Can gadoxetic acid–enhanced magnetic resonance imaging be used to avoid liver biopsy in patients with nonalcoholic fatty liver disease?
2021-01-13VivianeBrandAmorimDaniellaBrazParenteFernandoFernandesPaivaJaimeArajoOliveiraNetoAmandaAlmeidaMirandaCludiaCravoMoreiraFlviaFerreiraFernandesCarlosFredericoFerreiraCamposNathalieCarvalhoLeiteRenatadeMelloPerezRosanaSou
Viviane Brandão Amorim, Daniella Braz Parente, Fernando Fernandes Paiva,Jaime Araújo Oliveira Neto, Amanda Almeida Miranda, Cláudia Cravo Moreira, Flávia Ferreira Fernandes,Carlos Frederico Ferreira Campos, Nathalie Carvalho Leite, Renata de Mello Perez, Rosana Souza Rodrigues
Viviane Brandão Amorim, Daniella Braz Parente, Jaime Araújo Oliveira Neto, Renata de Mello Perez, Rosana Souza Rodrigues, Research Department, D’Or Institute for Research and Education, Rio de Janeiro 22281, Brazil
Viviane Brandão Amorim, Radiology Department, Brazilian National Cancer Institute, Rio de Janeiro 20230-130, Brazil
Viviane Brandão Amorim, Radiology Department, Fleury Group S.A., Rio de Janeiro 20765-000, Brazil
Daniella Braz Parente, Rosana Souza Rodrigues, Radiology Department, Hospital Universitário Clementino Fraga Filho, Federal University of Rio de Janeiro, Rio de Janeiro 21941-590, Brazil
Fernando Fernandes Paiva, São Carlos Institute of Physics, University of São Paulo, São Carlos 13560-970, Brazil
Jaime Araújo Oliveira Neto, Radiology Department, Quinta D'Or Hospital, Rio de Janeiro 20941-150, Brazil
Amanda Almeida Miranda, Radiology Department, Centro de Diagnóstico Médico do Maranhão, Maranhão 65074-441, Brazil
Cláudia Cravo Moreira, Nathalie Carvalho Leite, Department of Clinical Medicine, Hospital Universitário Clementino Fraga Filho, Federal University of Rio de Janeiro, Rio de Janeiro 21941-590, Brazil
Flávia Ferreira Fernandes, Gastroenterology and Hepatology Department, Hospital Federal de Bonsucesso, Rio de Janeiro 21041-030, Brazil
Carlos Frederico Ferreira Campos, Department of Pathology and Laboratories, University of the State of Rio de Janeiro, Rio de Janeiro 20551-030, Brazil
Renata de Mello Perez, Internal Medicine Department, Hospital Universitário Clementino Fraga Filho, Federal University of Rio de Janeiro, Rio de Janeiro 21941-590, Brazil
Renata de Mello Perez, Gastroenterology Department, Hospital Universitário Pedro Ernesto, University of the State of Rio de Janeiro, Rio de Janeiro 20551-030, Brazil
Abstract
Key Words: Fatty liver; Nonalcoholic fatty liver disease; Nonalcoholic steatohepatitis; Gadoxetic acid
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide, affecting individuals in all age groups and of all ethnicities.It affects 20%-40% of the population in Western countries and 5%-40% of the population in the Asia-Pacific region[1,2].The prevalence of NAFLD is increasing in parallel with the epidemics of overweight, obesity, type 2 diabetes mellitus, and metabolic syndrome[1,3-5].It is characterized by fatty liver infiltration in the absence of significant alcohol consumption or other known liver disease, and encompasses a spectrum of conditions that range from isolated steatosis to nonalcoholic steatohepatitis (NASH), fibrosis, and cirrhosis[1,3,6-8].Isolated steatosis is a reversible state of excessive fat accumulation in hepatocytes 9.NASH progresses to liver fibrosis and cirrhosis in up to 30% of cases within 10 years, and 4%-27% of these patients are at risk of hepatocellular carcinoma and cirrhosis-induced liver failure[1-3].NASH-induced cirrhosis is the second most common indication for liver transplantation in the United States 10, and it will become the most common indication in the near future[4].
The differentiation of relatively benign isolated steatosis from potentially progressive NASH is crucial and has prognostic and therapeutic implications[2,3,5,7,8].The reference standard for differentiation of these two entities is based on liver histopathological findings[2,4,7-9].However, liver biopsy has several limitations, including its invasive nature, with rare but potentially life-threatening complications, poor patient acceptance, sampling error, and intra-and interobserver variability in findings[3,4,7].In addition, liver biopsy is a high-cost procedure, requiring a day hospitalization, a specialist physician to perform the procedure and a pathologist with expertise in hepatobiliary disease for sample analysis.Given the high prevalence of NAFLD, liver biopsy is not a good option for routine clinical practice[10].
Abundant research has been performed to develop noninvasive diagnostic methods for the early detection of NASH and its accurate differentiation from isolated steatosis, due to the utmost clinical importance of this diagnosis.Plasma biomarkers of parenchymal liver disease, alone or combined with clinical parameters, have shown a high degree of accuracy for the differentiation of advanced fibrosis from mild or no fibrosis, but not for NASH diagnosis[2-4].Traditional imaging modalities [i.e., ultrasonography, computed tomography, and magnetic resonance imaging (MRI)] are routinely used to detect and stage diffuse liver disorders based on morphological features.These methods can be used to detect and quantify liver steatosis.However, they cannot differentiate NASH from isolated steatosis[2-5].Magnetic resonance elastography has shown promise for the detection of liver fibrosis, but it is not sensitive for the identification of steatohepatitis without fibrosis[4,8].In addition, MR elastography has limited availability due to the need for specialized equipment[11].
The introduction of gadoxetic acid (GA), a paramagnetic hepatobiliary MRI contrast agent, has expanded the role of MRI, particularly for functional imaging of chronic liver diseases such as NAFLD[3,9,12].GA is taken up by hepatocytes and excreted into the biliary system by hepatocyte membrane transporters[13].The expression of membrane transporters determines enhancement in the hepatobiliary phase[9,12].Two proof of concept studies yielded favorable results in terms of relative liver enhancement after GA administration for the differentiation of isolated steatosis from NASH[7,9].However, these studies were retrospective, and further validation with prospective studies is required.The purpose of this study was to evaluate gadoxetic acid–enhanced magnetic resonance imaging (GA-MRI) with analysis of the contrast enhancement index (CEI) based on muscle-corrected liver signal intensity (SI) as a noninvasive method for the diagnosis of NASH in patients with NAFLD, using histopathology as the reference standard.
MATERIALS AND METHODS
This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the local research ethics committees.Written informed consent was obtained from the study participants.
Patients
Patients aged ≥ 18 years with NAFLD diagnosed by liver biopsy were included in this study.The patients underwent MRI within 6 mo of liver biopsy, provided that the clinical conditions observed at the time of biopsy had been maintained.Exclusion criteria were: Secondary cause of chronic liver disease (viral hepatitis, autoimmune liver disorder, Wilson disease, hemochromatosis, alpha-1-antitripsin deficiency), use of drugs that may induce liver steatosis in the past 6 mo, hepatotoxin exposure, history of bariatric surgery, significant alcohol consumption (≥ 10 g alcohol/d in women and ≥ 20 g alcohol/d in men), and GA-MRI contraindication.Data on patients’ age, sex, weight, height, and body mass index (BMI) were collected.
Histological evaluation
All patients underwent subcostal biopsy of the right liver lobe with ultrasonography guidance and a 16-gauge Menghini biopsy needle.Specimens were fixed in 10% formaldehyde solution and embedded in paraffin.The sections were then stained with hematoxylin and eosin, Masson’s trichrome, and Perls’ stains.Only biopsy samples with lengths ≥ 10 mm and six complete portal tracts were included in the analysis.
An expert liver pathologist (C.F.F.C, with 15 years of experience in liver pathology) blinded to the imaging results evaluated the liver biopsy specimens.The extent of steatosis was evaluated semi-quantitatively by assessing the percentage of involvement by steatotic hepatocytes in the liver parenchyma: 0%-33% = mild, 34%-66% = moderate, and > 66% = severe[14].Steatohepatitis was defined and graded according to the NASH Clinical Research Network Scoring System, by assessment of variable degrees of steatosis accompanied by mixed-cell inflammatory infiltrates in the hepatic lobules and ballooning of hepatocytes, with or without apoptosis and necrosis[6].Fibrosis was scored from F0 to F4 (F0, absent; F1, perisinusoidal or portal/periportal fibrosis; F2, zone 3 perisinusoidal fibrosis and periportal fibrosis; F3, bridging fibrosis; and F4, cirrhosis)[6].
MRI protocol
All MRI examinations were performed on a 1.5-T Philips Achieva unit (Philips Healthcare, Best, The Netherlands) with a 16-channel SENSE XL Torso coil.Each patient received a bolus injection of GA (Primovist, 0.025 mmol/kg body weight; Bayer-Schering, Berlin, Germany) into a cubital or antecubital vein at a rate of 1 mL/s, followed by a 20-mL saline flush.A fat-suppressed three-dimensional T1-weighted gradient echo sequence was obtained without enhancement and in the hepatobiliary phase (20 min after contrast administration) with the following parameters: TR/TE = 4.0/2.0 ms, flip angle = 10o, field of view = 38 cm, matrix = 192 × 190, interpolation to 224 × 224, slice thickness = 4.0 mm, overlap = 2.0 mm, and anteroposterior phase direction.A multi-echo two-dimensional gradient echo sequence with a 120-ms TR, 20° flip angle, and 12 multiple echoes of 1.2 ms duration was also obtained.
Image analysis
A radiologist with 10 years of experience in abdominal MRI (V.B.A.) who was blinded to the patients’ clinical history, laboratory data, and histopathological findings measured SI using commercially available software (Horos version 2.0.1; https://www.horosproject.org/).SI was measured in regions of interest (ROIs) in the liver and paravertebral muscles on unenhanced and hepatobiliary-phase images.Four ROIs were drawn in the liver [three in the right lobe (one in segment V) and one in the left lobe, 2.5 cm2each], with avoidance of blood vessels, biliary structures, motion artifacts, and partial volume effects.ROIs (1.0 cm2each) were also drawn in the paravertebral muscles (Figure 1).The ROIs were first defined on post-contrast images and then copied onto the corresponding pre-contrast images using the software’s copy and paste function, to ensure the same ROI positions for CEI calculation.Average pre- and post-contrast values for liver and muscle ROIs were obtained for each subject.
The liver signal intensity ratio (SIR) was calculated as the ratio of the liver SI to the paraspinal muscle SI separately for unenhanced (SIRpre) and hepatobiliary-phase (SIRpost) images.The CEI was calculated as SIRpost/ SIRpre.The Youden index was used to determine the optimal CEI value for NASH prediction, and two alternative cut-off points were established.
The MRQuantif software (available at https://imagemed.univrennes1.fr/en/mrquantif/download.php from the imaging department of the University Hospital of Rennes) was used to detect and quantify hepatic iron overload using a multi-echo two-dimensional gradient echo sequence.
Statistical analysis
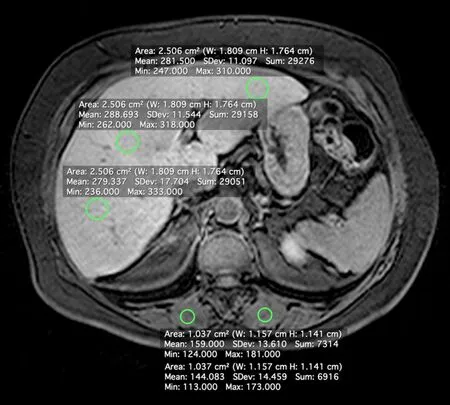
Figure 1 Axial magnetic resonance image showing the positions of regions of interest for the measurement of signal intensity of the liver parenchyma and paravertebral muscles.
Statistical analyses were performed with SPSS software (version 23; SPSS Inc., Chicago, IL, United States).Categorical variables are presented as numbers and percentages.The Shapiro–Wilk test was used to assess the distribution of continuous data.Differences between patients with NASH and those with isolated steatosis were examined using mean values and Student’sttest for normally distributed continuous variables, median values and the Mann–Whitney test for non - normally distributed continuous variables, and the chi-squared test for categorical variables.To evaluate the performance of GA-MRI in differentiating between NASH and isolated steatosis, receiver operating characteristic curve analysis was performed to calculate the area under the curve, sensitivity, and specificity.Cut-off points with 80%-90% sensitivity and specificity were identified.Pvalue < 0.05 were considered to be significant.
RESULTS
Demographic and clinical characteristics
During the study period, 59 consecutive patients diagnosed with NAFLD by histopathology were prospectively invited to undergo MRI.Three patients were excluded due to severe respiratory imaging artifacts.Of the 56 subjects included in the analysis, 18 patients had isolated steatosis (33% male, 67% female; mean age, 57 ± 8 years) and 38 patients had NASH (24% male, 76% female; mean age, 57 ± 9 years).The mean body weight of all patients was 84.2 ± 14.9 kg, and the mean BMI was 32.5 ± 5.1 kg/m2.The prevalences of diabetes, dyslipidemia (elevated triglyceride and decreased high-density lipoprotein cholesterol concentrations), and hypertension were 73%, 86%, and 88%, respectively, with no difference between the NASH and isolated steatosis groups.Sex, age, BMI, and most laboratory values were also similar in the two groups.The mean plasma level of alanine aminotransferase was significantly higher in patients with NASH than in those with isolated steatosis.Baseline characteristics of the overall study population are summarized in Table 1.
Histological characteristics
The biopsies were performed at a mean interval of 2.7 ± 1.5 mo before MRI.The mean length of the biopsy specimens was 17.8 ± 4.1 mm.Among the 18 patients with isolated steatosis, 5.6% had severe steatosis, 22.2% had moderate steatosis, and 72.2% had mild steatosis.No patient with isolated steatosis showed fibrosis.In the NASH group, 23.7% of the 38 patients had severe steatosis, 65.8% had moderate steatosis, and 10.5% had mild steatosis.The proportion of moderate or severe steatosis was higher among patients with NASH (P< 0.001).The distribution of fibrosis in the NASH group was: F4, 7.9%; F3, 10.5%; F2, 5.3%; F1, 65.8%; and F0, 10.5%.The histological characteristics of the liver biopsy samples are summarized in Table 2.

Table 1 Baseline features of the study population and comparison of the isolated steatosis and nonalcoholic steatohepatitis groups
MRI characteristics
The mean CEI in the study population was 1.82 ± 0.19.The mean CEI was significantly lower in the NASH group (1.78 ± 0.16) than in the isolated steatosis group (1.92 ± 0.22;P= 0.008; Figure 2).The area under the receiver operating characteristic curve for the distinction of the two groups was 0.68.The optimal CEI cut-off value was 1.76, which resulted in a specificity of 83% and sensitivity of 47%.The CEI cut-off value of 1.66 showed 94% specificity and that of 2.00 showed 92% sensitivity for the diagnosis of NASH (Table 3).The CEI did not differ according to the steatosis degree, hepatocellular ballooning, lobular inflammation, or fibrosis stage.
The majority (89%) of patients did not present liver iron overload, with an estimated liver iron concentration of 22.8 ± 10.4 µmol/g.The remaining patients (11%) had mild iron overload, with no significant difference between the isolated steatosis and NASH groups.
DISCUSSION
NAFLD is a major cause of liver disease worldwide (1) NASH is the most severe form of NAFLD and is the leading cause of end-stage liver disease in many countries; (2) The diagnosis of NASH is based on liver biopsy.Given its high prevalence (affecting about 1/3 of the global population) and the potential for cirrhosis, noninvasive methods for the early diagnosis of NASH are needed.In this study, we demonstrated that GA-MRI could be useful for the evaluation of patients with NAFLD, as it allows stratification according to the probabilities of NASH and isolated steatosis, thereby avoiding liver biopsy in many patients.
We found that the CEI was significantly lower in patients with NASH than in those with isolated steatosis, which facilitates differentiation between these entities.A main factor that may explain this difference is the expression of liver cell transporters.Multidrug resistance protein (MRP) transporters are responsible for GA excretion into the biliary system and sinusoidal backflow[3,15].Some rodent studies have shown greater MRP transporter excretion expression in NASH models compared with steatosis models[16,17].Other rodent studies have demonstrated the ability to differentiate isolated steatosis from NASH by comparing liver SI on images obtained by GA-MRI[18,19].We believe that expression of the MRP transporter is also increased in patients with NASH, causing an increase in GA excretion by hepatocytes with a consequent decrease in hepatobiliary-phase liver SI relative to that of patients with isolated steatosis, reflected by the difference in the CEI between these groups.
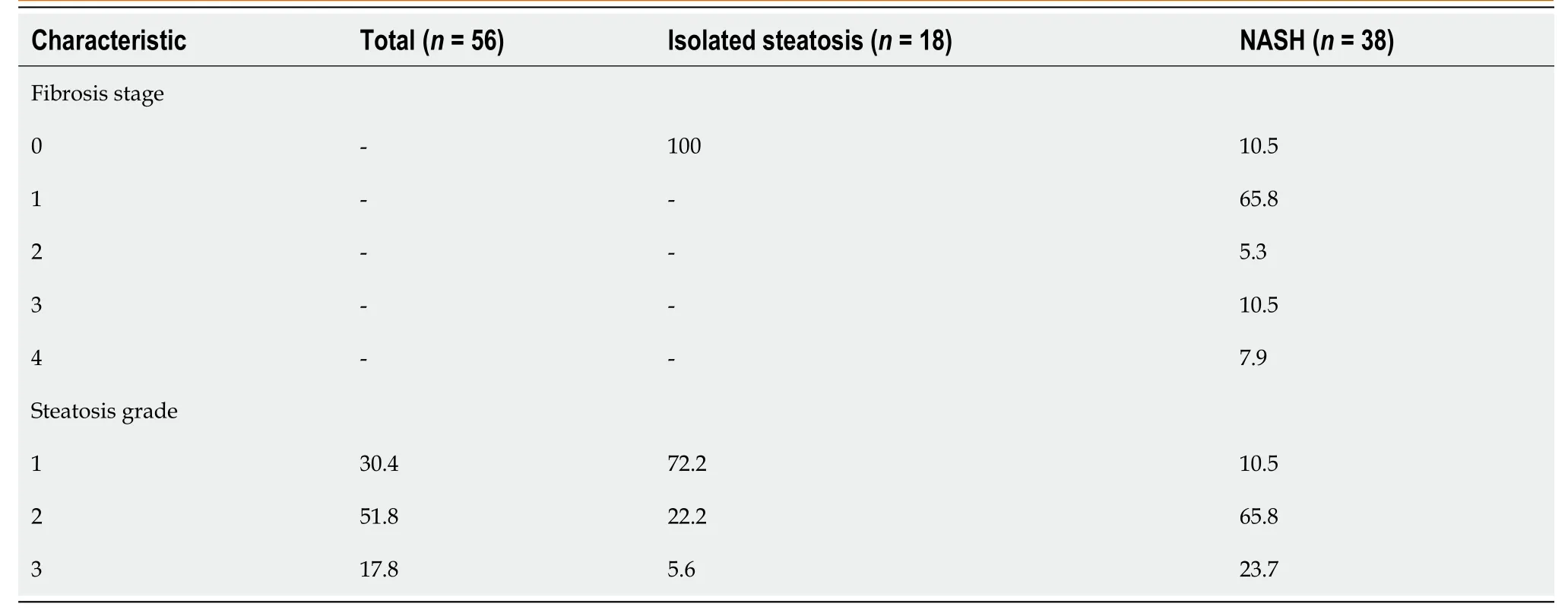
Table 2 Histological characteristics by study group

Table 3 Differentiation of nonalcoholic steatohepatitis from isolated steatosis using different contrast enhancement index cut-offs
Although the CEI differed significantly between the study groups, the cut-off point identified by the Youden index showed low sensitivity, despite good specificity.Therefore, we suggest two more extreme CEI cut-offs, one with greater sensitivity and the other with greater specificity, which appear be more clinically useful for the identification of patients with greater probability of having NASH or isolated steatosis.The combined use of these cut-offs can avoid liver biopsy in 40% of patients who undergo MRI, as it enables the identification of patients who should undergo more aggressive treatment (i.e., those with steatohepatitis) and those who could be managed mainly with lifestyle changes (i.e., those with isolated steatosis; Figure 3).Considering the high prevalence of NAFLD in the global population, biopsy avoidance in 40% of cases would be of great benefit, as liver biopsy is a procedure with high cost, risk of complications, sampling error, and inter- and intra-rater variability.In addition, the frequency of patients’ refusal to undergo biopsy exceeds 50% in some centers, and the frequency of physician reluctance is as high as 30%[4].The results of this study suggest that the use of these two extreme CEI cut-off values allows the detection and exclusion of NASH while maintaining high compliance among physicians and patients, as liver biopsy would be indicated only for patients with CEIs falling between the suggested cut-offs.
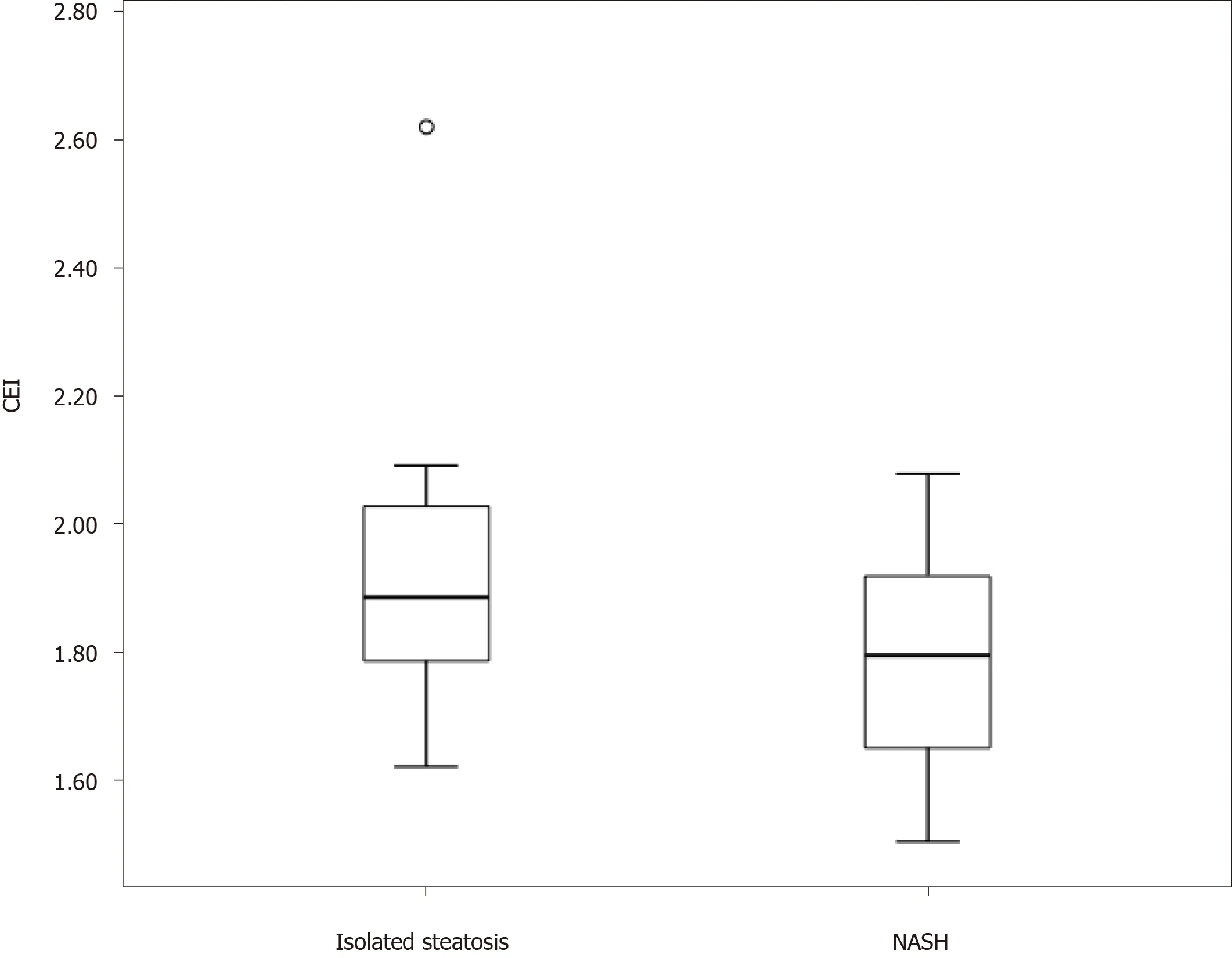
Figure 2 Boxplot of contrast enhancement indices in the isolated steatosis and nonalcoholic steatohepatitis groups.
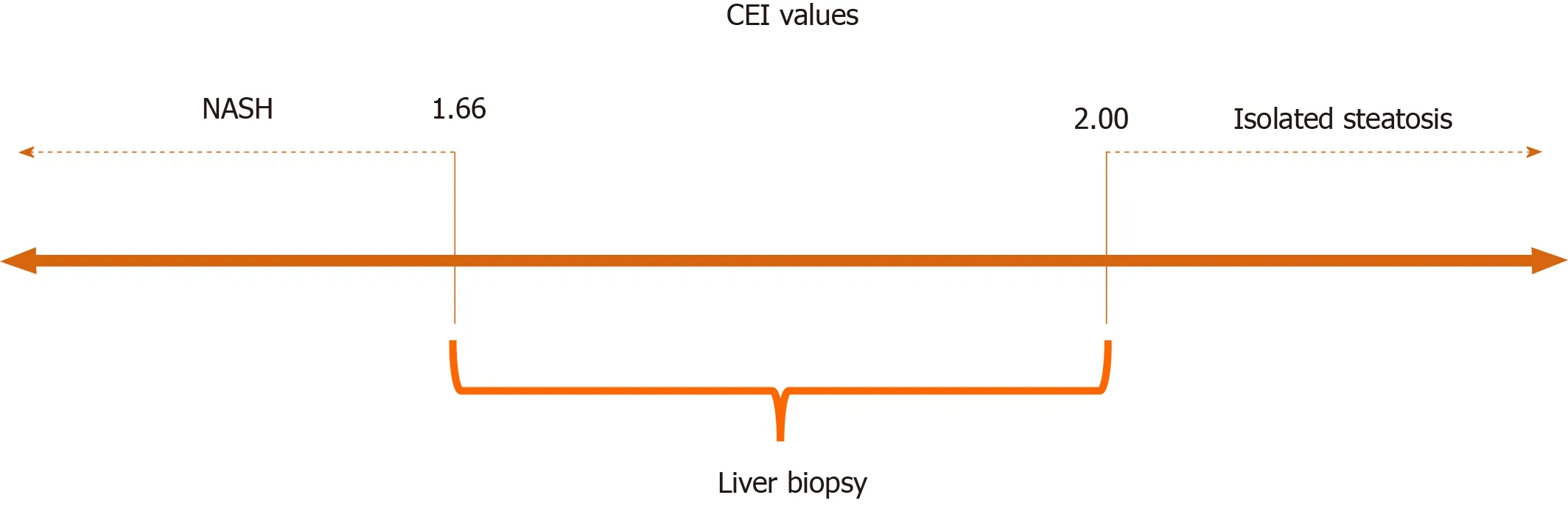
Figure 3 Contrast enhancement index cut-offs values suggested.
Only two human studies have examined the ability to differentiate isolated steatosis from steatohepatitis using the liver SI after GA injection, with promising results[7,9].However, these studies have limitations, including their retrospective designs.In addition, the liver SI measurements were defined by an arbitrary scale, without internal correction, in both studies, which prevents comparison of repeated examinations in the same patient or between different patients.In our study, we used internal muscle correction, which increases reproducibility.In addition, neither of the previous studies involved evaluation of the hepatic iron concentration, which could lead to misclassification.The sample examined in one of the studies was small (25 patients)[9].In the other study, heterogeneity of the groups impaired the analysis, as the isolated steatosis group included patients in whom fibrosis was detected by histopathology[7].In addition, they did not propose cut-off points.
Given their average size, the biopsy samples examined in this study were representative[20-22].CEI values did not differ according to the steatosis degree (P= 0.45), hepatocellular ballooning (P= 0.33), or lobular inflammation (P= 0.84); the proportion of each of these histopathological variables varied greatly in each NAFLD patient, presumably influencing the outcomes of their separate analysis.In addition, the CEI did not differ according to liver fibrosis (P= 0.18), probably due to the low prevalence of fibrosis scores ≥ F2, and especially F4, which was observed in only three patients; in previous studies, the degree of fibrosis has correlated inversely with liver enhancement in patients with chronic liver disease of various etiologies[7,9,12,23,24].As the aim of the present study was to evaluate the ability of the CEI to differentiate simple steatosis from NASH, we consider the inclusion of few cases of advanced fibrosis in our group to be an advantage.
The presence and level of liver iron concentration have been reported to influence liver enhancement by GA in the hepatobiliary phase[25].In our sample, only a few patients presented mild hepatic iron overload (11%), with no significant difference between groups.Liver iron deposition does not appear to have influenced our results.
This work has a few potential limitations.The major limitation is the relatively small sample size.The sample was small due to the strict inclusion and exclusion criteria, especially the selection only of patients who had undergone biopsy.However, our sample was larger than the optimal sample size (n= 51), calculated with a high significance level (0.01) and statistical power (0.99).In addition, most (68%) patients in our sample had NASH, which is not typical for an NAFLD population.This distribution occurred because patients selected for biopsy had more severe NAFLD.We believe that this factor did not interfere with the achievement of our objective, which was to assess the diagnostic ability of the CEI, derived from GA-MRI examination, to differentiate NASH from isolated steatosis, rather than to examine the prevalence of either disease.Similar to the situation in other studies[7-9], the interval between liver biopsy and MRI was longer than ideal in this study.
To our knowledge, this work was the first prospective study to examine the ability to differentiate isolated steatosis from NASH in patients with NAFLD using GA-MRI.In addition to the prospective design, our methodology had other advantages; all histological samples were of sufficient size and were analyzed by a single pathologist with experience in hepatobiliary diseases, and all patients underwent MRI with the same device and examination protocol, avoiding bias due to differences in histological analysis, examination protocol, and MRI field strength.Furthermore, we used liver SI values corrected by paravertebral muscle SI to increase reproducibility and allow comparison of examinations in the same patient and between patients.In addition, we used a simple method that is highly reproducible and broadly accessible for all patients undergoing GA-MRI.Also, this is the first study that proposed cut-off points of CEI to identify and exclude NASH, easily performed by radiologists.
Whereas patients with NAFLD usually undergo imaging examinations for overall assessment of the hepatic parenchyma, the possibility of using GA-MRI as a noninvasive and comprehensive diagnostic modality holds great promise.Furthermore, the MRI scan has lower risk of complications, is cheaper, easier to perform and more widely available when compared to liver biopsy.Although the costs of medical procedures are highly variable in different countries, MRI scan seems to result in a more favorable cost-benefit ratio considering all the complexity of liver biopsy procedure.
In conclusion, patients with NASH have significantly lower CEIs in the hepatobiliary phase of GA-MRI than do patients with isolated steatosis.Thus, this preliminary study suggests that GA-MRI may be an effective noninvasive method for the identification of patients for whom early intervention and more aggressive therapy should be implemented and those with low probability of having NASH, avoiding liver biopsy in up to 40% of the NAFLD population.Liver biopsy, the gold standard method, would still be required for cases in which GA-MRI findings are inconclusive.However, further studies with a larger sample size are warranted to validate the proposed cut-off points.
ARTICLE HIGHLIGHTS
Research background
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide, affecting up to 40% of the world population.It is characterized by fatty liver infiltration, and encompasses a wide clinical spectrum, ranging from a relatively benign isolated steatosis from potentially progressive nonalcoholic steatohepatitis(NASH), liver fibrosis and cirrhosis.
Research motivation
The diagnosis of NASH is crucial and has prognostic and therapeutic implications.Liver biopsy is currently the gold standard for diagnosing progressive NASH and has several limitations, such as sampling error, cost, and risk of complications.
Abundant research has been performed to develop noninvasive diagnostic methods for the early detection of NASH and its accurate differentiation from isolated steatosis,due to the utmost clinical importance of this diagnosis.
Research objectives
To evaluate the performance of gadoxetic acid–enhanced magnetic resonance imaging (GA-MRI) to differentiate NASH in patients with NAFLD using histopathology as the reference standard.
Research methods
In this prospective study, 56 patients with NAFLD (18 with isolated steatosis and 38 with NASH) underwent GA-MRI.Contrast enhancement index (CEI) was calculated as the rate of increase of the liver-to-muscle signal intensity ratio before and 20 min after intravenous GA administration.Between-group differences in mean CEI were tested with the Student'st-test.Area under the receiver operator characteristic curve, and the diagnostic performance of GA-MRI were evaluated.
Research results
The mean CEI for all subjects was 1.82 ± 0.19.The mean CEI was significantly lower in patients with NASH than in those with isolated steatosis (P= 0.008).Two CEI cut-off points were used: < 1.66 (94% specificity) to characterize NASH and > 2.00 (89% sensitivity) to characterize isolated steatosis.CEI values between 1.66 and 2.00 indicated liver biopsy, and the procedure could be avoided in 40% of patients with NAFLD.
Research conclusions
Patients with NASH have significantly lower CEIs in the hepatobiliary phase of GAMRI than do patients with isolated steatosis.This study suggests that GA-MRI may be an effective noninvasive method for the identification of patients for whom early intervention and more aggressive therapy should be implemented, avoiding liver biopsy in up to 40% of the NAFLD population.
Research perspectives
The possibility of using GA-MRI as a noninvasive and comprehensive diagnostic modality holds great promise.As it is a preliminary study, further prospective studies with a larger sample size are warranted.
ACKNOWLEDGEMENTS
The authors thank The Department of Radiology at Quinta D'or Hospital for providing technical support.
杂志排行
World Journal of Hepatology的其它文章
- Clinical implications, diagnosis, and management of diabetes in patients with chronic liver diseases
- Biomarkers for hepatocellular cancer
- Comprehensive review of hepatotoxicity associated with traditional Indian Ayurvedic herbs
- N-acetylcysteine and glycyrrhizin combination: Benefit outcome in a murine model of acetaminopheninduced liver failure
- Transaminitis is an indicator of mortality in patients with COVID-19: A retrospective cohort study
- Clinical efficacy of direct-acting antiviral therapy for recurrent hepatitis C virus infection after liver transplantation in patients with hepatocellular carcinoma
