance and lower solubility.Hence,the column temperature of C18column should be controlled at -30--40 ℃,while the content of EtOAc in mobile phase was ranging from 45% to 55%.

Table 1 Retention of the two main peaks of lycopene isomers on L-T C18 column
2.2 Solubility test of the mixture of lycopene isomers
An appropriate injection solvent was very important for the separation procedure by chromatography,which was closely correlated with the sample solubility and solvent effect.As shown in
Fig.3A,the color of solution gradually turns to reder with the increase of content of EtOAc.Obviously,the solubility of lycopene in EtOAc was higher than that in ACN.In
Fig.3B,the peak area of all the lycopene isomers(Peak 1 to 6) increased with the increase of EtOAc content in mobile phase.However,it is worth noting that the increasing rate was different,which suggests that lycopene mixture could be priliminarly separated by the solvent discrimination.Fig.3C presented the relative solubility of lycopene isomers in EtOAc-ACN.As the content of EtOAc increased,peak area of Peak 3,5 and 6(all-translycopene) significantly increased,but slightly waved for Peak 1,2 and 4.Except for all-translycopene,the solubility ofcis-isomers was relatively high when the percentage of EtOAc was 50% in mobile phase.Additionally,EtOAc-ACN(50∶50) was also a suitable choice used as mobile phase for the L-T C18column.Consequently,we hence used it as the appropriate solvent to enrichcis-isomers from the mixture.No precipitation occurred when cooling the saturated solution of lycopene in it at -40 ℃.Considering the compatibility between solvents and sample solubility,EtOAc-ACN(50∶50) was used as the injection solvent and mobile phase at the same time,while the temperature was kept at -40 ℃.
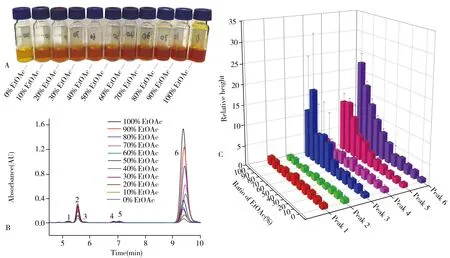
Fig.3 Solubility test results of lycopene isomers mixture in EtOAc-ACN A.color of the solution of lycopene;B.analytical chromatograms of lycopene on C30 column;C.relative contents of lycopene isomers in solution prepared with different proportions of EtOAc-ACN(the peak area of lycopene isomers in the solution prepared with 100% ACN marked inFig.2B was defined as 1)
2.3 Orthogonality analysis of L-T C18 column×C30 column
It is still impossible to separate all lycopene isomers on L-T C18column.To solve this problem,an orthogonal method using L-T C18and C30columns was constructed to obtain lycopene isomers with high purity,while the orthogonality was analyzed in
Fig.4.Three components(C1,C2,C3) collected from L-T C18column could be baseline separated by C30column to prepare seven types of lycopene isomers.Mutual interferences of Peak 3-2,Peak 1-1 and Peak 1-2 on C30column were well removed.For actual separation,we consolidate C1and C2together,because Peak 2 in C2did not create any interference around Peak 1-1(3-1),3-2,and 1-2 on C30column.Furthermore,due to the excellent resolution,the loading capacity of L-T C18column and C30column for lycopene isomers remarkably improved after the orthogonal separation was constructed.Fig.5A and
Fig.5B separately demonstrate the separation effects of lycopene isomers on L-T C18and C30column with different loading capacity.On C30column,peaks began to merge as loading volume of the saturated solution of lycopene reaches to 40 μL.However,the separation effect of lycopene isomers remains satisfactory on L-T C18column as the loading volume increased to 400 μL.When L-T C18column was adopted as the first dimensional(1D) separation,the collected fraction could be well separated on C30column.Moreover,the solubility of lycopene in EtOAc-ACN was higher than that in MTBE-methanol,which meant that L-T C18column was more appropriate to be used for 1D separation.
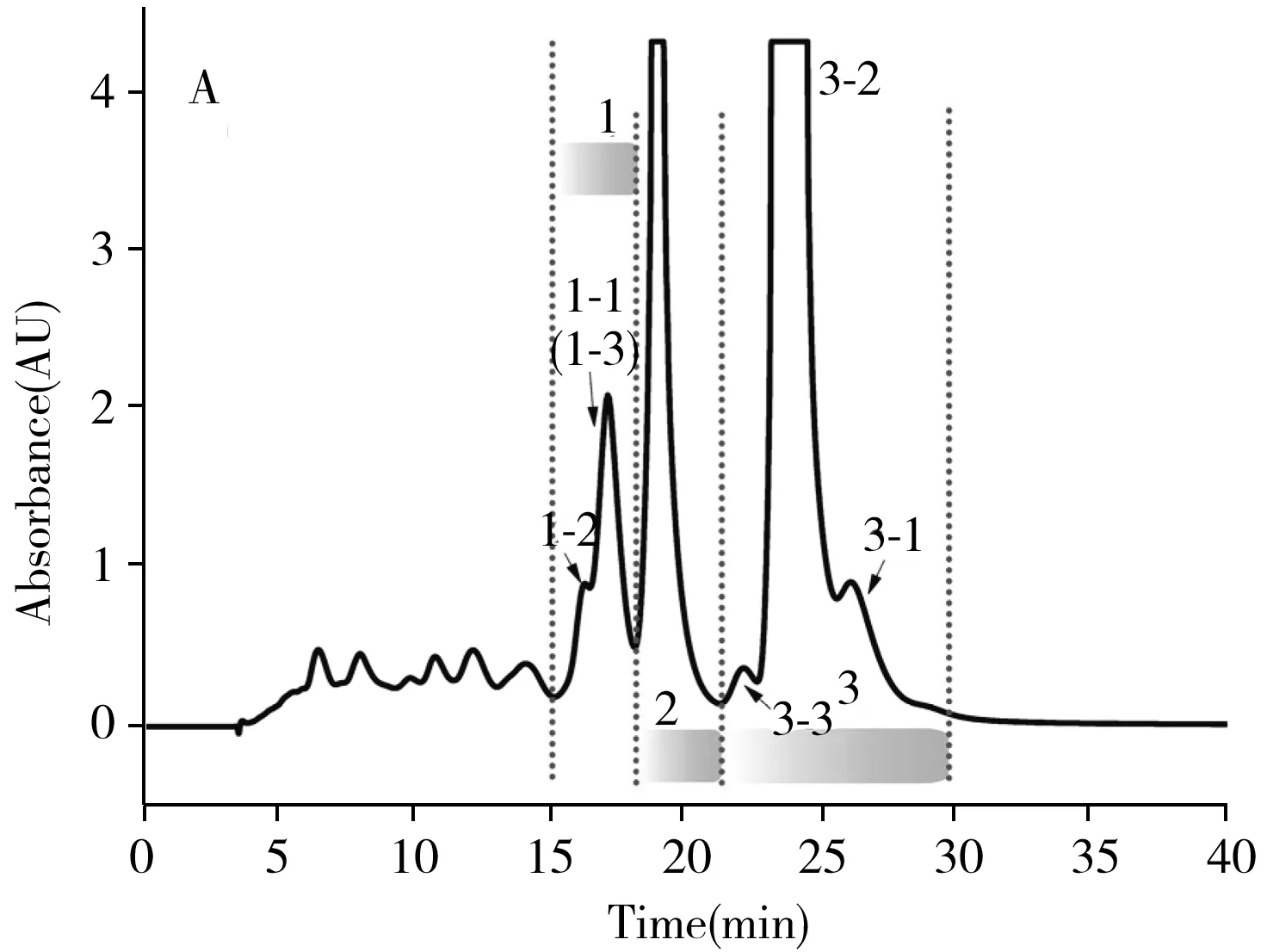
2.4 Low temperature preparation of lycopene isomers

Fig.6 Stability of seven lycopene isomers in three fractions (C1,C2,C3) collected from L-T C18 column at room temperature(25±1 ℃)
2.4.1 Stability test of lycopene in mobile phaseAs demonstrated in
Fig.6,the content of various lycopene isomers in the collected fraction from L-T C18column vigorously waved over time at(25 ±1) ℃,perhaps due to the mutal isomerization between isomers.However,most of them were stable within 1 h even at room temperature of(25±1) ℃.Therefore,to maintain the content of lycopene from the 1D separation,the collected fractions was fast concentrated.Interestingly,the isomerization rate of lycopene isomers with high purity began to slow down as the temperature decreased to -20 ℃.To maintain the purity,the prepared lycopene from the 2D separation was dried by N2blowing under low temperature(-20 ℃).
2.4.2 Preparation of lycopene isomers by L-T C18×C30columnBy using the term of L-T C18×C30column,seven types of lycopene isomers were separated and L-T dried,and then identified by UV-Vis spectrum based on the value ofλmaxand the ratio ofλmax/λ360 nm[18].The purities of all the obtained isomers(>97.0%) were listed in
Table 2,which were much higher than those prepared only with C30column,indicating that the term of L-T C18×C30column was highly efficient to obtain high-purity isomers of lycopene.

Table 2 Comparison of the purity of lycopene isomers prepared by two methods
2.5 Study of isomerization and degradation of lycopene in edible oils
The study of isomerization of lycopene in edible oils provides the basic for the resource utilization of lycopene.Table 3 showed the isomerization of all-translycopene in walnuts,black seed,almonds,grape seed,pumpkin seed and peach kernel oils.Cis-isomers rapidly generated,but gradually depredated with heating over time.The contents ofcis-isomers heating-isomerized from all-translycopene was listed in
Table 3 and
Table 4.The highest content ofcis-isomer was found after heating all-translycopene in grape seed oil at 0.5 h.The result suggests that grape seed oil have the greatest potential for developing active pharmaceutical ingredient(API) of lycopene due to the high content and short heating time,which are very important in the industrial application.In constrast,black seed oil and peach kernel oil were less valuable due to the long heating time and low conversion ratio tocis-isomers.
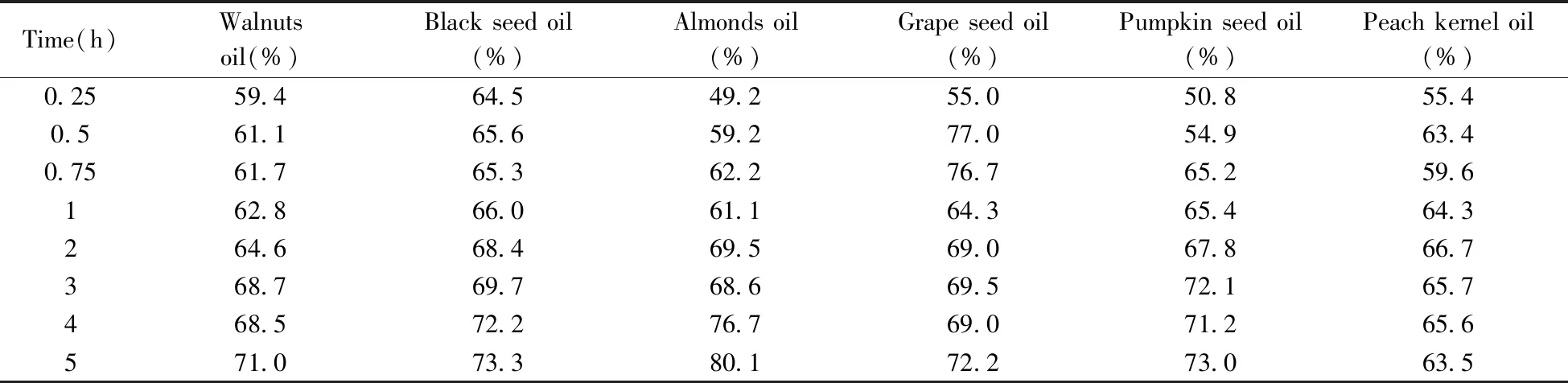
Table 3 Proportion of cis-isomers by heating all-trans lycopene in edible oils

Table 4 Content of cis-isomers of lycopene with heating time in edible oils
As discrible above,lycopene was more stable at L-T condition,which could be adopted for the development of storage technique.Hence,the stability of lycopene isomers in edible oils at L-T condition was investigated.Table 5 showed that no remarkable isomerization and degradation of all isomers of lycopene was found within 1 day.However,when the storage time comes to the fourth day,6.5%-32.3% of lycopene had been degradated.New technique like anaerobic operation,or preparation technology should be further developed to hinder this degradation if lycopene products in edible oils was promoted.The turning of color of lycopene from red to yellow in the continuous storage also confirmed the degradation perhaps from the oxidation.Interconversion between these isomers occurs with the increase of storage time,as listed in
Table 6.(13Z)-lycopene was the most stablecis-isomer in edible oil,which was worth to pay more attention during the heating isomerization proedure.These results were expected to provide beneficial information for producing API based on lycopene.
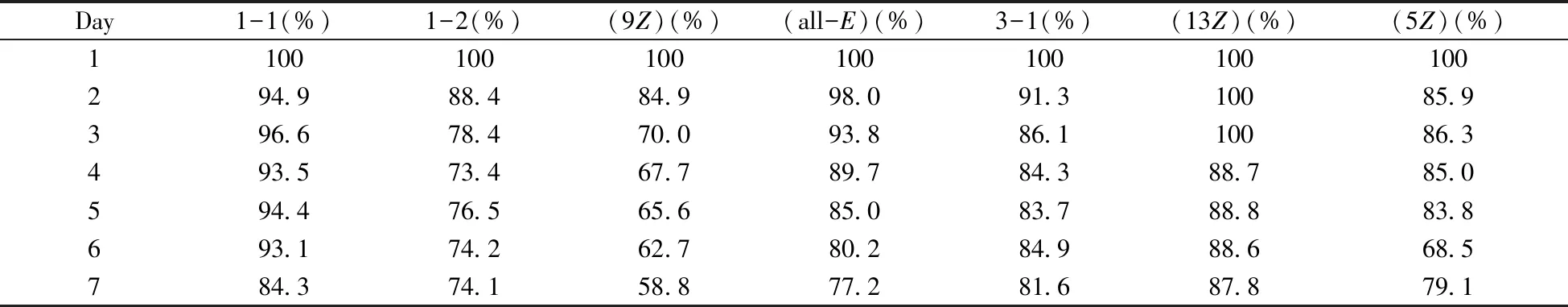
Table 5 Stability of seven kinds of lycopene isomers in walnut oil at 4 ℃
3 Conclusions
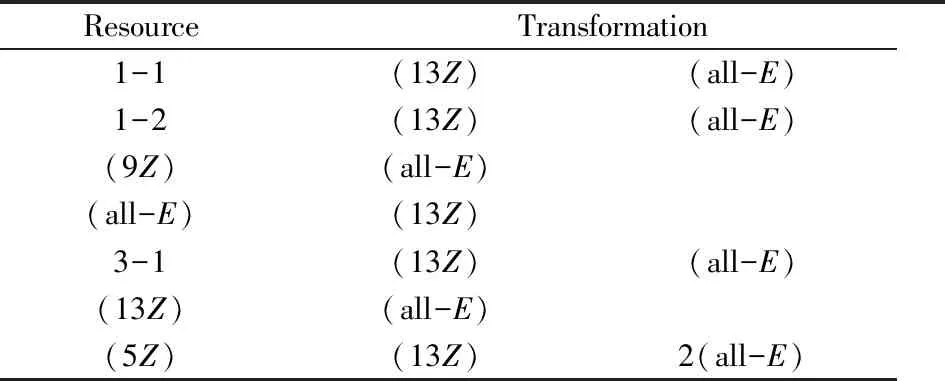
Table 6 Transformation of lycopene isomers in walnut oil at 4 ℃
In this study,L-T preparation technique was developed to separate lycopene isomers.By using the term of L-T C18and C30columns,seven kinds of isomers with high purity were prepared to study their isomerization and stability in edible oils.Critically for the separation perspective,the performance of a common C18column for lycopene isomers was significantly improved with the aid of low temperature.Moreover,considerable loading capacity of lycopene mixture could be achieved by combining the L-T C18and C30columns.It is reasonable for us to believe that the term of L-T C18× C30column have the potential in the separation of other carotenoids.In addition,the isomeric results determined from high-purity isomers accurately revealed their interconversion and degradation procedure in various oils,which was expected to provide beneficial information for industrial application of lycopene.
