N-acetylcysteine and glycyrrhizin combination: Benefit outcome in a murine model of acetaminopheninduced liver failure
2021-01-13CharlotteMinsartSandrineRoriveArnaudLemmersEricQuertinmontThierryGustot
Charlotte Minsart,Sandrine Rorive, Arnaud Lemmers, Eric Quertinmont, Thierry Gustot
Charlotte Minsart, Arnaud Lemmers, Eric Quertinmont, Thierry Gustot, Laboratory of Experimental Gastroenterology, Erasme Hospital, Université Libre de Bruxelles, Brussels 1070, Belgium
Sandrine Rorive, Department of Pathology, Erasme Hospital, Université Libre de Bruxelles, Brussels 1070, Belgium
Sandrine Rorive, DIAPATH-Center for Microscopy and Molecular Imaging, Université Libre de Bruxelles, Gosselies 6041, Belgium
Arnaud Lemmers, Thierry Gustot, Department of Gastroenterology, Hepato Pancreatology and Digestive Oncology, Erasme Hospital, Université Libre de Bruxelles, Brussels 1070, Belgium
Thierry Gustot, Inserm Unité 1149, Centre de Recherche sur l’inflammation, Paris 75006, France
Thierry Gustot, UMR S_1149, Université Paris Diderot, Paris 75006, France
Abstract
Key Words: Acetaminophen; Acute liver injury; Glycyrrhizin; N-acetylcysteine; Nacetylcysteine/glycyrrhizin combination; Murine model; High mobility group box 1
INTRODUCTION
Acetaminophen, also known as N-acetyl-p-aminophenol (APAP), is one of the most widely used drugs for its analgesic and antipyretic properties.Although acetaminophen is a safe and effective drug at recommended doses, it can cause hepatotoxicity and acute liver failure in the event of overdose[1,2].The hepatotoxicity of acetaminophen remains the leading cause of acute liver failure in the United States and Europe but the mechanism of hepatotoxicity is still incompletely understood and therapeutic options are limited[3,4].After ingestion, a majority (> 90%) of acetaminophen is metabolized by glucuronidation and sulfation reactions to produce non-toxic metabolites.A small fraction (< 10%), undergoing oxidation, is metabolized by CYP450 isoforms, mainly CYP2E1, to N-acetyl-p-benzoquinone imine (NAPQI), a toxic metabolite.Under normal conditions NAPQI, which binds covalently to cysteine groups on proteins (APAP adducts), is rapidly detoxified by glutathione (GSH)[5].There is strong evidence that depletion of hepatic GSH and the covalent binding of NAPQI to cellular macromolecule contribute to protein modification and mitochondrial dysfunction with ATP depletion, leading to massive centrilobular necrosis[6].
N-acetylcysteine (NAC) is the standard therapy for treatment of APAP overdose.This drug counters acetaminophen toxicity by increasing the detoxification of NAPQI by direct conjugation with GSH or by increasing GSH synthesis[7].In this way, NAC acts to prevent the accumulation of the toxic metabolites of APAP in hepatocytes and thereby prevents hepatocytes necrosis.However, to ensure effective treatment, NAC should be administered within 8-10 h after ingestion of acetaminophen[8,9].Since the symptoms of APAP overdosage are often overlooked, the administration of NAC is often insufficient or ineffective due to its short therapeutic window.In addition, restoration of the GSH store is not sufficient to stop the progression of APAP-induced hepatotoxicity[10-12].Thus, in case of acute liver failure, the only alternative remains liver transplantation, a rare resource associated with significant consequences (longterm immunosuppression, frequent medical follow-up, cost).New therapies are clearly needed.
As a medicinal resource, traditional Chinese herbs have attracted attention as food with health benefits and as herbal medicines.Glycyrrhizin (GL), an aqueous extract of licorice root, is composed of glycyrrhetinic acid and two molecules of glucuronic acid.In patients with chronic hepatitis, it is already commonly used in Japan and has been evaluated in therapeutic trials in Europe[13,14].GL has various pharmacological actions, including anti-inflammatory, anti-viral, antioxidative, anti-liver cancer, immunomodulatory and cardioprotective activities.GL is also known for its hepatoprotective effects[15].The different mechanisms of action of GL are not yet all known.However, GL has been described as an inhibitor of the high mobility group box 1 (HMGB1) protein that binds directly to both HMGB boxes and inhibits its cytokine activities[16].In our previousin vitroexperiments, we observed that APAP induced release of HMGB1 from damaged hepatocytes and we demonstrated the released HMGB1 contributed to the death of neighboring hepatocytes[17].
In the present study, we focused on the effects of GL, NAC, or co-administration of these two drugs in a murine model of APAP hepatotoxicity.The aim was to explore the efficacy of the combination of two drugs that act at different stages of the acetaminophen metabolism process and to evaluate the potential protective role of this combination in acute liver injury induced by APAP overdose.
MATERIALS AND METHODS
Animal model of APAP-induced liver injury and treatments.
Eight-week-old C57BL/6J wild-type female mice were obtained from The Jackson Laboratory (Bar Harbor, ME, United States).Upon arrival, the mice were acclimatized to laboratory conditions (21 °C, humidity 50%) for 1 wk prior to experimentation.Mice were maintained on 12-h light-dark cycle with free access to food and water in accordance with the Guide for the Care and Use of Laboratory Animals.Animal protocols were approved by the local Ethic Committee of the Université Libre de Bruxelles (Protocol Identifiers: 488N).
In all experiments, after 15 h fasting with free access to water, mice received an intraperitoneal injection of APAP at the dose of 500 mg/kg body weight.In some experiments, GL (200 mg/kg), NAC (150 mg/kg) or phosphate-buffered saline, as vehicle, was administered to the animals at various times after APAP injection.Mice were sacrificed at different time points after APAP challenge by cervical dislocation under anesthesia; blood was collected, and the liver was removed.Blood samples were centrifuged at 13523 ×gfor 5 min and supernatants were stored at -20 °C.Upon removal, the biggest lobe of each liver was fixed in 4% formaldehyde and three other lobes were snap-frozen and stored at -80 °C for RNA isolation.For survival experiments, animals were followed for 172 h.Mice were euthanized when they became moribund per the criteria of lack of response to stimuli or lack of righting reflex.
Assessment of hepatotoxicity
Liver injury was determined by measuring plasma levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) but also by histological examination of hematoxylin and eosin (H&E)-stained tissues sections.
ALT, AST and LDH measurements were performed using commercially available kits (Roche/Hitachi, Brussels, Belgium), based on methods recommended by the International Federation of Clinical Chemistry.Briefly, blood was collected by retroorbital puncture and centrifuged in a cold room (13523 ×g; 5 min) before storage at -20 °C for 24 h.ALT and AST concentrations were then measured in plasma samples at 37 °C using a photometric method based on the rate of decrease of NADH, which is directly proportional to the rate of pyruvate formation and therefore to the activity of ALT or AST.The latter, directly proportional to the quantity of ALT or AST present in the cells, is expressed in International Units per liter (IU/L).The reading of the kinetics was conducted during 4 min at 340 nm.LDH concentration was measured by ultraviolet assay.Briefly, lactate dehydrogenase catalyzes the conversion of lactate to pyruvate; NAD is reduced to NADH in the process.The initial rate of the NADH formation is directly proportional to the catalytic LDH activity.It is determined by photometrically measuring the increase in absorbance.
Necrosis score was determined by histological examination of H&E-stained tissue sections.Briefly, the large lobes of the liver, taken after each sacrifice, were fixed in a buffered isotonic solution of pH 7.4 of 4% formaldehyde for at least 24 h.Then, they were cut in half widthwise and placed in cassettes which were placed directly into the formalin.After that, pieces of liver were dehydrated by successive baths of isopropanol and toluene and impregnated with paraffin to form blocks.Sections 5 µm thick were made using the microtome and deposited on glass slides, which were then soaked in gelatinous water.Finally, slides were incubated for at least 30 min in an oven at 35-40 °C before being stained with H&E to reveal the cell structures, respectively the nucleus and the cytoplasm.
H&E-stained slides obtained were then analyzed under an optical microscope in a blinded manner.Centrilobular necrosis following treatment with APAP was scored by a grading system as described previously[18].
Assessment of hepatic GSH
Assessment of hepatic GSH levels was performed using Bioxytech GSH-400 colorimetric assay kit and following the manufacturer’s protocol (OxisResearchTM, Foster City, CA, United States).Briefly, the lobe of the liver was washed with 0.9% NaCl before being blotted on paper and weighed.Then, tissue was homogenized in 5% ice cold metaphosphoric acid and centrifuged at 3000 ×gfor 10 min at 4 °C.Finally, the clear upper aqueous layer was collected for the assay.The enzyme concentration obtained is expressed as nmol of enzyme per milligram of protein using bovine serum as a standard.The protein concentration was evaluated in liver homogenates using Quick Start Bradford Protein Assay (Bio-Rad, Hercules, CA, United States).
Assessment of ratio GSH/the oxidized state (GSSG)
Assessment of the GSH/GSSG ratio was performed using GSH/GSSG Ratio Detection Assay Kit (Fluorometric - Green) and following the manufacturer’s protocol (Abcam, Cambridge, United Kingdom).Briefly, the liver lobe was washed with 0.9% NaCl before being blotted on paper and weighed (20 mg of tissue was required by the protocol).Then, the tissue was homogenized in 5% ice cold metaphosphoric acid and centrifuged at 14000 ×gfor 10 min at 4 °C.The clear upper aqueous layer was collected and sample deproteinization was performed using trichloroacetic acid and sodium bicarbonate.After this step, thiol green indicator reaction mix was added to the deproteinized samples and the fluorescence measurement was performed (Ex/Em = 490/520 nm).In two separate assay reactions, GSH (reduced) was measured directly with a GSH standard and total GSH (GSH + GSSG) was measured by using a GSSG standard.
Enzyme-linked immunosorbent assay
HMGB1 concentrations in the plasma of mice were measured by a sandwich-enzyme immunoassay (IBL International GmbH, Hamburg, Germany) following the manufacturer’s protocol.Briefly, with the wells of the plate being coated with purified anti-HMGB1 antibody, the protein of interest binds specifically to the immobilized antibody during the first incubation (24 h, 37 °C).After the washing step to remove all unbound components of the starting sample, a second peroxidase-labelled antibody was distributed to the wells.After incubation (2 h, room temperature), the enzyme substrate (solution containing TMB and buffer with 0.005 M hydrogen peroxide) was added.The enzyme reaction took place for 30 min and was stopped by addition of a 0.35 M hydrogen sulfate solution.The intensity of the light produced, directly proportional to the amount of HMGB1 present in our sample, was measured using a spectrophotometer (Multiskan Ascent) at a wavelength of 450 nm.Concentration of HMGB1 is expressed as ng/mL.
Immunohistochemical staining of HMGB1 in the liver
Serial sections (5 µm thickness) of formalin-fixed and paraffin-embedded liver were immunostained for HMGB1 (1:1000) by indirect immuno-peroxidase method using Discovery Ventana (Roche Diagnostics GmbH, Mannheim, Germany).
Quantification of HMGB1 in the liver
Immunohistochemical expression of nuclear HMGB1 was quantified as previously described[19,20].The immunostained sections were acquired at 20 × using a Hamamatsu NanoZoomer HT2.0 whole slide scanner (Hamamatsu Photonics, Hamamatsu City, Japan).Finally, semi-quantitative image analysis software (Tissue Map 3.0; Definiens, Munich, Germany) was independently applied to all corresponding digitalized slides.An average of 695146.9 ± 238143.2 nuclei was analyzed per liver and HMGB1 staining intensity, expressed as the labelling index, which represented the percentage of stained pixels in the nuclear area, was quantified.
RNA extraction and RT-qPCR
Frozen liver samples were homogenized in lysis buffer by MagNa Lyser (Roche Diagnostics, Brussels, Belgium).mRNA was extracted by High Pure RNA Tissue kit (Roche Diagnostics).Briefly, the homogenates were first centrifuged (15871 ×g) for 10 min.Chloroform was added to the supernatant recovered and the mixture was centrifuged for 15 min at 4 °C.Ethanol 70% was added before transfer to a column (high pure spin filter tubes) and centrifuged for 30 s at 13000 ×g.DNAse was added to the column and incubated for 15 min at room temperature.Then, three successive washes of the column were performed.Finally, the column was washed with elution buffer to remove all the RNA retained in the filter and recover it in a clean Eppendorf.The mRNA quality/purity of each sample was evaluated before RT-qPCR using the NanoDrop™ 1000 Spectrophotometer (ThermoFisher Scientific, Waltham, MA, United States).We evaluated the concentration of RNA in each sample as well as the ratio 260/280 (to exclude the presence of protein, phenol and other contaminants) and the ratio 260/230 (to exclude the presence of co-purified contaminants).None of the samples used had a ratio less than 1.8.
Retro-transcription of the mRNA into cDNA was performed as follows: 4 µL of oligo-dT primer (0.1 µg/µL; Eurogentec, Liege, Belgium) was joined to 9 µL of H2O containing 1 µg of RNA.This mixture was incubated at 65 °C for 5 min and then cooled on ice.After that, 7 µL of RT mix, consisting of 5 × buffer, deoxyribonucleotide triphosphates (10 mmol/L), porcine RNAse inhibitor (50 U/µL) and reverse transcriptase (20 U/µL), were added.Finally, the mixture was incubated at 42 °C for 1 h, and then at 70 °C for 15 min.
Quantification of cDNA was performed by real time PCR using the LightCycler (Roche Diagnostics).Detection of the amplified product was carried out using a fluorescent probe (TaqMan; Roche) and the relative expression of the gene of interest was calculated against β-actin and GAPDH gene (housekeeping gene) following the Pfaffi method[21].The sequences of primers used are listed in Table 1.
Reagents
GL was purchased from Sigma-Aldrich (Darmstadt, Germany).NAC (Lysomucil®) was provided by Zambon (Brussels, Belgium).Acetaminophen (Paracetamol Fresenius Kabi) was purchased from Fresenius Kabi (Homburg, Germany).
Statistical analysis
Statistical analyses were performed using SPSS Statistics 18.0 (Chicago, IL, United States).Difference testing between groups was performed using the Mann-WhitneyUor Student’sttests, as appropriate.We assessed mice rates of survival using the Kaplan-Meier method and compared survival between groups using the log-rank test.APvalue of < 0.05 was considered statistically significant.
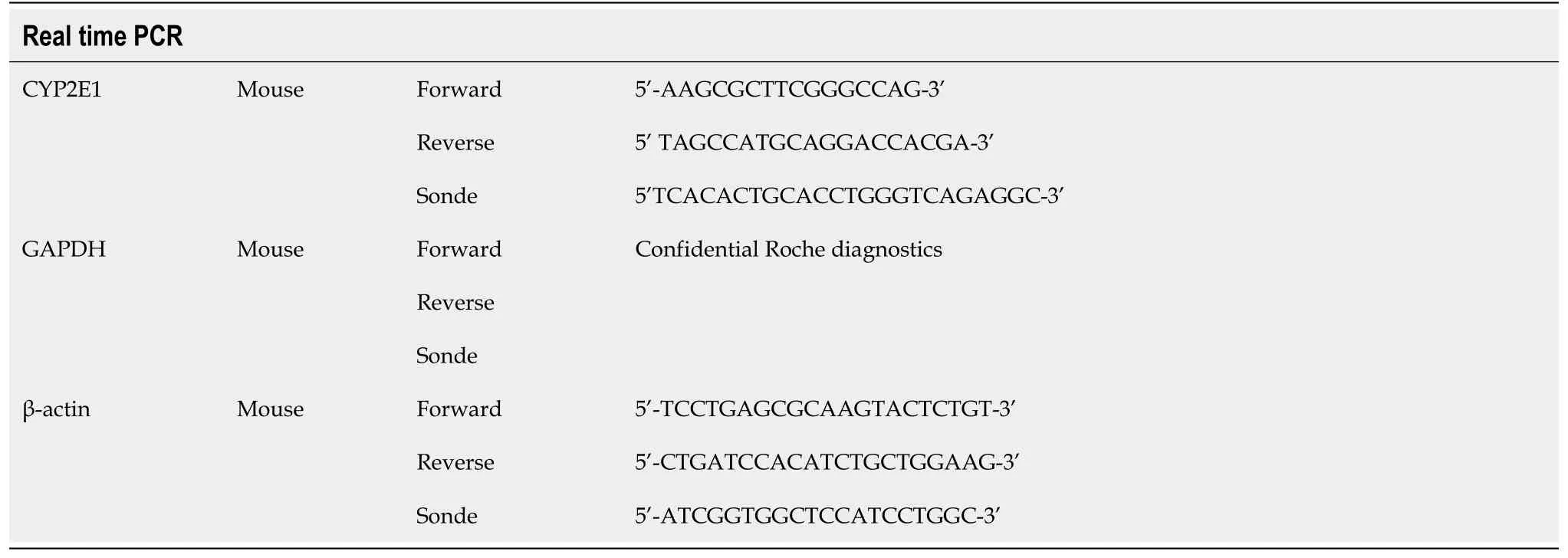
Table 1 List and sequence of primers used for qPCR analysis
RESULTS
Administration of GL, at the same time as APAP, reduced the severity of liver injury
Female C57BL/6J mice treated with overdose of APAP (500 mg/kg) showed evidence of severe hepatic injury as indicated by significantly increased ALT values (Figure 1A), necrosis of centrilobular hepatocytes (Figure 1B and F) and GSH depletion (Figure 1C).Moreover, increased HMGB1 concentrations were observed with a peak 6 h after APAP administration (Figure 1D).Parallel to this phenomenon, a decrease in the nuclear staining of HMGB1 in hepatocytes was observed from 6 h after APAP injection (Figure 1E and F).
Concomitant administration of GL (200 mg/kg) and APAP (500 mg/kg) reduced the severity of the liver injury, as shown by ALT levels (Figure 2A), AST levels (Figure 2B), LDH levels (Figure 2C), and hepatocyte necrosis (Figure 2D and E) in mice sacrificed after 12 hours.In addition, a reduction in HMGB1 concentration was observed (Figure 2F) as well as maintenance of nuclear HMGB1 immunostaining in hepatocytes (Figure 2E and G).
Administration of NAC/GL combination, at the same time as APAP, reduced the severity of liver injury as well as GL or NAC alone
The efficacy of NAC treatment after APAP overdose is well documented in the literature and we have confirmed these results in our murine model.Indeed, after coadministration of NAC (150 mg/kg) and APAP (500 mg/kg), a significant decrease in ALT levels (Figure 3A), AST levels (Figure 3B), LDH levels (Figure 3C), and necrosis score (Figure 3D and E) was observed in mice sacrificed after 12 h.In addition, a reduction in HMGB1 concentration was observed (Figure 2F) as well as the maintenance of nuclear HMGB1 immunostaining in hepatocytes (Figure 2E and G).
Co-administration of NAC/GL and APAP was then investigated in the same murine model (APAP 500 mg/kg; sacrificed after 12 h).As shown in Figure 4, significant decreases in ALT levels (Figure 4A), AST levels (Figure 4B), LDH levels (Figure 4C), and centrilobular hepatocytes necrosis (Figure 4D and E) were observed.In addition, a reduction in HMGB1 concentration was observed (Figure 4F) as well as the maintenance of nuclear HMGB1 immunostaining in hepatocytes (Figure 4E and G).The latter results demonstrated that the NAC/GL combination is as effective as NAC alone when treatment is administered at the same time of APAP.
GSH levels (Figure 5A) and GSH/GSSG ratio (Figure 5B) were also assessed.As expected, administration of GL did not influence GSH levels, while administration of NAC restored GSH stores.When mice were given NAC/GL combination, partial restoration of GSH stores was observed.GSH is known to reduce NAPQI and protect against oxidative damage.As expected, after an overdose of APAP, we observed an increase in oxidative stress resulting in a drastic decrease in the GSH/GSSG ratio.This situation returned to normal after administration of NAC and NAC/GL, as shown by the high ratio, whereas GL offered less protection.
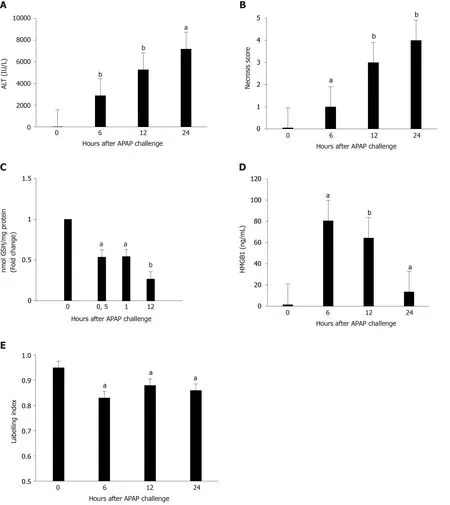

Figure 1 Murine model of acetaminophen-induced liver injury: Hepatotoxicity assessment.
Delayed administration of NAC/GL combination reduced APAP-induced hepatocytes necrosis compare to GL or NAC alone
We explored the effect of delayed administration of GL, NAC or NAC/GL combination under the same conditions (APAP 500 mg/kg; mice sacrificed after 12 h).As shown in Figure 6A and B, all treatments (GL, NAC and NAC/GL) administered 2 h or 6 h after APAP administration, resulted in significant decreases in ALT and AST at similar levels.Regarding the plasma concentration of LDH, NAC and the NAC/GL combination remained effective at 2 h and 6 h, in contrast to GL which no longer showed a protective effect at 6 h after APAP administration.Furthermore, in Figure 6D and E, we observed a decrease in the necrosis score in all treatment groups.It is interesting to note that when treatment was administered 2 h and 6 h after APAP, NAC/GL combination was associated with a lower necrosis score than NAC or GL alone.
Regarding the HMGB1 protein, all treatments (GL, NAC and NAC/GL) given at 2 h or 6 h after APAP administration decreased HMGB1 concentration (Figure 7A).GL and NAC/GL combination continued to maintain the nuclear localization of HMGB1 immunostaining in hepatocytes (Figure 7B and C).
The administration of NAC and NAC/GL was still effective in restoring GSH stores and protecting against oxidative stress, as shown in Figure 8A and B, respectively.On the other hand, delayed administration of GL, 2 h and 6 h after acetaminophen, was no longer effective in restoring GSH stores and protecting against oxidative stress.
Delayed administration of NAC/GL combination increased mice survival following APAP-induced liver injury compared to GL or NAC alone
Survival rates were then analyzed for each treatment using Kaplan-Meier curves.As shown in Figure 9, administration of NAC/GL combination was associated with improved survival rates.Indeed, we observed that when treatment (GL, NAC or NAC/GL) was administered at the same time as APAP, the survival of mice was significantly increased regardless of the treatment.However, if treatment (GL, NAC or NAC/GL) was given 2 h after APAP administration, both NAC and NAC/GL significantly improved survival in mice, but the GL lost its protective effect.
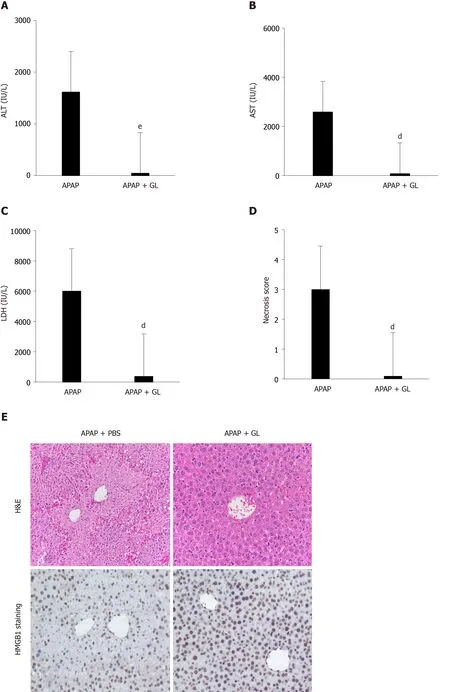
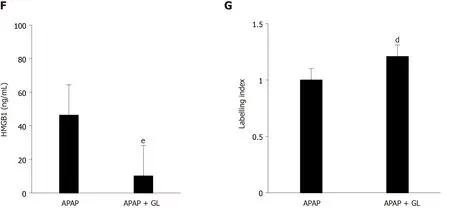
Figure 2 Administration of glycyrrhizin at the same time as acetaminophen, reduced the severity of liver injury.
Thereafter, if treatment (GL, NAC or NAC/GL) was administered 6 h after APAP administration, only the NAC/GL combination showed significant survival efficacy.
Protective effects of the NAC/GL combination do not result from inhibition of hepatic expression of CYP2E1
Yanget al[22]observed a decrease in CYP2E1 mRNA expression after glycyrrhetinic acid administration in a murine model of APAP-induced liver injury; these results suggested the influence of glycyrrhetinic acid, a metabolite of GL, on APAP metabolism.To exclude this possibility in our murine model, RT-qPCR was performed on liver extracts.Decreased expression of CYP2E1 was observed over time in mice after APAP administration, as shown in Figure 10A.However, this decrease is no longer observed in mice treated with GL, NAC or NAC/GL combination.These results, consistent with others[23], demonstrated the lack of inhibition of CYP2E1 mRNA expression by the treatments used in our murine model.
DISCUSSION
Since NAC is less efficient for delayed treatment of acetaminophen-induced liver injury, other therapies need to be explored.One such a drug is GL, the main biologically active component of licorice.This drug has already shown, in other diseases, a variety of pharmacological effect resulting from anti-inflammatory and antioxidant activities.We have previously confirmed the hepatoprotective effect of GL in a murine model of APAP-induced liver injury and these results have been replicated in a human hepatocyte cell line[17].
The aim of this study was to compare the potential efficacy of the NAC/GL combinationvsGL or NAC alone.This study is based on the desire to combine an antioxidant drug that acts on the early phase of acetaminophen toxicity and an antiinflammatory drug that acts on the late phase, after hepatocyte necrosis induced by the accumulation of the acetaminophen toxic metabolite.
We compared these three additional treatments on the liver injury by analysis of biochemical and histopathological parameters.At first, we studied the efficacy of each treatment when administered at the same time of APAP.Regardless of the treatment administered, an improvement in liver injury was observed.Next, we investigated the delayed administration of these three treatments.We observed a similar improvement in ALT, AST and LDH levels.Interestingly, the administration of NAC/GL, 2 h and 6 h after APAP, decreased centrilobular hepatocyte necrosis, in contrast to NAC and GL.Third, we assessed treatment-dependent survival rates.We observed that GL already lost its efficacy when administered 2 h after APAP and NAC when administered 6 h after APAP.It is important to note that the survival benefit was only observed in mice receiving NAC/GL.These observations suggest potential alternative mechanisms for this survival benefit.

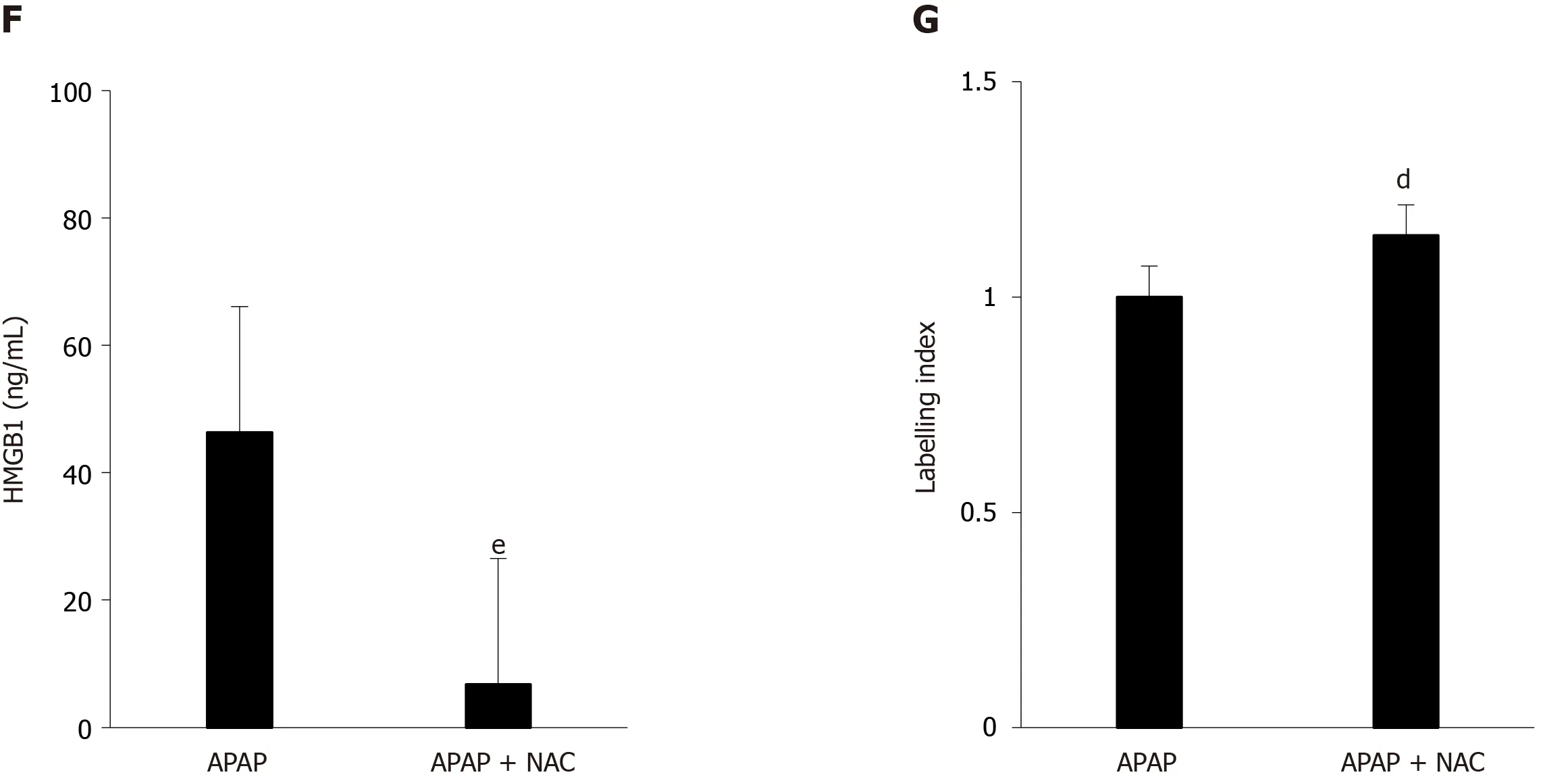
Figure 3 Administration of N-acetylcysteine, at the same time as acetaminophen, reduced the severity of liver injury.
In view of the encouraging results obtained with the NAC/GL combination in the prevention of APAP-induced liver damage, it was important to examine whether the properties of each drug were maintained.Therefore, in each experiment we focused on the HMGB1 protein and the level of GSH.Regardless of when the NAC/GL combination was injected, the concentration of HMGB1 in the plasma of our mice was significantly reduced.In addition, we observed the maintenance of the immunohistochemical staining of the protein in hepatocytes.Knowing the properties of HMGB1 as a “damage associated molecular pattern” protein, these two observations confirm in us the idea of a protective effect.The main mechanism of action of NAC is to promote hepatic GSH synthesis which supports the detoxification of NAPQI and reduces protein binding[24].In our model, a complete recovery of hepatic GSH content was observed when treatment, NAC or NAC/GL, was administered at the same time of APAP.In addition, hepatic GSSG levels are decreased compared to APAP alone, as shown by the GSH/GSSG ratio, suggesting the absence of increasing levels of oxidative stress.When treatment was administered later than APAP, partial recovery of hepatic GSH levels was observed while the GSH/GSSG ratio remained similar.Thus, it appears that NAC loses its efficacy in the synthesis of GSH when administered at later times, when oxidative stress does not appear to be higher.These results need further explorations.
To our knowledge, this combination was not already tested in mice.Xuet al[25]had investigated this association in rats, however.They showed no benefit of NAC/GL combinationvsthe use of NAC alone in the APAP-induced liver injury.However, these results are to be interpreted with caution.Indeed, as described in the literature, rats are defined as resistant to the liver-damaging effects of APAP due to low mitochondrial dysfunction which prevented oxidative stress[26-28].This could explain why this combination works in our murine model.
By browsing the literature, we observed that female mice are described as resistant to acetaminophen.In order to rule out the possibility of impact of sex on the efficacy of the NAC/GL combination on paracetamol-induced liver lesions, we confirmed our results on male mice (Supplementary data).
This study opens a potential new therapeutic pathway in the prevention of acetaminophen hepatotoxicity.
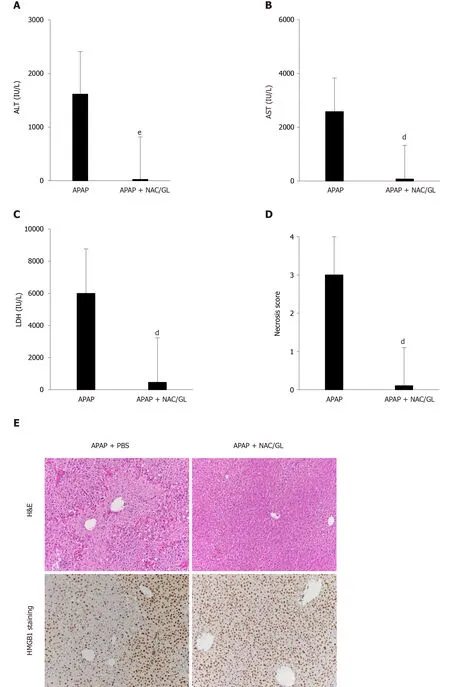
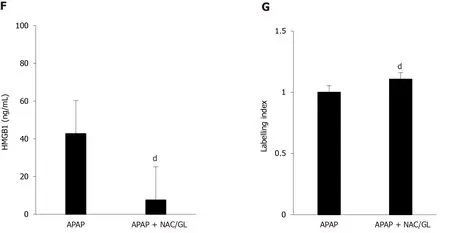
Figure 4 Administration of N-acetylcysteine/glycyrrhizin combination, at the same time as acetaminophen, reduced the severity of liver injury as well as glycyrrhizin or N-acetylcysteine alone.
CONCLUSION
In conclusion, compared to NAC alone, concomitant administration of GL decreased the liver necrosis score and improved the survival during acetaminophen-induced liver injury in mice.These results suggest, for the first time, that the combination of an antioxidant like NAC and an anti-inflammatory drug like GL prevents the liver damage induced by acetaminophen intoxication.
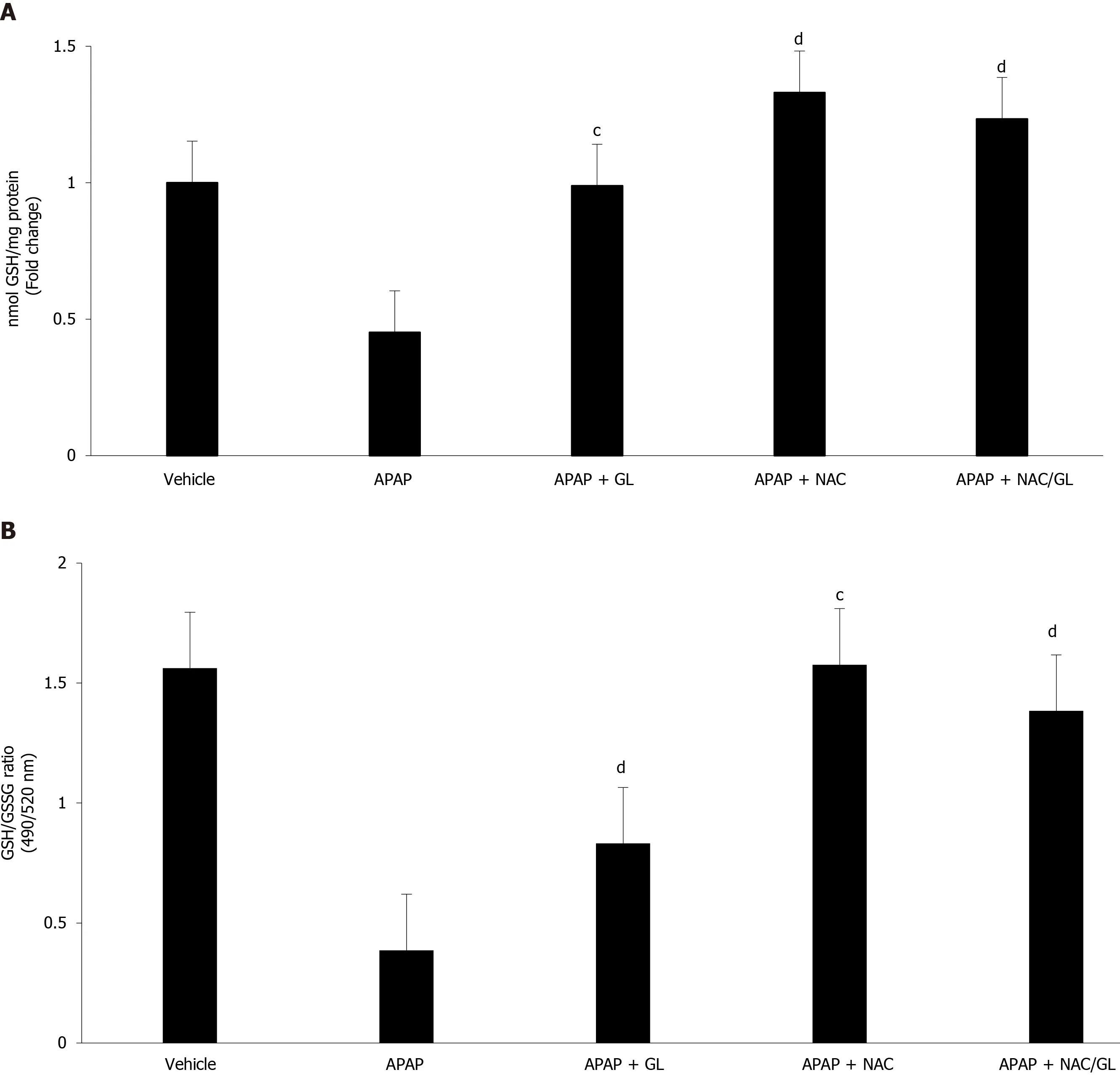
Figure 5 Acetaminophen overdose induced hepatic glutathione depletion and oxidative stress.
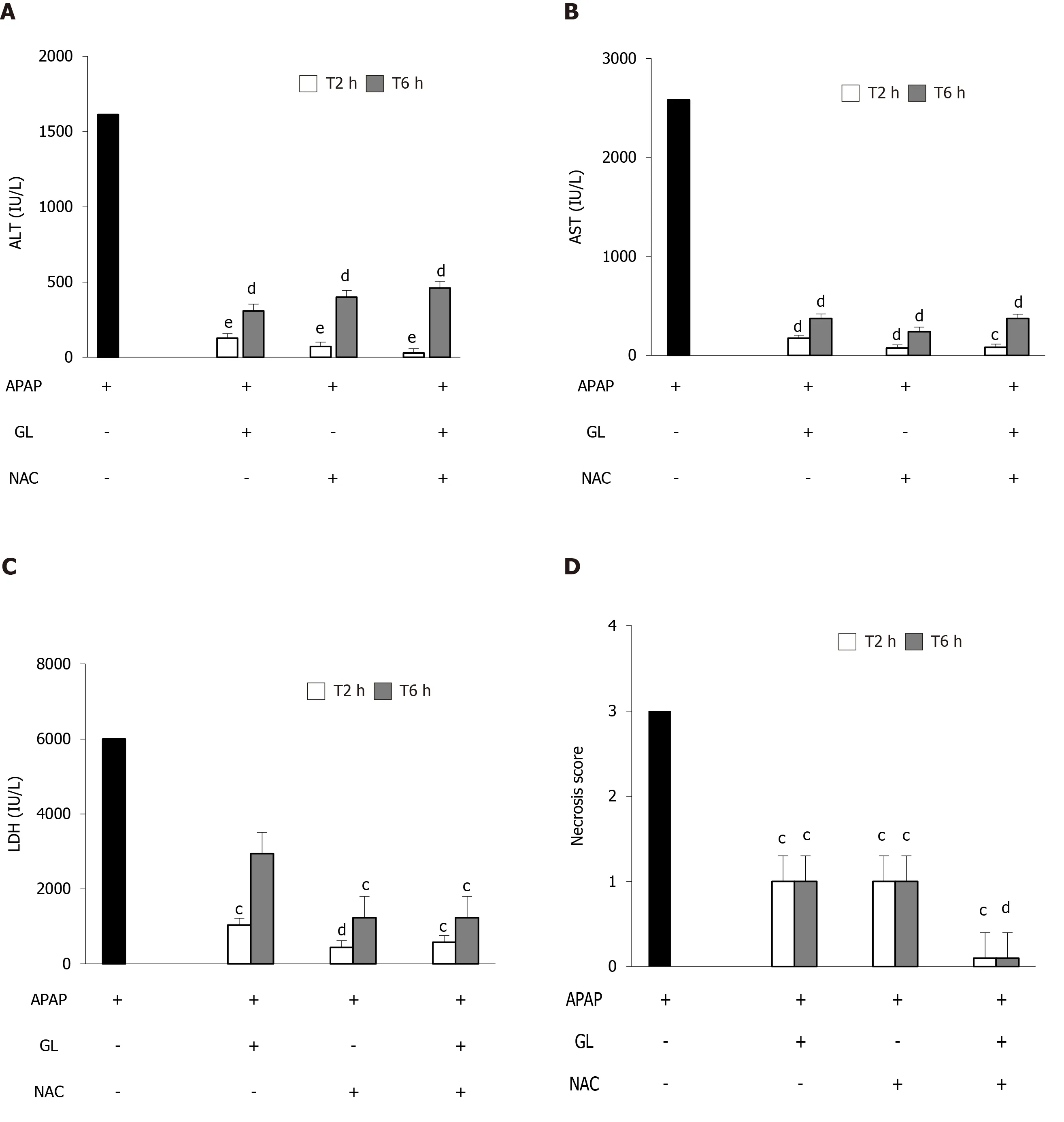

Figure 6 Delayed administration of N-acetylcysteine/glycyrrhizin combination reduced acetaminophen-induced hepatocytes necrosis compare to glycyrrhizin or N-acetylcysteine alone.

Figure 7 Delayed administration of N-acetylcysteine/glycyrrhizin combination reduced high mobility group box 1 release as well as glycyrrhizin alone.
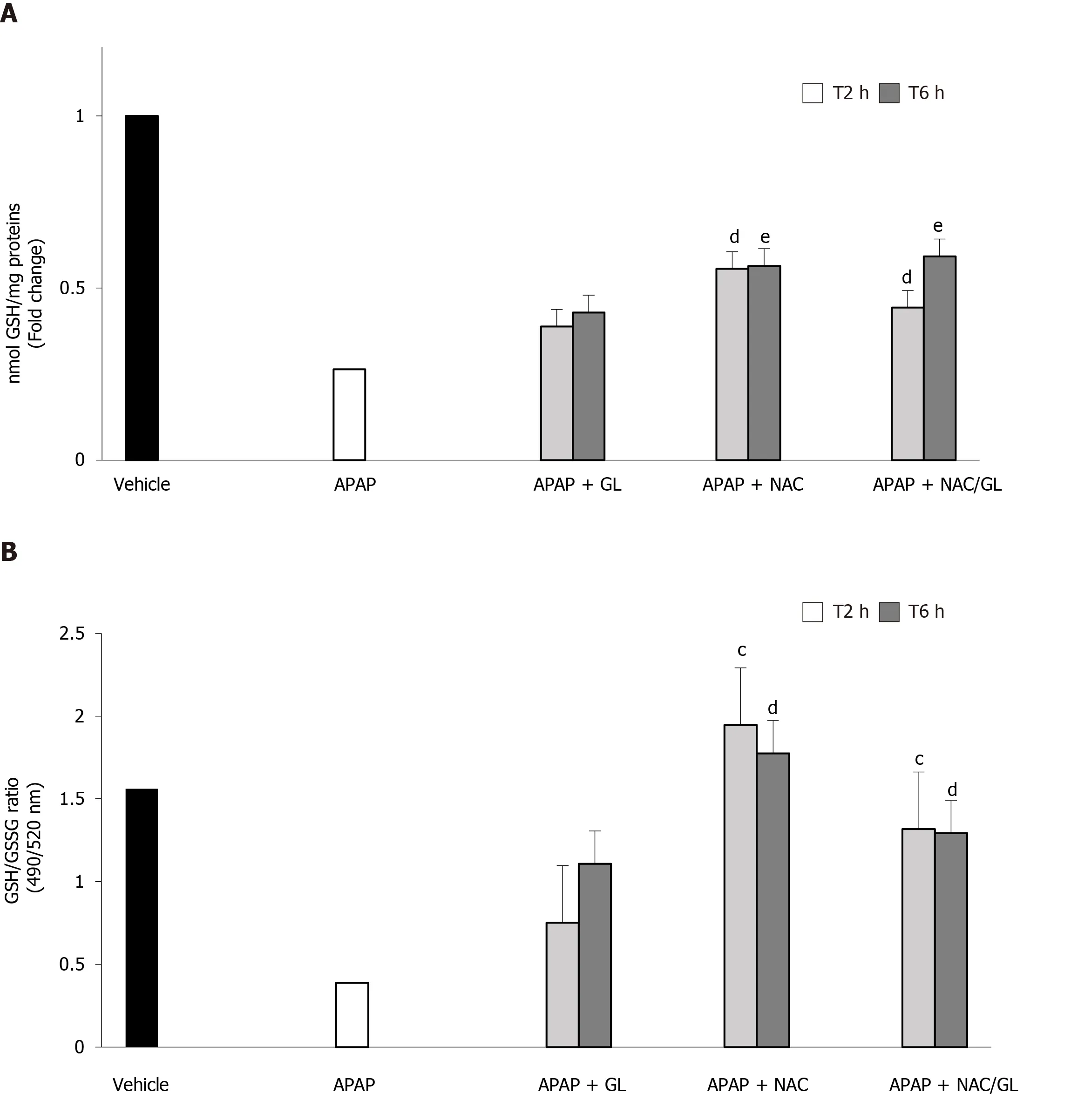
Figure 8 Delayed administration of N-acetylcysteine/glycyrrhizin combination restores partially glutathione stores.

Figure 9 Delayed administration of N-acetylcysteine/glycyrrhizin combination increased mice survival following acetaminophen-induced liver injury compared to glycyrrhizin or N-acetylcysteine alone.

Figure 10 Protective effects of the N-acetylcysteine/glycyrrhizin combination do not result from inhibition of hepatic expression of CYP2E1.
ARTICLE HIGHLIGHTS
Research background
Acetaminophen overdose is the most frequent cause of drug-induced liver failure in the developed countries.Despite substantial progress in the understanding of the mechanism of hepatocellular injury, N-acetylcysteine remains the only effective treatment if administered within 8 h to 10 h of acetaminophen ingestion.Thus, other hepatoprotective drugs are needed for the delayed treatment of acetaminopheninduced hepatotoxicity.
Research motivation
Our interest focused on glycyrrhizin for its role as an inhibitor of high mobility group box 1 protein, a member of the family of damage associated molecular pattern, known to play important pathological roles in different diseases.
Research objectives
The present study aimed to investigate the efficacy of the N-acetylcysteine/glycyrrhizin combination compared to N-acetylcysteine alone in the prevention of liver toxicity.
Research methods
Eight-week-old C57BL/6J wild-type female mice were used for all our experiments.Mice fasted for 15 h were treated with acetaminophen (500 mg/kg) or vehicle (phosphate-buffered saline) by intraperitoneal injection and separated into the following groups: Glycyrrhizin (200 mg/kg); N-acetylcysteine (150 mg/kg); and Nacetylcysteine/glycyrrhizin.Hepatotoxicity was assessed by biochemical and histopathological analyses.Survival rates were also compared.
Research results
In C57BL/6J mice, glycyrrhizin administration was shown to reduce the release of HMGB1 and to significantly decrease the severity of acetaminophen-induced liver injury.Thus, the co-administration of glycyrrhizin and N-acetylcysteine was investigated.Administered concomitantly with acetaminophen, the combination significantly reduced the severity of liver injury.Delayed administration of the combination of drugs, 2 h or 6 h after acetaminophen, also induced a significant decrease in hepatocyte necrosis compared to mice treated with N-acetylcysteine alone.In addition, administration of N-acetylcysteine/glycyrrhizin combination was associated with an improved survival rate compared to mice treated with only Nacetylcysteine.
Research conclusions
Compared to N-acetylcysteine alone, co-administration of glycyrrhizin decreases the liver necrosis score and improves survival in our murine model of acetaminopheninduced liver injury.
Research perspectives
Further experiments are needed to better investigate the efficacy of the Nacetylcysteine/glycyrrhizin combination, but these results suggest for the first time that the combination of an antioxidant like N-acetylcysteine and an anti-inflammatory drug like glycyrrhizin prevents the liver damage induced by acetaminophen intoxication.
ACKNOWLEDGEMENTS
We thank Justine Allard and Mélanie Derock (DIAPATH-Center for Microscopy and Molecular Imaging (CMMI), Gosselies, Belgium) for technical assistance in histological and immunohistological staining.We also thank Christine Decaestecker (DIAPATHCMMI) for technical assistance in immunohistological staining quantification and statistical analysis.We would like to acknowledge the contribution of a medical writer, Sandy Field, PhD, for the English-language editing of this manuscript.
杂志排行
World Journal of Hepatology的其它文章
- Clinical implications, diagnosis, and management of diabetes in patients with chronic liver diseases
- Biomarkers for hepatocellular cancer
- Comprehensive review of hepatotoxicity associated with traditional Indian Ayurvedic herbs
- Transaminitis is an indicator of mortality in patients with COVID-19: A retrospective cohort study
- Clinical efficacy of direct-acting antiviral therapy for recurrent hepatitis C virus infection after liver transplantation in patients with hepatocellular carcinoma
- Surgical treatment of gallbladder cancer: An eight-year experience in a single center
