Clinical implications, diagnosis, and management of diabetes in patients with chronic liver diseases
2021-01-13WaihongChungKittichaiPromratJackWands
Waihong Chung, Kittichai Promrat, Jack Wands
Waihong Chung, Division of Gastroenterology, Department of Medicine, Rhode Island Hospital, Providence, RI 02905, United States
Kittichai Promrat, Division of Gastroenterology and Hepatology, Providence VA Medical Center, Providence, RI 02908, United States
Jack Wands, Liver Research Center, The Warren Alpert Medical School of Brown University, Providence, RI 02903, United States
Abstract Diabetes mellitus (DM) negatively affects the development and progression of chronic liver diseases (CLD) of various etiologies.Concurrent DM and CLD are also associated with worse clinical outcomes with respect to mortality, the occurrence of hepatic decompensation, and the development of hepatocellular carcinoma (HCC).Unfortunately, early diagnosis and optimal treatment of DM can be challenging, due to the lack of established clinical guidelines as well as the medical complexity of this patient population.We conducted an exploratory review of relevant literature to provide an up-to-date review for internists and hepatologists caring for this patient population.We reviewed the epidemiological and pathophysiological associations between DM and CLD, the impact of insulin resistance on the progression and manifestations of CLD, the pathogenesis of hepatogenic diabetes, as well as the practical challenges in diagnosis and monitoring of DM in this patient population.We also reviewed the latest clinical evidence on various pharmacological antihyperglycemic therapies with an emphasis on liver disease-related clinical outcomes.Finally, we proposed an algorithm for managing DM in patients with CLD and discussed the clinical and research questions that remain to be addressed.
Key Words: End stage liver disease; Diabetes mellitus; Liver cirrhosis; Insulin resistance; Non-alcoholic fatty liver disease; Liver diseases
INTRODUCTION
The liver plays a major role in maintaining blood glucose homeostasis, including glycogenesis and lipogenesis under feeding conditions as well as glycogenolysis and gluconeogenesis under fasting conditions.The liver is also the primary site of insulin clearance.Not surprisingly, diabetes mellitus (DM), a metabolic disease characterized by impaired blood glucose regulation and altered insulin sensitivity, is strongly associated with the development, progression, and consequence of chronic liver diseases (CLD), as illustrated in Figure 1.
DIABETES AND CHRONIC LIVER DISEASES
Insulin resistance, occurring in the context of metabolic syndrome, is a wellestablished independent pathophysiological driver for the development of nonalcoholic fatty liver disease (NAFLD)[1].An early study on non-obese patients revealed that fasting insulin level and index of insulin resistance were nearly double in those with NAFLD compared to healthy controls[2].Subsequent cross-sectional studies have shown that as much as 69%-87% of patients with type 2 DM (T2DM) have evidence of NAFLD on imaging or histology[3,4], while 21%-45% of patients with NAFLD were also found to have DM[5].It is generally accepted that the presence of T2DM increases the risk of developing NAFLD by 2-5 folds[6].Indeed the most recent practice guidelines from the American Diabetes Association (ADA) now make specific recommendations regarding screening patients with prediabetes or T2DM for NAFLD[7], representing a step up from prior guidelines published by the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL), which only included a qualified recommendation about screening high-risk patients due to significant gaps in knowledge concerning the treatment of NAFLD at the time[8,9].
The impact of insulin resistance and DM holds true for other etiologies of CLD as well.Insulin resistance, for example, was found to be an independent predictor of fibrosis in non-diabetic patients with chronic hepatitis C virus (HCV) infection[10].Hyperinsulinemia and hyperglycemia have also been associated with the development of more severe fibrosis in patients with chronic HCV infection[11,12].A recent Taiwanese nationwide cohort study has further identified new-onset DM as an independent predictor for cirrhosis and decompensation in patients with chronic hepatitis B virus (HBV) or chronic HCV[13,14].In alcoholic liver disease (ALD), BMI and fasting blood glucose levels have been demonstrated to correlate with fibrosis score after adjusting for daily alcohol intake and the total duration of alcohol use[15].

Figure 1 Impact of diabetes on various stages of chronic liver diseases.N
The relationship between DM and HCV infection is particularly noteworthy because of its possible two-way association.There is epidemiological evidence to suggest that diabetic patients are nearly 3.5 folds more likely to acquire HCV infection[16].Conversely, HCV infection is associated with a 1.5-1.7 fold excess risk of new-onset T2DM development[17,18].Although the exact mechanisms of action remain elusive, it is postulated that the metabolic disturbances associated with DM may favor HCV survival as evidenced by reports that insulin resistance positively correlates with HCV viral load[10].Meanwhile, insulin resistance and DM are also increasingly being recognized as metabolic extrahepatic manifestations of chronic HCV infection.HCV may exert a direct cytopathic effect on pancreatic islets and induce β-cell deathviaa caspase 3-dependent pathway[19,20].In addition, HCV core protein has been shown to promote ubiquitination of insulin receptor substrate (IRS)-1 and -2 in transfected hepatoma cells and a transgenic mouse model[21,22], thereby interrupting hepatic insulin signaling resulting in hepatic insulin resistance.Finally, dipeptidyl peptidase-4 (DPP-4) expression was found to be upregulated by HCV nonstructural protein while interferon therapy led to decrease serum DPP-4 activity in HCV-infected patients[23,24], providing support for another mechanism of HCV-induced insulin resistanceviathe glucagon-like peptide-1 (GLP-1) pathway[25].
DIABETES AND END-STAGE LIVER DISEASES
Once CLD progresses to end-stage liver disease (ESLD), the presence of DM continues to predict more severe diseases and worse clinical outcomes.On the one hand, the prevalence of DM positively correlates with the severity of liver disease based on the Child-Pugh (CP) class or Model for End-Stage Liver Disease (MELD) score[26,27].On the other hand, multiple prospective studies on patients with compensated/ decompensated cirrhosis of various etiologies have consistently demonstrated lower survival, as much as a 40% reduction in 5 years, in the diabetic group compared to the non-diabetic group[28-30].There is evidence that even the presence of subclinical impaired glucose tolerance (IGT) predicts worse short-term and long-term survival in cirrhotic patients[29,31,32].It is noteworthy that the higher mortality in these diabetic patients is due not to the classical DM-related micro-/macrovascular diseases, but complications of liver failure[33].Clinically, baseline DM status was shown to be independently associated with the development of ascites, renal dysfunction, and bacterial infections during long-term follow-up in a cohort of cirrhotic patients with chronic HCV infection[34].DM is also associated with more severe hepatic encephalopathy in cirrhotic patients independent of the severity of liver disease[35].
Most importantly, the incidence of hepatocellular carcinoma (HCC) and HCCspecific mortality are nearly double in patients with preexisting DM based on a metaanalysis of 28 prospective epidemiological studies[36].The risk for HCC is further increased by up to 10 folds when combined with alcohol consumption and/or viral hepatitis[37,38].The impact of DM on the risk for HCC is particularly dramatic in patients with certain single nucleotide polymorphisms in the patatin-like phospholipase domain containing 3 (PNPLA3) gene, which was known to increase the risk for HCC in alcoholic cirrhosis[39].A case-control study of nearly 500 HCC patients revealed an adjusted odds ratio of 19.11 for diabetic patients with the GG genotype compared to that of 2.65 for non-diabetic controls with the same genotype[40].Remarkably, cirrhosis is only present in 50% of patients with NAFLD-related HCC[41], suggesting that the mechanism of hepatocarcinogenesis in the context of metabolic syndrome may be different from the classical mechanism involved in cirrhosis.It is postulated that hyperinsulinemia is central to the pathophysiological effects of DM on HCC development by upregulating growth signaling pathways[42], promoting an inflammatory milieu[43], activating hepatic progenitor cells[44], and stimulating angiogenesis[45].
Regardless of the etiology of underlying CLD, the diagnosis of HCC carries a poor prognosis with median survival measured in the order of months[46].A recent metaanalysis of over 9000 HCC cases concluded that DM status is associated with lower overall/disease-free survival in patients receiving hepatic resection as well as lower overall survival in patients receiving non-surgical treatments[47].This reduction in overall survival may partly be attributable to an increased risk of postoperative complications, including hepatic decompensation, intractable ascites, and intraperitoneal infection, as demonstrated by several regression studies on HCC patients undergoing hepatic resection[48,49].Similarly, there is evidence that diabetic patients undergoing transarterial chemoembolization (TACE) are at an increased risk for hepatic decompensation, liver abscess formation, and prolonged acute renal failure[50,51].
HEPATOGENIC DIABETES
It is worth noting that not only is DM a risk factor for ESLD but it can also be a complication of ESLD.Hepatogenic diabetes is characterized by the development of hyperinsulinemia, insulin resistance, and β-cell dysfunction after the onset of cirrhosis, often in the absence of traditional risk factors, such as a family history of DM or obesity.As illustrated in Figure 2, it is postulated that hyperinsulinemia occurs in cirrhosis as a result of decreased insulin clearance by the damaged liver and increased portosystemic shunting.This notion is supported by evidence that hyperinsulinemia worsens after transjugular intrahepatic portosystemic shunt (TIPS) placement in diabetic patients[52], yet improves with portosystemic shunt occlusion by balloonoccluded retrograde transvenous obliteration (BRTO)[53].There is also evidence that cirrhotic patients may develop exaggerated β-cell responsiveness to glucose as well as pancreatic islet hypertrophy, resulting in increased insulin secretion[54].
Sustained hyperinsulinemia can, by means of an array of negative feedback mechanisms[55-57], give rise to insulin resistance in peripheral adipose tissues and skeletal muscles, resulting in reduced insulin-stimulated non-oxidative glucose disposal[58].The hyperglycemic effect of reduced glucose disposal can be further amplified by cirrhosis-induced sarcopenia and impaired glucose effectiveness[59].Moreover, certain etiologies of CLD may exert additional disease-specific mechanisms of insulin resistance, in a similar fashion to HCV-induced DM[21].Chronic alcohol exposure, for instance, may inhibit insulin signaling pathways by altering intracellular calcium level within hepatocytes[60], resulting in impaired insulin-mediated suppression of hepatic glucose production[61].Finally, there is evidence that hepatic expression of DPP-4 is increased and GLP-1 is decreased in both chronic HCV and NAFLD[25,62,63], resulting in hepatic insulin resistance and IGT.
As in the pathogenesis of T2DM, the development of pancreatic β-cell dysfunction signals the body’s diminishing capacity to compensate for worsening insulin resistance and marks the progression from IGT to frank DM[27].Similar to the development of insulin resistance, a number of disease-specific mechanisms of β-cell dysfunction may be operating even before the onset of cirrhosis.Chronic alcohol consumption and hereditary hemochromatosis, for instance, cause down-regulation of glucokinase and increased oxidative stress, respectively, leading to increased β-cell apoptosis and decreased glucose-induced insulin secretion[64,65].In NAFLD, the combination of chronic hyperglycemia and elevated free fatty acid levels lead to dysfunction and death of pancreatic β-cells by glucolipotoxicity[66].Meanwhile, chronic HCV infection is thought to induce pancreatic islet destructionviaa combination of autoimmune-mediated and direct cytopathic mechanisms as abovementioned[19,20,67].Eventually, the development of cirrhosis and hepatic decompensation further exacerbates β-cell dysfunction due to the accumulation of advanced glycation end products[68], which can inhibit glucose-stimulated insulin secretion and induce apoptosis of β-cells[69,70].

Figure 2 Mechanisms of action of hepatogenic diabetes.
DIABETES AND LIVER TRANSPLANTATION
In many cases, orthotopic liver transplantation (OLT) remains the only curative option for patients with ESLD.Given the pathogenic effects of insulin resistance on oxidative and inflammatory stresses, it is not surprising that multiple large-scale retrospective studies have repeatedly demonstrated pre-transplant DM to be associated with decreased post-transplant survival, independent of BMI or MELD score[71-73].Pretransplant DM is also an independent predictor for postoperative complications, including cardiovascular events, infections, renal dysfunction, and acute graft rejection[71].There is even evidence that the donor’s history of DM correlates with increased recipient’s mortality as well as increased risk of graft failure[73], suggesting that DM can exert biologically-significant damages to the liver without causing clinically-detectable hepatic dysfunction.
Similarly, the presence of post-transplant DM, which occurs in about 30% of patients undergoing OLT[74], has been linked to increased mortality as well as a higher incidence of postoperative complications and acute rejection[73,75,76].Although preexisting overt DM may disappear in more than half of the patients after OLT[77], pre-transplant DM remains one of the most important predictors for post-transplant DM[78].This observation is consistent with the findings that peripheral insulin resistance is improved, but not completely normalized, after OLT[79].Other recognized risk factors for new-onset DM after transplantation, which affects 7%-18% of OLT patients[77,80], include male gender[78,80], use of tacrolimus[80,81], and HCV infection[78,81].Besides DM, metabolic syndrome and its other individual components, namely hypertension, dyslipidemia, and obesity, are also found to be highly prevalent posttransplant[82].Not only does it increases the incidence of cardiovascular events[83], but the presence of post-transplant metabolic syndrome may also contribute to the development of new-onset NAFLD after transplantation for non-NAFLD cirrhosis[84].While there have been case reports of combined liver and islet transplantation in cirrhotic patients with concurrent type 1 DM[85], the safety and utility of this technique in patients with hepatogenic diabetes or T2DM is yet to be determined[86].
TRADITIONAL GLYCEMIC MARKERS FOR PATIENTS WITH LIVER DISEASES
Given the abovementioned detrimental effects of DM on the development and progression of CLD as well as the manifestation and management of ESLD, it is reasonable to hypothesize that early diagnosis and optimal treatment of DM in patients with CLD would be beneficial.Nonetheless, IGT and DM were identified in as much as 38.5% and 19.2%, respectively, of patients with normal fasting plasma glucose (FPG) and compensated cirrhosis of various etiologies in a small prospective study[87], which alluded to the practical challenges of managing DM in this patient population.The first challenge is to establish an accurate diagnosis and to assess disease severity.As summarized in Figure 3, the utility and accuracy of most glycemic markers are restricted in patients with CLD.
Fasting plasma glucose
FPG is a simple and inexpensive test for diagnosing DM but its widespread adoption is limited by the need for prolonged fasting and stringent processing requirements[88].Although the test offers a reasonable intra-individual reproducibility[89], FPG can be affected by stress, acute illness, and alcohol consumption.Moreover, the optimal FPG threshold for diagnosing DM in patients with CLD/ESLD remains controversial.Certain studies have reported that using thresholds of 100-125 mg/dL and ≥ 126 mg/dL, the sensitivities of FPG in detecting prediabetes and DM in high-risk populations were as low as 28.9% and 55.7%, respectively[90,91].In a study on patients with HCV cirrhosis, 21% of patients with FPG < 110 mg/dL met the diagnostic criteria for DM based on oral glucose tolerance test (OGTT) and regression analysis showed that a threshold of 107 mg/dL, instead of 126 mg/dL should be used for diagnosing DM in these patients[92].It is unclear if the same threshold can be applied to patients with other etiologies or severities of liver diseases.
Oral glucose tolerance test
OGTT is often considered the gold standard for diagnosing DM.It is the preferred method for detecting gestational DM, cystic fibrosis-related DM, and post-transplant DM[93].Furthermore, it is the only way to formally diagnose IGT, which may be particularly relevant to cirrhotic patients because of the implications on short-term and long-term prognosis as abovementioned[29,31,32].The major drawbacks of OGTT are the need for prolonged fasting as well as the length and complexity of the test, which may contribute to the poor reproducibility of OGTT in clinical practice[94].Most importantly, OGTT cannot be used to assess disease severity or treatment effectiveness because of practical limitations and the lack of established OGTT-based glycemic targets.
Hemoglobin A1c
Since it was first proposed as a measure of glucose intolerance in 1976[95], glycated hemoglobin (A1c) has evolved into one of the most broadly deployed tests for diagnosing and monitoring DM.Its major advantages are the absence of a fasting requirement and the relative ease of sample handling.A1c is particularly useful as a marker for treatment effectiveness because it reflects average blood glucose over a period of months instead of a single point in time.While it remains a useful glycemic marker in most patients with mild liver diseases, the accuracy and validity of A1c in patients with advanced liver diseases remained controversial.A small study involving 15 patients with compensated cirrhosis found 40% of subjects to have A1c results below the non-diabetic reference range[96].Another retrospective study on a cohort decompensated cirrhotic patients undergoing liver transplantation evaluation revealed a similar discordance between measured A1c and average blood glucose[97].The poor diagnostic performance of A1c is attributable to the well-described curvilinear correlation between A1c and erythrocyte turnover[98], which can occur in patients with CLD/ESLD as a result of hemorrhage related to portal hypertension and coagulopathy, hemolysis caused by splenomegaly as well as impaired erythropoiesis due to marrow suppression and nutritional deficiency[99].A1c value can also be affected unpredictably by blood transfusion[100,101].More importantly, the inherent biochemical characteristics of the glycation process limit the ability of A1c to capture the excessive blood glucose lability exhibited by patients with CLD, as demonstrated in a recent study that compared A1c against continuous glucose monitoring in diabetic patients with cirrhosis[102].This shortcoming may have crucial clinical implications since glycemic variability was shown to be an independent predictor for fibrosis in NAFLD as well as cardiovascular disease and all-cause mortality in the general population[103,104].Finally, there is evidence that alcohol consumption is independently associated with lower A1c values, even adjusting for confounding factors such as FPG, obesity, and anemia[105-107], likely as a result of an increase in the size of the hemoglobin A1 fraction[108].
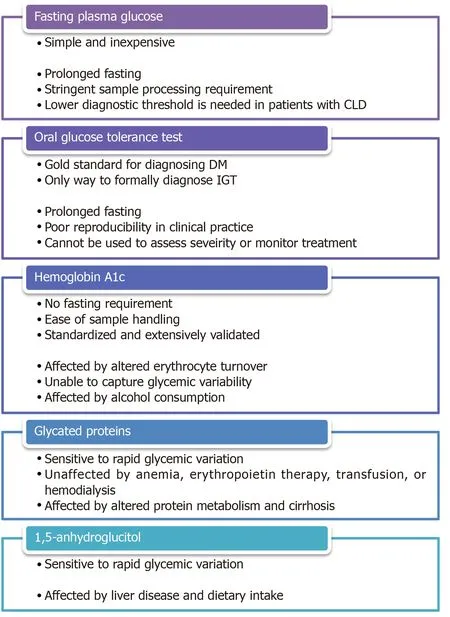
Figure 3 Pros and cons of various glycemic markers.
NON-TRADITIONAL GLYCEMIC MARKERS FOR PATIENTS WITH LIVER DISEASES
Glycated proteins
Glycated albumin (GA) and fructosamine are ketoamines that are formed by nonenzymatic glycation of glucose to serum proteins in a similar fashion to the glycation of hemoglobin.Due to the shorter half-life of albumin and other serum proteins, the faster rate of albumin glycation compared to hemoglobin glycation, and the direct exposure of serum proteins to serum glucose, GA and fructosamine are typically taken to reflect glycemic control over 2-3 wk[109,110].GA has proven to be a particularly useful glycemic marker in patients with chronic kidney disease and/or on dialysis because, unlike A1c, GA level is not affected by anemia, erythropoietin therapy, blood transfusion, or hemodialysis[111,112].Not surprisingly, the accuracy of GA and fructosamine is negatively impacted by disease states that affect protein metabolisms.Indeed, an older study on a small cohort of cirrhotic patients demonstrated an inverse correlation between fructosamine levels and serum albumin levels[113].A more recent study comparing fructosamine against continual glucose monitoring confirmed the poor association in diabetic patients with cirrhosis[102].Several entities, including albumin-corrected fructosamine, total protein-corrected fructosamine, and CLD-A1c, have been proposed in an attempt to improve the diagnostic performance of GA and fructosamine by correcting for variations in serum protein concentrations[114,115].Unfortunately, the validity of these entities outside the study populations has not been externally or prospectively verified.
Serum 1,5-anhydroglucitol
Serum 1,5-anhydroglucitol (1,5-AG) is a dietary monosaccharide that is normally reabsorbed by the proximal renal tubules, but its reabsorption is competitively inhibited by glucosuria in the setting of hyperglycemia.Serum 1,5-AG was found to be a sensitive day-to-day glycemic marker in diabetic patients as well as a sensitive diagnostic test in those at high risk of DM[116,117].Nonetheless, there is evidence that 1,5-AG metabolism is affected in patients with liver diseases[118,119].There is also a concern that dietary intake may affect serum 1,5-AG level[120], which is particularly relevant to the cirrhotic population due to the high prevalence of malnutrition.
MANAGEMENT OF DIABETES IN PATIENTS WITH LIVER DISEASES
The second challenge in the management of DM in patients with liver diseases is to identify a safe and effective treatment strategy for this medically complicated population, especially those with decompensated cirrhosis.It is well accepted that basic lifestyle interventions, such as healthful diet, physical activity, alcohol/smoking cessation as well as psychosocial support are fundamental to the medical management of both DM and liver diseases.Nonetheless, antihyperglycemic medications are often needed when patients fail to achieve targeted glycemic control through lifestyle interventions alone.Attention must be paid to consider the unique mechanisms of action, the side effect profiles, and the implications on liver diseases associated with the use of these medications, as summarized in Figure 4.An up-to-date summary, with a focus on liver-disease related outcomes, of the major clinical trials involving these medications is provided in Supplementary Table 1.
Metformin
Metformin, which is often considered first-line oral therapy in T2DM due to its favorable safety profile and cardioprotective effects[121], has been studied in both diabetic and non-diabetic patients with biopsy-proven NAFLD[122-128].Despite the limitations of these small, open-label, or non-randomized published trials, the overall evidence indicates that metformin use is associated with improvement in insulin resistance, aminotransferase levels, and liver morphology[129].Furthermore, several large retrospective studies on diabetic patients with CLD of various etiologies have demonstrated a 50%-70% reduction in HCC risk among those treated with metformin[130-132], with one recent case-control study showing a dose-dependent association[133].Similar results, including a reduction in 5-year HCC incidence, liverrelated mortality, and transplantation, were obtained in a prospective study involving specifically diabetic patients with HCV cirrhosis[134].Additionally, metformin has been shown to reduce the incidence of overt hepatic encephalopathy by 8 folds through inhibition of glutaminase activity[135].Most strikingly, a retrospective study on 250 diabetic patients with cirrhosis of various etiologies revealed that the continuation of metformin after cirrhosis diagnosis nearly doubles the overall median survival across all Child-Pugh classes[136].
Despite its remarkable morbidity and mortality benefits, metformin is often withheld from patients with liver diseases due to an exaggerated concern for metformin-associated lactic acidosis (MALA).It is worth noting, however, that metformin is not intrinsically hepatotoxic and can be safely administered even in the context of mildly abnormal transaminases[137].In fact, animal studies have revealed a protective potential of metformin against acetaminophen-induced hepatotoxicity[138,139].Additionally, MALA is an exceedingly rare condition with an estimated incidence of < 10 per 100000 patient-years of exposure in patients without significant renal impairment[140].A recent retrospective study of over 132000 patients with newly diagnosed T2DM found no significant difference in the risk of lactic acidosis in patients prescribed metformin compared to those prescribed other antihyperglycemic medications or no medication[141].This is in contrast to its predecessor, phenformin, which carries a significantly higher risk of lactic acidosis even at therapeutic drug levels[142].Finally, it is important to bear in mind that although metformin can increase lactate production by promoting intestinal glucose utilizationviathe anaerobic pathway as well as inhibiting hepatic lactate uptake and gluconeogenesis[143,144], lactate is not toxic in itself and hyperlactatemia does not directly cause the clinical condition of lactic acidosis[145].
Pioglitazone
The thiazolidinedione pioglitazone, an insulin-sensitizing peroxisome proliferatoractivated receptor-γ (PPAR-γ) agonist, is another extensively studied antihyperglycemic agent that shows promise in the treatment of nonalcoholic steatohepatitis (NASH).A small pilot study on non-diabetic patients with biopsyproven NASH resulted in normalization of aminotransferase levels in 72% of patients as well as improvement in histological features of hepatic steatosis, hepatocellular injury, parenchymal inflammation, and fibrosis in 67% of patients after 48 wk of treatment[146].Similar results except for a decrease in fibrosis score, which failed to achieve statistical significance in two studies[147,148], were replicated in subsequent randomized, placebo-controlled trials of various study designs involving both diabetic and non-diabetic patients[147-150].It is interesting to note that although its beneficial effects are greater in patients with T2DM, pioglitazone use is associated with a reduction in NAFLD activity score and resolution of NASH in 46% and 26%, respectively, of patients without frank DM[151].Given its low risk of inducing hypoglycemia, pioglitazone may be uniquely suited in the treatment of selected NASH patients with normoglycemia at baseline.Finally, there is limited data suggesting that pioglitazone may inhibit HCC development[152,153], but these findings have not been confirmed in human studies[154].
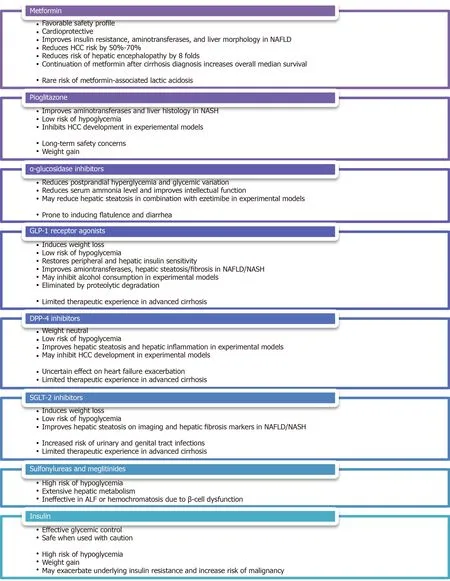
Figure 4 Pros and cons of various antihyperglycemic medications.
Lingering concerns regarding the long-term safety of thiazolidinedione therapy stemmed from potential troglitazone-related hepatotoxicity, rosiglitazone-related cardiovascular risks, and pioglitazone-related bladder cancer[155-157], remain a barrier to the widespread use of pioglitazone in clinical practice.This is particularly relevant in the face of findings that long-term rosiglitazone or pioglitazone therapy does not impart additional improvement in metabolic or hepatic parameters compared to shortterm therapy[150,158].A recently published expert panel statement, however, does recommend the use of pioglitazone, either alone or in combination with a statin or ezetimibe, for the primary or secondary prevention of cardiovascular disease as well as the avoidance of cirrhosis, liver transplantation, or HCC in patients with NAFLD/NASH[159].Another barrier to the use of pioglitazone is its propensity to cause significant weight gain, which may be dose-dependent[160], as demonstrated by multiple systematic reviews and meta-analyses[161,162].Finally, other than a small pilot study that showed a reduction in hepatic steatosis on imaging in patients with human immunodeficiency virus (HIV)/HCV coinfection[163], the use of pioglitazone in other etiologies of CLD has not been thoroughly investigated.
α-glucosidase inhibitors
Although α-glucosidase inhibitors, such as acarbose, voglibose, and miglitol, enjoy limited adoption amongst the general diabetic population due to its gastrointestinal side effects of flatulence and diarrhea, they deserved to be recognized as a pluripotent antihyperglycemic agent in patients with CLD.A randomized, placebo-controlled trial of acarbose in diabetic patients with compensated cirrhosis, who are prone to postprandial hyperglycemia partly due to decreased hepatic glucose uptake and impaired glycogenesis[164], demonstrated a significant reduction in fasting glycemia, postprandial glycemia, mean glycemic variation, and A1c in the treatment group[165].By favoring saccharolytic, instead of proteolytic, intestinal bacterial flora, acarbose treatment has also been shown to reduce serum ammonia level as well as to improve intellectual function in a randomized, placebo-controlled trial, crossover study[166], making it an effective regimen for the consolidated treatment of hyperglycemia and mild hepatic encephalopathy.In a small pilot study of diabetic patients with biopsyconfirmed NASH, miglitol was found to reduce aminotransferase levels, hepatic steatosis, and histological inflammation after 12 months of therapy[167].Moreover, voglibose was found to be effective in minimizing pioglitazone-induced weight gain in the general diabetic population[168].It is important to note that although there are rare case reports of acarbose-related acute hepatitis[169], the medication is generally felt to be safe in patients with CLD/ESLD since it is exclusively metabolized in the gastrointestinal tract and has a very low systemic bioavailability[170].This notion is supported by a Taiwanese nationwide retrospective study, which discovered no increased risk of liver injury amongst diabetic patients with severe renal insufficiency regardless of the presence or absence of CLD[171].
GLP-1 receptor agonists
GLP-1 receptor agonists, such as exenatide and liraglutide, constitute an increasingly popular class of incretin-based therapy for the treatment of T2DM thanks to their ability to induce weight loss and their lower risk of hypoglycemia.The observations that glucose-induced GLP-1 secretion and hepatic GLP-1 receptor expression are deficient in NAFLD patients suggest GLP-1 agonism may exert a direct effect on hepatocytes and may play a role in the treatment of NAFLD[62,172,173].Several small open-label studies have demonstrated reductions in hepatic steatosis, aminotransferase levels, and liver fibrosis score after treatment with liraglutide in diabetic patients with imaging findings of hepatic steatosis[174-176].These findings were further supported by a Japanese single-arm, open-label study, and a British doubleblinded, randomized, placebo-controlled trial of liraglutide on patients with biopsyproven NASH.The former showed significant improvement in histological inflammation and fibrosis in 60%-70% of subjects after 96 wk of therapy[177], while the latter showed histological resolution of NASH in 39% of subjects after 48 wk of therapy[178].A reduction in hepatic triglyceride content was also observed in a small, open-label, randomized study of exenatide in diabetic patients with imaging findings suggestive of NAFLD[179].With regard to other etiologies of CLD, GLP-1 receptor agonists were found to exhibit an inhibitory effect on alcohol consumption in animal models[180,181].It has also been reported that fasting serum GLP-1 levels were decreased in patients with chronic HCV, but not those with chronic HBV[25].It is worth noting, however, that patients with HBV-, HCV-, or alcohol-related liver diseases were excluded from the original Liraglutide Effect and Action in Diabetes trials[182].The effects of GLP-1 agonism on other liver disease-related clinical outcomes, such as encephalopathy and HCC development, have yet to be thoroughly evaluated.Since GLP-1 receptor agonists are eliminated by proteolytic degradation and glomerular filtration, no dosage adjustment is necessary for patients with any degree of hepatic impairment[183].Caution is advised in patients with advanced cirrhosis given limited therapeutic experiences in this vulnerable population.
DPP-4 inhibitors
DPP-4 is a ubiquitously expressed membrane-bound and circulating glycoprotein that acts enzymatically on a variety of substrates, including GLP-1, gastric inhibitory polypeptide (GIP), insulin-like growth factor-1 (IGF-1), neuropeptide Y and substance P, as well as interacts non-enzymatically with many ligands, including adenosine deaminase, caveolin-1, and CD45[184].As such, DPP-4 inhibitors, such as linagliptin, saxagliptin, sitagliptin, and vildagliptin are thought to act upstream of GLP-1 by slowing its degradation, but they may also exert diverse metabolic, immunologic, and neurologic effectsviaother GLP-1-independent pathways.Similar to GLP-1 receptor agonists, DPP-4 inhibitors are considered weight neutral and carry a low risk of hypoglycemia[185], but they have the added advantage of being oral agents.In the context of liver diseases, hepatic DPP-4 expression was found to be upregulated in patients with NAFLD while serum DPP-4 activity was reported to correlate with the histopathologic grade of NASH as well as serological markers of liver damage[186-188].In addition, DPP-4 inhibition by specific inhibitors was demonstrated in several animal models of NAFLD to attenuate hepatic steatosis[189,190].There were two open-label trials of sitagliptin, one reported a reduction in intrahepatic lipid content in diabetic patients with clinical NAFLD, another reported improvements in hepatic steatosis and ballooning in patients with biopsy-proven NASH irrespective of DM status[176,191].Unfortunately, improvements in hepatic steatosis, liver fibrosis, or NAFLD activity score were not observed in other randomized, placebo-controlled trials of sitagliptin[192,193].It is worth noting that the hepatic protective effects of DPP-4 inhibitors, which may be mediated through direct actions on hepatocytesviaGLP-1 receptors[172], appear to occur irrespective of the degree of glycemic control[187].Moreover, the observations in obese patients of increased expression of membranebound DPP-4 on visceral adipose tissue-derived dendritic cells as well as increased release of soluble DPP-4 as adipokine from visceral adipocytes support the notion that DPP-4 inhibition may play a role in modulating adipose tissue inflammation and regulating adipose tissue metabolism[194,195], likely through a GLP-1-independent pathway[196].Finally, alteration in DPP-4 expression was observed in rat hepatoma cell lines and human HCC specimens[197,198], raising the possibility that DPP-4 activity may influence HCC development.It is prudent, however, to mention that although DPP-4 inhibitors were shown to be protective against cardiovascular diseases by improving endothelial function and reducing inflammation[199,200], a recent meta-analysis of five clinical trials revealed a slightly increased risk of hospital admission for heart failure attributable to DPP-4 inhibitor use amongst diabetic patients with existing cardiovascular risk factors[201].Most DPP-4 inhibitors, except vildagliptin, are considered safe in patients with mild or moderate hepatic impairment, although caution is advised in patients with severe hepatic impairment due to limited therapeutic experiences[183].
Sodium-glucose cotransporter-2 inhibitors
Instead of directly altering insulin availability or insulin sensitivity, sodium-glucose cotransporter-2 (SGLT-2) inhibitors, including canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin, exert their antihyperglycemic effect by blocking proximal renal tubular glucose reabsorption, thus leading to increased glucose excretion in the form of glucosuria.SGLT-2 inhibitors carry a low risk of hypoglycemia and are capable of inducing modest weight loss as well as lowering blood pressure[202].They have been demonstrated in several animal models of NAFLD/NASH to improve hepatic steatosis and liver fibrosis[203,204].Improvement in hepatic steatosis as well as reductions in various serological or imaging parameters of liver injury were also observed in clinical trials of several SGLT-2 inhibitors in diabetic NAFLD patients[205-211].Most impressively, preliminary evidence indicates SGLT-2 inhibitors may slow the proliferation of hepatoma cell lines and hinder xenograft tumor growth in nude mice independent of their antihyperglycemic effect, suggesting that SGLT-2 inhibitors may attenuate HCC developmentviasome unidentified mechanisms of action[212].The use of SGLT-2 inhibitors in other etiologies of CLD has not been thoroughly investigated.Long-term safety data on the use of SGLT-2 inhibitors in cirrhotic patients is relatively scarce given limited therapeutic experiences, but canagliflozin and ertugliflozin are generally considered safe in patients with mild or moderate hepatic impairment[213,214].Limited data is showing that empagliflozin can be used without dosage adjustment across all degrees of hepatic impairment[215], while dose reduction may be necessary for dapagliflozin in patients with severe hepatic impairment[216].
Sulfonylureas and meglitinides
The second- and third-generation sulfonylureas, including glipizide, glyburide, gliclazide, and glimepiride, and the meglitinides, repaglinide, and nateglinide, are insulin secretagogues that stimulate insulin secretion from pancreatic β-cells.While these medications are highly effective antihyperglycemic agents capable of lowering A1c by 1%-2%[217], they also carry a high risk of hypoglycemia[218].Patients with hepatic impairment are particularly susceptible due to reduced drug inactivation and elevated free drug concentration because both sulfonylureas and meglitinides are extensively metabolized by the liver and are tightly bound to serum proteins.The risk of hypoglycemia is further exacerbated in patients with cirrhosis as a result of impaired hepatic gluconeogenesis, reduced hepatic insulin clearance, and peripheral hyperinsulinism[219].Alcohol consumption can predispose patients to hypoglycemia in a similar fashion by impairing gluconeogenesis and glycogenolysis[220].Conversely, these medications may be rendered ineffective in chronic ALD or hemochromatosis due to β-cell dysfunction and apoptosis[64,65].Most notably, several case-control studies have meta-analyses revealed an increased odds of HCC development by up to 3 folds amongst patients with T2DM treated with sulfonylureas[131,221-223], possibly as a result of hyperinsulinemia.Expert opinions advise that insulin secretagogues be avoided or used with extreme caution in patients with CLD/ESLD[224].
Insulin
Despite the ever-expanding list of antihyperglycemic medications, insulin and insulin analogs remain the safest and most effective glycemic therapy in patients with DM.They can be used, with caution, even in patients with decompensated cirrhosis or severe hepatic impairment[225,226].Not surprisingly, hypoglycemia is a major limiting side effect.On the one hand, cirrhosis can induce or exacerbate a state of hyperinsulinemia and insulin resistance as abovementioned[219], which may increase a patient’s insulin requirement.On the other hand, cirrhotic patients may exhibit an exaggerated response to insulin due to impaired hepatic gluconeogenesis and sarcopenia[227].These opposing mechanisms of actions, together with symptoms of nausea, bloating, and poor appetite experienced by many patients with CLD, make it difficult to predict a patient’s day-to-day exogenous insulin requirement.Furthermore, some cirrhotic patients, who are on β-blockers for variceal bleeding prophylaxis, may experience an impaired hormonal counterregulatory response to insulin, resulting in delayed recovery from hypoglycemia[228].As such, expert opinions recommend the use of short-acting insulin analogs as well as frequent dose adjustment and close glucose monitoring to minimize the risk of hypoglycemia[229].
Weight gain of 3 to 9 kg associated with the initiation of insulin therapy, attributable to the intrinsic anabolic properties of insulin, the central effects of insulin on appetite and reward regulation, the non-physiological pharmacokinetics of exogenous insulin, and the behavioral changes related to fear of hypoglycemia[230], is a well-documented phenomenon amongst the general diabetic population[231].This weight gain can be particularly detrimental to patients with metabolic syndrome as it is predominantly represented by fat mass and is likely to further exacerbate the underlying insulin resistance, oxidative stress, and systemic inflammation[232], leading to worsening metabolic diseases as well as increased risk of malignancy.Similar to the case of sulfonylureas, data from observational studies suggest an association between insulin therapy and HCC development amongst patients with T2DM[130,131].Further studies are needed to further delineate the oncogenic risk of insulin therapy versus the cardiometabolic risk of uncontrolled hyperglycemia.Nonetheless, it may be prudent to reserve insulin therapy in patients with CLD to those who are unable to receive or inadequately managed by other antihyperglycemic medications.
GLYCEMIC TARGETS IN PATIENTS WITH LIVER DISEASES
After selecting an appropriate glycemic marker and formulating an effective antihyperglycemic therapy plan, the third challenge in the management of DM in patients with CLD is to determine a reasonable glycemic target.For the general nonpregnant adult population, the ADA recommends an A1c goal of < 7%[233], which is largely based on several landmark studies demonstrating decreased rates of microvascular complications, such as retinopathy, neuropathy, and nephropathy, in patients undergoing intensive glycemic control[234,235].It is important to note, however, that the higher mortality in patients with concurrent DM and CLD is predominantly attributable to complications of liver failure, not DM-related micro-/macrovascular diseases[33].Unfortunately, except for a small cohort study on NAFLD patients showing an association between improvement in A1c and improvement in liver fibrosis on biopsy[236], the impact of glycemic control on the natural history of various CLD etiologies has not been thoroughly investigated.It is also unclear if the degree of glycemic control directly correlates with the incidence or severity of liver disease complications.Although the ADA does recommend that less stringent A1c goals be considered in selected patients with limited life expectancy, extensive comorbid conditions, advanced micro-/macrovascular complications, or difficult to manage diabetes[233], there is little data to guide the clinical decision-making process in practice.Further studies are desperately needed to help determine the optimal glycemic targets, in relation to the etiology of liver disease and the degree of decompensation, in patients with CLD.
CONCLUSION
Given the abovementioned evidence, it is clear that DM plays a significant role in the development and progression of CLD.DM can also occur as a consequence of or be exacerbated by CLD.Most importantly, concurrent DM and CLD are associated with worse clinical outcomes, including reduced survival, more severe liver failure-related complications, and a higher incidence of HCC and HCC-specific mortality.It is, therefore, imperative that practitioners astutely identify and closely monitor the development of DM in any patient with CLD, bearing in mind that A1c may not be accurate in patients with advanced liver diseases.Alternative glycemic markers, such as FPG, OGTT, or CLD-A1c, may be needed to establish the diagnosis in patients with moderate-to-severe anemia, frequent transfusion requirement, chronic alcohol consumption, and/or fluctuating A1c levels.Once the diagnosis of DM is confirmed, efforts should be made to incorporate the management of DM into the patient’s overall treatment plan, with aims to slow the progression of CLD and to reduce the risk of liver failure-related complications.A proposed treatment algorithm is presented in Figure 5.Similar to the general diabetic population, lifestyle interventions involving a calorie-restricted diet and moderate-intensity exercise should be considered first-line treatment.If antihyperglycemic pharmacotherapy is deemed necessary, metformin should be included as the backbone, unless otherwise contraindicated, given its favorable safety profile, chemopreventive effect, and mortality benefit.GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT-2 inhibitors may be considered, even in patients with mild-to-moderate hepatic impairment, given its low risk of hypoglycemia, weight-neutral metabolic profile, and protective effect on hepatic steatosis and hepatic fibrosis.α-glucosidase inhibitors may be useful in patients with concurrent postprandial hyperglycemia and mild hepatic encephalopathy as a way to minimize polypharmacy.Insulin therapy should be reserved for patients who failed other antihyperglycemic medications and should entail frequent dose adjustment and close glucose monitoring to minimize the risk of hypoglycemia.Sulfonylureas and meglitinides should be avoided in most instances.
The aforementioned evidence also highlighted the importance of recognizing the impact of insulin resistance and DM on other etiologies of CLD besides NAFLD.A recent consensus statement endorsed by a panel of international experts has revitalized the decades-long effort to revise the definition and nomenclature of NAFLD[237], acknowledging the heterogeneity of the disease in relation to its natural history, risk factors, clinical manifestations, and responses to treatment[238].The newly proposed definition of metabolic dysfunction-associated fatty liver disease (MAFLD) no longer requires the exclusion of alcohol consumption or other concomitant liver diseases.It also serves to promote research on patients with coexisting metabolic and alcoholic liver disease, who were previously excluded from most NAFLD studies but are in fact at high risk of disease progression[239].

Figure 5 Proposed diabetes treatment algorithm in patients with chronic liver diseases.
FUTURE DIRECTIONS
Despite our improved understanding of the interplay between DM and CLD, thanks largely to research in the pathophysiology and management of NAFLD, many pressing clinical questions remain to be addressed.First, an alternative glycemic marker, whose diagnostic accuracy is not affected by altered erythrocyte turnover or excessive glycemic variability, is desperately needed for diagnosing and monitoring DM in patients with advanced liver diseases.Ideally, the test could be performed without prolonged fasting and the result could be easily converted back to an A1cequivalent value.Second, the optimal glycemic target for slowing CLD progression and preventing liver disease complications while minimizing the risk of hypoglycemia needs to be established.It is reasonable to suspect that patients with various degrees of decompensation would benefit from different glycemic targets.Third, a serological marker for DM-related liver diseases akin to the use of urine albumin excretion to screen for diabetic nephropathy should be investigated.A recent study has shown, for example, that the circulating level of osteopontin (OPN), a soluble pluripotent glycophosphoprotein and a proinflammatory cytokine, may be useful in diagnosing NAFLD in patients with DM[240].Fourth, given the impact of DM on the progressive of CLD and liver disease complications, it would be interesting to see if the inclusion of a glycemic marker in the calculation of the MELD score would improve its predictive value for the short-term survival and liver disease severity.Fifth, the long-term safety and efficacy of the novel antihyperglycemic medications, including GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT-2 inhibitors, need to be thoroughly investigated, especially in the non-NAFLD patient populations and with regard to liver diseaserelated clinical outcomes, such as encephalopathy and HCC development.Sixth, the oncogenic risk of insulin therapy in the context of insulin resistance and chronic hyperinsulinemia must be further evaluated.Seventh, considering the medical complexity of patients with CLD, the risk for drug-induced liver injury from polypharmacy, and the impact of glycemic control on transplant survival and complications, it is worth debating if DM in patients with CLD would be best managed by internists, endocrinologists, or hepatologists.
It is also important to acknowledge and address a number of systemic barriers in order to facilitate advances in this area of research.Clinical studies on the management of DM have traditionally focused on the micro-/macrovascular complications of prolonged hyperglycemia instead of liver disease-related clinical outcomes.In addition, patients with CLD are often excluded from clinical trials of novel medications due to overwhelming concerns regarding hepatic dysfunction and drug-induced liver injury.Conversely, there is an emphasis on the manifestations of portal hypertension as well as the relatively short-term changes in morbidity and mortality, instead of the systemic and long-term effects of insulin resistance, in the management of CLD.While the rapidly worsening epidemic of metabolic syndrome has certainly called to attention the impact of DM on the natural history of liver diseases, most research efforts, especially in terms of novel treatment options, remain restricted to the traditional definitions of NAFLD/NASH.We hope that the evolution from NAFLD to MAFLD would help broaden future studies in this area to include other etiologies of CLD as well over the next 5 to 10 years.
杂志排行
World Journal of Hepatology的其它文章
- Biomarkers for hepatocellular cancer
- Comprehensive review of hepatotoxicity associated with traditional Indian Ayurvedic herbs
- N-acetylcysteine and glycyrrhizin combination: Benefit outcome in a murine model of acetaminopheninduced liver failure
- Transaminitis is an indicator of mortality in patients with COVID-19: A retrospective cohort study
- Clinical efficacy of direct-acting antiviral therapy for recurrent hepatitis C virus infection after liver transplantation in patients with hepatocellular carcinoma
- Surgical treatment of gallbladder cancer: An eight-year experience in a single center
