密蒙花中的1个新的环烯醚萜苷类化合物
2021-01-05龙泽海王琦瑶李医明朱维良
龙泽海,王琦瑶,李 波,张 勇*,贾 琦*,李医明,朱维良
密蒙花中的1个新的环烯醚萜苷类化合物
龙泽海1,王琦瑶1,李 波2, 3,张 勇2, 3*,贾 琦1*,李医明1,朱维良2, 3
1. 上海中医药大学中药学院,上海 201203 2. 中国科学院上海药物研究所,上海 201203 3. 中国科学院大学,北京 100049
研究马钱科醉鱼草属密蒙花干燥花蕾及花序的化学成分。采用加热回流提取、溶剂萃取、多种柱色谱方法进行分离纯化,并运用NMR、MS等现代谱学技术进行化合物结构鉴定。从密蒙花水提取物中分离得到20个化合物,分别鉴定为6α-羟基-8β-羟甲基-1β,5α,6β,7β,9α-五氢-7(8)-环氧-2-oxaind-3-烯-1--α--吡喃鼠李糖基-(1→6)-β--吡喃葡萄糖苷(1)、6--甲基梓醇(2)、梓醇(3)、蒙花苷(4)、芹菜素-7--芦丁糖苷(5)、木犀草素-7--芦丁糖苷(6)、密蒙花新苷(7)、芹菜素-7--半乳糖醛酸苷(8)、芹菜素-7,4′--葡萄糖醛酸苷(9)、芹菜素-7--α-鼠李糖基- (1→2)-β-葡萄糖醛酸苷(10)、腺嘌呤核苷(11)、鸟嘌呤核苷(12)、香草酸(13)、鸢尾番红花素M(14)、苦藏花素(15)、二氢红花菜豆酸-3′--β--葡萄糖苷(16)、二氢红花菜豆酸钠盐-3′--β--葡萄糖苷(17)、()-芥子酸-4--β--吡喃葡萄糖苷(18)、咖啡酸(19)、绿原酸(20)。化合物1为新的环烯醚萜苷类化合物,命名为6′--α--鼠李糖基梓醇,化合物9、10、15~18均为首次从醉鱼草属植物中分离得到,化合物3、8、11、12、19和20为首次从密蒙花中分离得到。
醉鱼草属;密蒙花;环烯醚萜苷;6′--α--鼠李糖基梓醇;芹菜素-7,4′--葡萄糖醛酸苷;苦藏花素
中药密蒙花是马钱科(Loganiaceae)醉鱼草属(Buddleia auct.) Linn.植物密蒙花Maxim.的干燥花蕾及花序,广泛分布于山西、陕西、四川、贵州、云南和西藏等省区[1]。密蒙花始载于宋代的《开宝本草》,具有清热泻火、养肝明目及退翳的功效[2],临床上常用于治疗干眼症、糖尿病视网膜病变等眼部疾病。密蒙花中主要含有黄酮、苯乙醇苷、三萜及其皂苷等类化合物[3-5],还包括少量环烯醚萜、单萜、木脂素以及生物碱类化合物[6-9]。现代药理学研究发现,该中药提取物具有抗炎[10]、抗增生[4]和神经保护[11]等多种活性。目前,有关密蒙花化学成分的研究主要集中在乙醇提取物,而传统水煎剂中化学成分未见系统报道。为进一步研究密蒙花水煎液中化学成分的组成,为中药密蒙花药效物质研究奠定基础,本实验对密蒙花水提取物进行了系统的分离纯化,从中分离并鉴定了20个化合物,分别为6α-羟基-8β-羟甲基-1β, 5α,6β,7β,9α-五氢-7(8)-环氧-2-oxaind-3-烯-1--α--吡喃鼠李糖基-(1→6)-β--吡喃葡萄糖苷[6α- hydroxy-8β-hydroxymethyl-1β,5α,6β,7β,9α-pentahydro- 7(8)-epoxy-2-oxaind-3-ene-1--α--rhamnopyranosyl- (1→6)-β--glucopyranoside,1]、6--甲基梓醇(6-- methylcatalpol,2)、梓醇(catalpol,3)、蒙花苷(linarin,4)、芹菜素-7--芦丁糖苷(apigenin-7-- rutinoside,5)、木犀草素-7--芦丁糖苷(luteolin-7--rutinoside,6)、密蒙花新苷(neobudofficide,7)、芹菜素-7--半乳糖醛酸苷(luteolin-7-- galacturonide,8)、芹菜素-7,4′--葡萄糖醛酸苷(apigenin-7,4′-di--glucuronide,9)、芹菜素-7--α-鼠李糖基-(1→2)-β-葡萄糖醛酸苷[apigenin-7-- α-rhamnopyranosyl-(1→2)-β-glucuronide,10]、腺嘌呤核苷(adenosine,11)、鸟嘌呤核苷(guanosine,12)、香草酸(vanillic acid,13)、鸢尾番红花素M(crocusatin M,14)、苦藏花素(picrocrocin,15)、二氢红花菜豆酸-3′--β--葡萄糖苷(dihydrophaseic acid-3′--β--glucopyranoside,16)、二氢红花菜豆酸钠盐-3′--β--葡萄糖苷(dihydrophaseic acid sodium salt-3′--β--glucopyranoside,17)、()-芥子酸-4--β--吡喃葡萄糖苷[()-sinapic acid-4--β--glucopyranoside 18]、咖啡酸(caffeic acid,19)、绿原酸(chlorogenic acid,20)。其中,化合物1为新的环烯醚萜苷类化合物,命名为6′--α--鼠李糖基梓醇(图1);化合物9、10、15~18均为首次从醉鱼草属植物中分离得到,而化合物3、8、11、12、19、20为首次从密蒙花中分离得到。
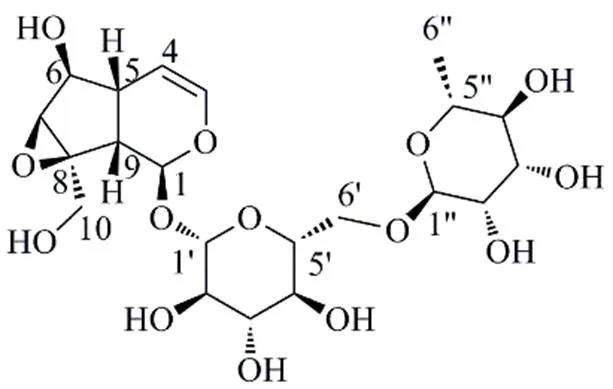
图1 化合物1的结构
1 仪器与材料
Autopol VI 90079型旋光仪(美国Rudolph公司);Agilent G6520 Q-TOF LC-MS型质谱仪(美国Agilent公司);Thermo nicolet 6700型红外光谱仪(美国Thermo公司);Bruker Avance III 500型核磁共振仪(瑞士Bruker公司),以TMS为内标;Shimadzu GCMS-QP2010E型气相色谱仪(日本Shimadzu公司),DB-5MS柱(30 m×0.25 mm×0.25 µm)。所用材料有D-101型大孔吸附树脂(国药集团化学试剂有限公司);ODS-A-HG 12 nm S-50μm(日本YMC公司);Sephadex LH-20型凝胶(瑞典GE Healthcare Bio-Sciences AB公司);硅胶GF254预制薄层色谱板(烟台化学工业研究所);所用试剂有-半胱氨酸甲酯盐酸盐(国药集团化学试剂有限公司);六甲基二硅氮烷(上海士锋生物科技有限公司);三甲基氯硅烷(萨恩化学技术有限公司);-葡萄糖标准品(上海阿拉丁生化科技有限公司);-鼠李糖标准品(上海源叶生物科技有限公司);甲醇、乙醇、醋酸乙酯、正丁醇、吡啶、二氧六环等试剂均为分析纯或色谱纯,由国药集团化学试剂有限公司提供。
本实验药材于2018年购于安徽省亳州药材市场,由上海中医药大学石燕红副研究员鉴定为马钱科醉鱼草属植物密蒙花Maxim.的干燥花蕾及花序。标本(No.MMH-20170427)保存于中国科学院上海药物研究所药物发现与设计中心实验室。
2 提取与分离
干燥的密蒙花花蕾及花序(10.2 kg)加入蒸馏水(80 L),在100 ℃加热回流提取,每次1 h,重复2次,合并滤液减压浓缩至12 L。依次用醋酸乙酯和正丁醇进行萃取,得到醋酸乙酯、正丁醇萃取物以及萃取后水层3个部位。正丁醇萃取部位采用D-101型大孔吸附树脂,依次以蒸馏水、20%乙醇、40%乙醇及95%乙醇进行梯度洗脱,按不同乙醇体积分数洗脱部位浓缩合并得到4个组分(Fr. A~D)。Fr. B(20%乙醇水组分)经ODS柱色谱分离,以蒸馏水、5%甲醇、20%甲醇、40%甲醇、60%甲醇和甲醇进行梯度洗脱后得到6个组分(Fr. B.1~B.6)。Fr. B.1经ODS柱色谱分离,洗脱流动相依次为蒸馏水及10%、20%、30%、40%甲醇,得到6个组分(Fr. B.1.1~B.1.6)。Fr. B.1.1经ODS柱色谱[甲醇-水(0∶100~40∶60)梯度洗脱]和Sephadex LH-20柱色谱(10%甲醇等度洗脱)的不断分离纯化后,得到化合物3(43.2 mg)、18(23.0 mg)、2(28.7 mg)、16(10.6 mg)、17(2.3 mg)。Fr. B.2经Sephadex LH-20柱色谱,5%甲醇为流动相进行等度洗脱后得到5个组分(Fr. B.2.1~B.2.5)。Fr. B.2.1经ODS柱色谱[甲醇-水(0∶100~40∶60)]梯度洗脱和Sephadex LH-20柱色谱(10%甲醇)等度洗脱后,得到化合物5(21.1 mg)、7(19.5 mg)、11(11.4 mg)。Fr. B.2.3和Fr. B.2.4经ODS柱色谱分离[甲醇-水(0∶100~40∶60)梯度洗脱]和Sephadex LH-20柱色谱(10%甲醇等度洗脱)纯化后分别得到化合物20(3.9 mg)和19(5.7 mg)。Fr.B.3经ODS柱色谱分离,依次以蒸馏水及10%、20%、40%、60%甲醇为流动相洗脱,得到6个组分(Fr. B.3.1~B.3.6)。Fr. B.3.2经Sephadex LH-20柱色谱(10%甲醇等度洗脱)和ODS柱色谱[甲醇-水(0∶100~20∶80)]梯度洗脱纯化后得到化合物15(2.1 mg)。Fr. B.3.5经Sephadex LH-20柱色谱(10%甲醇等度洗脱)的不断纯化后得到化合物8(2.4 mg)。Fr. B.4经ODS柱,以蒸馏水及20%、40%、60%甲醇为流动相梯度洗脱后得到4个组分(Fr. B.4.1~B.4.4)。Fr. B.4.1经Sephadex LH-20柱色谱(10%甲醇等度洗脱)和ODS柱色谱[甲醇水(0∶100~30∶70)梯度洗脱]纯化后得到化合物10(7.6 mg)和6(8.3 mg)。Fr. D(95%乙醇组分)经Sephadex LH-20柱色谱(80%甲醇等度洗脱)纯化后,得到化合物4(20.3 mg)。萃取后水层采用D-101型大孔吸附树脂分离,以蒸馏水、10%乙醇、20%乙醇为流动相梯度洗脱后分别得到3个组分(Fr. E~G)。Fr. F(10%乙醇组分)经ODS柱色谱分离,以甲醇-水(0∶100~20∶80)进行梯度洗脱后,得到2个组分(Fr. F.1~F.2)。Fr. F.1和Fr. F.2再经Sephadex LH-20柱色谱,以10%甲醇为流动相进行等度洗脱,分别得到化合物9(851.5 mg)和1(38.1 mg)。Fr. G(20%乙醇组分)经ODS柱色谱分离,以甲醇-水体系(0∶100~20∶80)进行梯度洗脱后,得到3个组分(Fr. G.1~G.3)。Fr. G.2经Sephadex LH-20柱色谱(10%甲醇等度洗脱)和ODS柱色谱[甲醇-水(0∶100~20∶80)梯度洗脱]纯化后得到化合物12(5.0 mg)。醋酸乙酯萃取部位用甲醇-二氯甲烷进行重结晶后过滤得到母液(Fr. H)和固体(Fr. I)。母液浓缩后用DMSO溶解,经ODS柱色谱分离,依次以10%、20%、30%甲醇为流动相梯度洗脱后,分别得到3个组分(Fr. H.1~H.3)。Fr. H.2和Fr. H.3经Sephadex LH-20柱色谱(30%甲醇等度洗脱)和ODS柱色谱[甲醇-水体系(0∶100~40∶60)梯度洗脱]反复纯化后,分别得到化合物14(3.4 mg)和13(26.2 mg)。
3 糖的绝对构型测定
通过酸水解和气相色谱(GC)分析方法对化合物1结构中糖的绝对构型进行确定,具体操作参考文献中的方法[12]。化合物1(1 mg)加入2 mL 10% HCl-二氧六环(1∶1)中,80 ℃加热搅拌2 h后,反应液减压浓缩后氮气吹干,再加入0.1 mol/L-半胱氨酸甲酯盐酸盐(200 μL)和无水吡啶(100 μL),60 ℃加热反应1 h,然后加入2.6 mL三甲基硅烷化试剂六甲基二硅氮烷-三甲基氯硅烷-吡啶(2∶1∶10),继续在60 ℃下加热反应30 min,反应液再加入水和环己烷(各2 mL),摇匀并静置后取上层环己烷层进行GC分析。GC条件为:Shimadzu GCMS-QP2010E型气相色谱仪;DB-5MS柱(30 m×0.25 mm×0.25 µm);FID检测器;载气为氮气;检测温度为 280 ℃;程序升温条件:初始温度100 ℃保持2 min,然后升温至280 ℃(升温速率35 ℃/min),280 ℃保持9 min。-葡萄糖和-鼠李糖作为标准品均按照上述制备方法和检测方法进行测定,并与样品保留时间进行比较。
4 结构鉴定
根据化合物1的HR-ESI-MS/: 531.167 8 [M+Na]+(计算值531.168 4,C21H32O14Na),确定该化合物的分子式为C21H32O14,不饱和度为5。IR(KBr)光谱中3417 cm−1和1655 cm−1处的特征吸收峰表明其结构中含有羟基和不饱和碳-碳双键。1H- NMR数据(表1)显示,低场区有1组烯烃氢信号 [H6.35 (1H, dd,= 5.7, 1.6 Hz, H-3), 5.00 (1H, dd,= 5.7, 4.9 Hz, H-4)],1个与氧连接的次甲基氢信号H4.73 (1H, d,= 9.8 Hz, H-1),2个糖端基氢信号H4.57 (1H, d,= 7.9 Hz, Glc-H-1′), 4.53 (1H, d,= 0.7 Hz, Rha-H-1′′) 和高场区的甲基氢信号H1.11 (3H, d,= 6.2 Hz, Rha-6′′-CH3)。13C-NMR数据(表1)显示化合物含有21个碳原子,结合氢谱和DEPT135谱可推断出1组吡喃葡萄糖基信号C98.4, 76.2, 75.2, 73.4, 69.8, 65.9;1组吡喃鼠李糖基信号C100.5, 70.7, 70.6, 72.0, 68.4, 18.0;1组烯烃碳信号C140.6 (C-3), 103.6 (C-4);苷元上的5个次甲基信号C93.9 (C-1), 77.6 (C-6), 60.7 (C-7), 42.0 (C-9), 37.5 (C-5);1个亚甲基信号C59.5 (C-10) 以及1个季碳信号C64.9 (C-8)。化合物1的这些特征信号与本实验分离得到的已知化合物3(梓醇)[13]的信号相似,不同之处在于,化合物1比梓醇多了1个鼠李糖基的信号,且葡萄糖基的C-6位仲碳的信号C65.9 (Glc-C-6′) 与梓醇的对应信号C61.3相比明显往低场方向移动,推测化合物1含有鼠李糖基,并且与葡萄糖基的C-6′位相连构成芸香糖基。这一推测经HMBC谱(图2)得到验证,其中H3.76 (1H, dd,= 11.0, 1.5 Hz, Glc-H-6′)、H3.45 (1H, dd,= 11.3, 5.5 Hz, Glc-H-6′) 与C100.5 (Rham-C-1′′) 具有远程相关。葡萄糖基和鼠李糖基通过两个端基氢的偶合常数值7.9 Hz和0.7 Hz可确定其相对构型分别为β构型和α构型。结合1D和2D NMR谱,完成了对化合物1碳氢信号的归属(表1)及一维平面结构的确认(图2)。
在ROESY谱(图3)中可以看到,H-1与H-6和H-7均有NOE相关,与Glc-H-1′α没有NOE相关,而H-5与H-9有NOE相关,与H-1、H-6和H-7均没有NOE相关,由此可确定H-1与H-6和H-7在同一平面的同一侧,均为β-H,而H-5与H-9均为α-H。糖的绝对构型是通过化合物1酸水解后进行衍生,用GC分析保留时间(R=13.47、14.53 min),并与相同方法下制备的标准-鼠李糖和-葡萄糖的衍生物的保留时间(R=13.49、14.51 min)进行比较,确定为β--葡萄糖和α--鼠李糖。

表1 化合物1的1H-和13C-NMR数据(500/125 MHz, DMSO-d6)
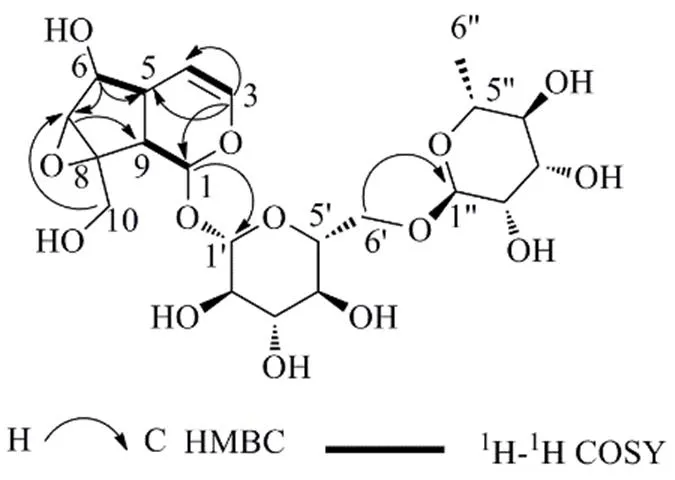
图2 化合物1的HMBC和1H-1H COSY关键相关信号
综上,化合物1的结构鉴定为6α-羟基-8β-羟甲基-1β,5α,6β,7β,9α-五氢-7(8)-环氧-2-oxaind-3-烯- 1--α--吡喃鼠李糖基-(1→6)-β--吡喃葡萄糖苷,为1个新化合物,命名为6′--α--鼠李糖基梓醇。

图3 化合物1的ROESY关键相关信号
化合物2:淡黄色无定形粉末;ESI-MS/: 375 [M-H]−;1H-NMR (500 MHz, DMSO-6): 6.36 (1H, d,= 5.9 Hz, H-3), 4.95 (1H, d,= 5.5 Hz, H-4), 4.93 (1H, d,= 9.9 Hz, H-1), 4.57 (1H, d,= 7.9 Hz, Glc-H-1), 3.89 (1H, d,= 13.2 Hz, H-10), 3.69 (1H, overlapped, H-10), 3.66 (1H, overlapped, Glc-H-6), 3.60 (1H, br s, H-7), 3.57 (1H, d,= 8.2 Hz, H-6), 3.39 (1H, dd,= 11.9, 6.7 Hz, Glc-H-6), 3.35 (3H, s, 6-OCH3), 3.18 (1H, t,= 8.9 Hz, Glc-H-3), 3.15~3.12 (1H, m, Glc-H-4), 3.03~3.00 (1H, overlapped, Glc-H-2), 3.03~3.00 (1H, overlapped, Glc-H-5), 2.33 (1H, dd,= 9.2, 8.1 Hz, H-9), 2.20~2.17 (1H, m, H-5);13C-NMR (125 MHz, DMSO-6): 140.7 (C-3), 103.0 (C-4), 97.9 (Glc-C-1), 93.2 (C-1), 86.3 (C-6), 77.5 (Glc-C-3), 76.4 (Glc-C-5), 73.4 (Glc-C-2), 70.2 (Glc-C-4), 65.1 (C-8), 61.3 (Glc-C-6), 58.9 (C-10), 57.1 (C-7), 57.0 (6-OCH3), 41.8 (C-9), 35.5 (C-5)。以上数据与文献报道基本一致[14],故鉴定化合物2为6--甲基梓醇。
化合物3:白色无定形粉末;ESI-MS/: 361 [M-H]−;1H-NMR (500 MHz, DMSO-6): 6.35 (1H, d,= 5.9 Hz, H-3), 5.00 (1H, t,= 5.2 Hz, H-4), 4.89 (1H, d,= 9.7 Hz, H-1), 4.58 (1H, d,= 7.9 Hz, Glc-H-1), 3.87 (1H, d,= 13.1 Hz, H-10), 3.77 (1H, d,= 8.1 Hz, 6-H), 3.68 (1H, d,= 11.5 Hz, Glc-H-6), 3.63 (1H, d,= 13.1 Hz, H-10), 3.40 (1H, overlapped, Glc-H-6), 3.34 (1H, brs, H-7), 3.20~3.16 (1H, m, Glc-H-3), 3.14~3.11 (1H, m, Glc-H-4), 3.03~3.00 (1H, overlapped, Glc-H-2), 3.03~3.00 (1H, overlapped, Glc-H-5), 2.33~2.28 (1H, m, H-9), 2.11 (1H, dd,= 12.2, 7.7 Hz, H-5);13C-NMR (125 MHz, DMSO-6): 140.3 (C-3), 103.4 (C-4), 97.9 (Glc-C-1), 93.3 (C-1), 77.5 (Glc-C-3), 77.2 (C-6), 76.4 (Glc-C-5), 73.4 (Glc-C-2), 70.2 (Glc-C-4), 64.8 (C-8), 61.3 (Glc-C-6), 60.7 (C-7), 59.0 (C-10), 42.1 (C-9), 37.4 (C-5)。以上数据与文献报道基本一致[13],故鉴定化合物3为梓醇。
化合物4:淡黄色无定形粉末;ESI-MS/: 615 [M+Na]+;1H-NMR (500 MHz, DMSO-6): 8.01 (2H, d,= 8.8 Hz, H-2′, 6′), 7.13 (2H, d,= 8.8 Hz, H-3′, 5′), 6.85 (1H, s, H-3), 6.79 (1H, d,= 1.5 Hz, H-8), 6.46 (1H, d,= 2.0 Hz, H-6), 5.04 (1H, d,= 7.4 Hz, Glc-H-1), 4.55 (1H, d,= 0.8 Hz, Rha-H-1), 3.87 (1H, overlapped, Glc-H-6), 3.84 (3H, s, 4′-OCH3), 3.67~3.66 (1H, m, Rha-H-2), 3.62~3.59 (1H, m, Glc-H-5), 3.47 (1H, overlapped, Rha-H-3), 3.45 (1H, overlapped, Glc-H-6), 3.42 (1H, overlapped, Rha-H-5), 3.33 (1H, overlapped, Glc-H-3), 3.29 (1H, overlapped, Glc-H-2), 3.18 (1H, d,= 9.7 Hz, Glc-H-4), 3.14 (1H, d,= 9.5 Hz, Rha-H-4), 1.06 (3H, d,= 6.2 Hz, Rha-6-CH3);13C-NMR (125 MHz, DMSO-6): 182.4 (C-4), 164.5 (C-2), 163.3 (C-9), 162.9 (C-4′), 161.2 (C-5), 157.4 (C-7), 128.9 (C-2′, 6′), 123.0 (C-1′), 115.2 (C-3′, 5′), 105.9 (C-10), 104.2 (C-3), 100.8 (C-6), 100.3 (Rha-C-1), 100.1 (Glc-C-1), 95.4 (C-8), 76.4 (Glc-C-3), 76.0 (Glc-C-5), 73.3 (Glc-C-2), 72.4 (Rha-C-4), 71.0 (Rha-C-2), 70.6 (Rha-C-3), 69.9 (Glc-C-4), 68.7 (Rha-C-5), 66.4 (Glc-C-6), 56.0 (4′-OCH3), 18.1 (Rha-C-6)。以上数据与文献报道基本一致[15],故鉴定化合物4为蒙花苷。
化合物5:淡黄色无定形粉末;ESI-MS/: 577 [M-H]−;1H-NMR (500 MHz, CD3OD): 7.89 (2H, d,= 8.8 Hz, H-2′, 6′), 6.97 (2H, d,= 8.8 Hz, H-3′, 5′), 6.78 (1H, d,= 2.0 Hz, H-8), 6.67 (1H, s, H-3), 6.54(1H, d,= 2.0 Hz, H-6), 5.07 (1H, d,= 7.1 Hz, Glc-H-1), 4.74 (1H, d,= 1.3 Hz, Rha-H-1), 4.06 (1H, d,= 9.5 Hz, Glc-H-6), 3.92 (1H, dd,= 3.4, 1.6 Hz, Rha-H-2), 3.73 (1H, dd,= 9.5, 3.4 Hz, Rha-H-3), 3.69~3.65 (3H, overlapped, Rha-H-5, Glc-H-6, 5), 3.53~3.51 (2H, overlapped, Glc-H-2, 3), 3.45~3.42 (1H, m, Glc-H-4), 3.35 (1H, overlapped, Rha-H-4), 1.20 (3H, d,= 6.2 Hz, Rha-6-CH3);13C-NMR (125 MHz, CD3OD): 184.1 (C-4), 166.8 (C-2), 164.7 (C-7), 163.0 (C-5), 162.9 (C-4′), 158.9 (C-9), 129.7 (C-2′, 6′), 123.1 (C-1′), 117.1 (C-3′, 5′), 107.1 (C-10), 104.2 (C-3), 102.1 (Glc-C-1), 101.5 (Rha-C-1), 101.1 (C-6), 96.3 (C-8), 77.8 (Glc-C-3), 77.1 (Glc-C-5), 74.7 (Glc-C-2), 74.1 (Rha-C-4), 72.4 (Rha-C-3), 72.1 (Rha-C-2), 71.3 (Glc-C-4), 69.8 (Rha-C-5), 67.4 (Glc-C-6), 17.9 (Rha-C-6)。以上数据与文献报道基本一致[16],故鉴定化合物5为芹菜素-7--芦丁糖苷。
化合物6:黄色无定形粉末;ESI-MS/: 593 [M-H]−;1H-NMR (500 MHz, CD3OD): 7.38 (2H, overlapped, H-2′, 6′), 6.91 (1H, d,= 8.8 Hz, H-5′), 6.71 (1H, brs, H-8), 6.57 (1H, s, H-3), 6.50 (1H, d,= 1.7 Hz, H-6), 5.03 (1H, d,= 7.0 Hz, Glc-H-1), 4.72 (1H, brs, Rha-H-1), 4.05 (1H, d,= 9.7 Hz, Glc-H-6), 3.91 (1H, dd,= 1.8 Hz, Rha-H-2), 3.74 (1H, dd,= 9.5, 3.4 Hz, Rha-H-3), 3.68~3.61 (3H, overlapped, Rha-H-5, Glc-H-6, 5), 3.52~3.45 (2H, overlapped, Glc-H-2, 3), 3.40 (1H, t,= 8.8 Hz, Glc-H-4), 3.35 (1H, overlapped, Rha-H-4), 1.19 (3H, d,= 6.2 Hz, Rha-6-CH3);13C-NMR (125 MHz, CD3OD): 184.0 (C-4), 166.9 (C-2), 164.7 (C-7), 162.9 (C-5), 158.9 (C-9), 151.2 (C-4′), 147.0 (C-3′), 123.5 (C-1′), 120.6 (C-6′), 116.9 (C-2′), 114.3 (C-5′), 107.1 (C-10), 104.3 (C-3), 102.1 (Glc-C-1), 101.6 (Rha-C-1), 101.1 (C-6), 96.2 (C-8), 77.8 (Glc-C-3), 77.2 (Glc-C-5), 74.8 (Glc-C-2), 74.1 (Rha-C-4), 72.4 (Rha-C-3), 72.1 (Rha-C-2), 71.3 (Glc-C-4), 69.8 (Rha-C-5), 67.5 (Glc-C-6), 17.9 (Rha-C-6)。以上数据与文献报道基本一致[17],故鉴定化合物6为木犀草素-7--芦丁糖苷。
化合物7:黄色无定形粉末;ESI-MS/: 737 [M-H]−;1H-NMR (500 MHz, CD3OD): 7.98 (2H, d,= 8.3 Hz, H-2′, 6′), 7.12 (2H, d,= 8.3 Hz, H-3′, 5′), 6.75 (1H, s, H-3), 6.71 (1H, brs, H-8), 6.52 (1H, brs, H-6), 5.31 (1H, brs, Rha-H-1′′′), 5.20 (1H, d,= 7.2 Hz, Glc-H-1′′), 4.72 (1H, brs, Rha-H-1′′′′), 4.06 (1H, d,= 9.5 Hz, Glc-H-6′′), 3.97 (2H, overlapped, Rha-H-2′′′, 2′′′′), 3.91 (3H, s, 4′-OCH3), 3.72~3.65 (8H, overlapped, Rha-H-3′′′′, 5′′′′, Glc-H-2′′, 3′′, 5′′, 6′′, Rha-H-3′′′, 5′′′), 3.46~3.42 (2H, overlapped, Rha-H-4′′′, Glc-H-4′′), 3.37 (1H, overlapped, Rha-H-4′′′′), 1.37 (3H, d,= 5.8 Hz, Rha-6′′′-CH3), 1.19 (3H, d,= 5.8 Hz, Rha-6′′′′-CH3);13C-NMR (125 MHz, CD3OD): 184.0 (C-4), 166.5(C-2), 164.5 (C-7), 164.4 (C-4′), 163.1 (C-5), 158.9 (C-9), 129.5 (C-2′, 6′), 124.4 (C-1′), 115.7 (C-3′, 5′), 107.2 (C-10), 104.7 (C-3), 102.5 (Rha-C-1′′′′), 102.1 (Rha-C-1′′′), 101.0 (C-6), 99.8 (Glc-C-1′′), 96.1 (C-8), 79.0 (Glc-C-3′′), 79.0 (Glc-C-2′′), 77.0 (Glc-C-5′′), 74.1 (Rha-C-4′′′′), 74.0 (Rha-C-4′′′), 72.4 (Rha-C-3′′′′), 72.2 (Rha-C-2′′′, 2′′′′), 72.1 (Rha-C-3′′′), 71.4 (Glc-C-4′′), 70.0 (Rha-C-5′′′′), 69.8 (Rha-C-5′′′), 67.4 (Glc-C-6′′), 56.1 (4′-OCH3), 18.3 (Rha-C-6′′′), 17.9 (Rha-C-6′′′′)。以上数据与文献报道基本一致[18],故鉴定化合物7为密蒙花新苷。
化合物8:淡黄色无定形粉末;ESI-MS/: 447 [M+H]+;1H-NMR (500 MHz, DMSO-6): 7.94 (2H, d,= 5.7 Hz, H-2′, 6′), 6.93 (2H, d,= 5.7 Hz, H-3′, 5′), 6.85 (1H, s, H-3), 6.82 (1H, brs, H-8), 6.43 (1H, brs, H-6), 5.10 (1H, brs, H-1′′), 3.68~3.15 (4H, overlapped, H-2′′~5′′);13C-NMR (125 MHz, DMSO-6): 182.0 (C-4), 171.2 (C-6′′), 164.3 (C-2), 163.0 (C-7), 161.5 (C-5), 161.1 (C-4′), 157.0 (C-9), 128.6 (C-2′, 6′), 120.9 (C-1′), 116.1 (C-3′, 5′), 105.3 (C-10), 103.1 (C-3), 99.6 (C-6), 99.6 (C-1′′), 94.8 (C-8), 76.4 (C-5′′), 74.3 (C-3′′), 72.9 (C-2′′), 71.9 (C-4′′)。以上数据与文献报道基本一致[19],故鉴定化合物8为芹菜素-7--半乳糖醛酸苷。
化合物9:淡棕色无定形粉末;ESI-MS/: 621 [M-H]−;1H-NMR (500 MHz, DMSO-6): 12.90 (1H, s, 5-OH), 8.08 (2H, d,= 9.0 Hz, H-2′, 6′), 7.22 (2H, d,= 9.0 Hz, H-3′, 5′), 6.98 (1H, s, H-3), 6.89 (1H, d,= 2.1 Hz, H-8), 6.48 (1H, d,= 2.1 Hz, H-6), 5.27 (1H, d,= 7.2 Hz, H-1′′), 5.24 (1H, d,= 7.3 Hz, H-1′′′), 4.05 (1H, d,= 9.6 Hz, H-5′′), 4.00 (1H, d,= 9.6 Hz, H-5′′′), 3.44~3.29 (6H, overlapped, H-2′′~4′′, 2′′′~4′′′);13C-NMR (125 MHz, DMSO-6): 182.1 (C-4), 170.0 (C-6′′), 170.0 (C-6′′′), 163.6 (C-2), 162.6 (C-7), 161.2 (C-5), 160.0 (C-4′), 157.0 (C-9), 128.4 (C-2′, 6′), 124.1 (C-1′), 116.5 (C-3′, 5′), 105.6 (C-10), 104.3 (C-3), 99.5 (C-6), 99.3 (C-1′′), 99.2 (C-1′′′), 94.8 (C-8), 75.8 (C-5′′), 75.7 (C-5′′′), 75.4 (C-3′′), 75.4 (C-3′′′), 72.9 (C-2′′′), 72.8 (C-2′′′), 71.3 (C-4′′′), 71.3 (C-4′′′)。以上数据与文献报道基本一致[20],故鉴定化合物9为芹菜素-7,4′--葡萄糖醛酸苷。
化合物10:淡黄色无定形粉末;ESI-MS/: 591 [M-H]−;1H-NMR (500 MHz, DMSO-6): 7.89 (2H, d,= 8.0 Hz, H-2′, 6′), 6.92 (2H, d,= 8.0 Hz, H-3′, 5′), 6.82 (1H, s, H-3), 6.77 (1H, brs, H-8), 6.36 (1H, brs, H-6), 5.27 (1H, d,= 6.6 Hz, H-1′′), 5.12 (1H, s, H-1′′′), 3.77 (1H, d,= 9.3 Hz, H-5′′), 3.72 (1H, dd,= 9.3, 6.3 Hz, H-5′′′), 3.68 (1H, brs, H-2′′′), 3.50 (1H, overlapped, H-2′′), 3.45 (1H, overlapped, H-3′′), 3.31 (1H, overlapped, H-3′′′), 3.20 (1H, overlapped, H-4′′), 3.20 (1H, overlapped, H-4′′′), 1.19 (3H, d,= 6.2 Hz, 6′′′-CH3);13C-NMR (125 MHz, DMSO-6): 182.0 (C-4), 171.5 (C-6′′), 164.3 (C-2), 162.5 (C-7), 161.6 (C-4′), 161.1 (C-5), 157.0 (C-9), 128.5 (C-2′, 6′), 120.8 (C-1′), 116.0 (C-3′, 5′), 105.4 (C-10), 103.1 (C-3), 100.6 (C-1′′′), 99.3 (C-6), 97.4 (C-1′′), 94.3 (C-8), 77.2 (C-3′′), 76.2 (C-2′′), 74.0 (C-5′′), 71.9 (C-4′′), 71.9 (C-4′′′), 70.5 (C-2′′′), 70.4 (C-3′′′), 68.4 (C-5′′′), 18.1 (C-6′′′)。以上数据与文献报道基本一致[21],故鉴定化合物10为芹菜素-7-- α-鼠李糖基-(1→2)-β-葡萄糖醛酸苷。
化合物11:黄色无定形粉末;ESI-MS/: 268 [M+H]+;1H-NMR (500 MHz, D2O): 8.22 (1H, s, H-8), 8.14 (1H, s, H-2), 5.96 (1H, s, H-1′), 4.38 (1H, s, H-3′), 4.25 (1H, s, H-4′), 3.87 (1H, overlapped, H-5′a), 3.80 (1H, overlapped, H-5′b);13C-NMR (125 MHz, D2O): 155.4 (C-6), 152.4 (C-2), 148.3 (C-4), 140.5 (C-8), 119.0 (C-5), 88.3 (C-1′), 85.8 (C-4′), 73.7 (C-2′), 70.6 (C-3′), 61.5 (C-5′)。以上数据与文献报道基本一致[22],故鉴定化合物11为腺嘌呤核苷。
化合物12:淡黄色胶状固体;ESI-MS/: 282 [M-H]−;1H-NMR (500 MHz, DMSO-6): 7.93 (1H, s, H-8), 6.48 (2H, brs, 2-NH2), 5.68 (1H, d,= 6.0 Hz, H-1′), 4.38 (1H, t,= 6.0 Hz, H-2′), 4.07 (1H, t,= 4.0 Hz, H-3′), 3.86 (1H, d,= 3.6 Hz, H-4′), 3.60 (1H, m, H-5′a), 3.51 (1H, m, H-5′b);13C-NMR (125 MHz, DMSO-6): 156.8 (C-6), 153.7 (C-2), 151.3 (C-4), 135.6 (C-8), 116.7 (C-5), 86.4 (C-1′), 85.2 (C-4′), 73.7 (C-2′), 70.4 (C-3′), 61.4 (C-5′)。以上数据与文献报道基本一致[23],故鉴定化合物12为鸟嘌呤核苷。
化合物13:淡黄色无定形粉末;ESI-MS/: 167 [M-H]−;1H-NMR (500 MHz, CD3OD): 7.60~7.55 (2H, m, H-2, 6), 6.84 (1H, d,= 8.7 Hz, H-5), 3.89 (3H, s, 3-OCH3);13C-NMR (125 MHz, CD3OD): 170.0 (C-7), 152.7 (C-4), 148.6 (C-3), 125.3 (C-6), 123.1 (C-1), 115.8 (C-2), 113.8 (C-5), 56.4 (3-OCH3)。以上数据与文献报道基本一致[24],故鉴定化合物13为香草酸。
化合物14:黄色油状物;ESI-MS/: 207 [M+Na]+;1H-NMR (500 MHz, CD3OD): 5.95 (1H, brs, H-4), 3.80 (1H, d,= 11.5 Hz, H-7), 3.66 (1H, d,= 11.5 Hz, H-7), 2.95 (1H, d,= 17.3 Hz, H-2), 2.10 (1H, d,= 17.3 Hz, H-2), 2.05 (3H, s, 10-CH3), 1.14 (3H, s, 9-CH3), 1.04 (3H, s, 8-CH3);13C-NMR (125 MHz, CD3OD): 201.8 (C-3), 167.7 (C-5), 128.3 (C-4), 78.9 (C-6), 65.9 (C-7), 51.0 (C-2), 41.3 (C-1), 25.3 (C-8), 24.1 (C-9), 20.1 (C-10)。以上数据与文献报道基本一致[7],故鉴定化合物14为鸢尾番红花素M。
化合物15:白色无定形粉末;ESI-MS/: 353 [M+Na]+;1H-NMR (500 MHz, CD3OD): 10.09 (1H, s, H-7), 4.43 (1H, d,= 7.7 Hz, Glc-H-1), 4.09 (1H, m, H-4), 3.86 (1H, d,= 12.4 Hz, Glc-H-6), 3.67 (1H, m, Glc-H-6), 3.36~3.30 (3H, overlapped, Glc-H-5, Glc-H-4, Glc-H-3), 3.16 (1H, t,= 8.2 Hz, Glc-H-2), 2.69 (1H, dd,= 18.8, 4.6 Hz, H-3a), 2.32 (1H, dd,= 18.8, 8.9 Hz, H-3b), 2.15 (3H, s, 10-CH3), 1.86 (1H, d,= 12.5 Hz, H-5a), 1.54 (1H, t,= 12.5 Hz, H-5b), 1.24 (3H, s, 8-CH3), 1.22 (3H, s, 9-CH3);13C-NMR (125 MHz, CD3OD): 193.5 (C-7), 155.7 (C-2), 141.1 (C-1), 102.6 (Glc-C-1), 78.1 (Glc-C-5), 77.9 (Glc-C-3), 75.1 (Glc-C-2), 72.0 (C-4), 71.6 (Glc-C-4), 62.7 (Glc-C-6), 48.3 (C-5), 42.3 (C-3), 36.6 (C-6), 29.3 (C-8), 27.9 (C-9), 19.3 (C-10)。以上数据与文献报道基本一致[25],故鉴定化合物15为苦藏花素。
化合物16:淡黄色无定形粉末;ESI-MS/: 443 [M-H]−;1H-NMR (500 MHz, CD3OD): 7.77 (1H, d,= 16.0 Hz, H-4), 6.31 (1H, d,= 16.0 Hz, H-5), 5.82 (1H, s, H-2), 4.36 (1H, d,= 7.8 Hz, Glc-H-1), 4.24 (1H, m, H-3′), 3.86 (1H, d,= 11.1 Hz, Glc-H-6), 3.79 (1H, dd,= 7.2, 1.7 Hz, H-7′a), 3.74 (1H, d,= 7.2 Hz, H-7′b), 3.68~3.66 (1H, m, Glc-H-6), 3.34 (1H, overlapped, Glc-H-3), 3.29 (1H, overlapped, Glc-H-4), 3.27 (1H, overlapped, Glc-H-5), 3.14 (1H, dd,= 9.0, 8.0 Hz, Glc-H-2), 2.17 (1H, m, H-4′ax), 1.97 (3H, s, 6-CH3), 1.95 (1H, m, H-2′ax), 1.82 (1H, m, H-4′eq), 1.79 (1H, m, H-2′eq), 1.16 (3H, s, 9′-CH3), 0.93 (3H, s, 10′-CH3);13C-NMR (125 MHz, CD3OD): 174.3 (C-1), 143.3 (C-3), 132.8 (C-5), 131.3 (C-4), 126.2 (C-2), 103.1 (Glc-C-1), 87.6 (C-5′), 83.2 (C-8′), 78.0 (Glc-C-3), 77.9 (Glc-C-5), 77.1 (C-7′), 75.1 (Glc-C-2), 74.0 (C-3′), 71.6 (Glc-C-4), 62.7 (Glc-C-6), 42.8 (C-2′), 42.7 (C-4′), 20.7 (C-6), 19.7 (C-9′), 16.4 (C-10′)。以上数据与文献报道基本一致[26],故鉴定化合物16为二氢红花菜豆酸-3′--β--葡萄糖苷。
化合物17:淡黄色无定形粉末;ESI-MS/: 466 [M-H]−;1H-NMR (500 MHz, DMSO-6): 7.94 (1H, d,= 15.9 Hz, H-4), 6.25 (1H, d,= 15.9 Hz, H-5), 5.69 (1H, s, H-2), 4.19 (1H, d,= 7.8 Hz, Glc-H-1), 4.08 (1H, m, H-3′), 3.66 (1H, overlapped, Glc-H-6), 3.64 (1H, overlapped, H-7′a), 3.57 (1H, d,= 7.1 Hz, H-7′b), 3.37 (1H, overlapped, Glc-H-6), 3.12 (1H, t,= 8.4 Hz, Glc-H-3), 3.09 (1H, m, Glc-H-4), 3.03 (1H, t,= 9.0 Hz, Glc-H-5), 2.89 (1H, t,= 8.4 Hz, Glc-H-2), 2.05 (1H, dd,= 13.3, 6.7 Hz, H-4′ax), 1.96 (3H, s, 6-CH3), 1.82 (1H, dd,= 13.3, 6.7 Hz, H-2′ax), 1.64 (1H, overlapped, H-4′eq), 1.63 (1H, overlapped, H-2′eq), 1.05 (3H, s, 9′-CH3), 0.84 (3H, s, 10′-CH3);13C-NMR (125 MHz, DMSO-6): 130.4 (C-5), 130.4 (C-4), 101.5 (Glc-C-1), 85.5 (C-5′), 81.2 (C-8′), 76.8 (Glc-C-3), 76.7 (Glc-C-5), 75.1 (C-7′), 73.4 (Glc-C-2), 71.5 (C-3′), 70.1 (Glc-C-4), 61.1 (Glc-C-6), 47.8 (C-1′), 41.7 (C-2′), 41.5 (C-4′), 20.7 (C-6), 19.6 (C-9′), 16.1 (C-10′)。在13C-NMR中未检测到C-1、C-2和C-3的信号,可能是其羧基形成了钠盐,碳原子弛豫时间较长的关系。以上数据与文献报道基本一致[27],故鉴定化合物17为二氢红花菜豆酸钠盐-3′--β--葡萄糖苷。
化合物18:淡黄色无定形粉末;ESI-MS/: 385 [M-H]−;1H-NMR (500 MHz, DMSO-6): 7.20 (1H, d,= 15.8 Hz, H-7), 6.87 (2H, s, H-2, 6), 6.43 (1H, d,= 15.8 Hz, H-8), 4.97 (1H, d,= 6.3 Hz, Glc-H-1), 3.78 (6H, s, 3, 5-OCH3), 3.57 (1H, d,= 11.4 Hz, Glc-H-6), 3.42 (1H, dd,= 11.4, 5.2 Hz, Glc-H-6), 3.21~3.20 (2H, m, Glc-H-3, 4), 3.15 (1H, m, Glc-H-2), 3.03 (1H, m, Glc-H-5);13C-NMR (125 MHz, DMSO-6): 169.5 (C-9), 152.7 (C-3, 5), 138.3 (C-7), 135.0 (C-4), 131.5 (C-1), 125.8 (C-8), 105.7 (C-2, 6), 102.5 (Glc-C-1), 77.3 (Glc-C-5), 76.6 (Glc-C-3), 74.2 (Glc-C-2), 69.9 (Glc-C-4), 60.8 (Glc-C-6), 56.4 (3, 5-OCH3)。以上数据与文献报道基本一致[28],故鉴定化合物18为()-芥子酸-4-- β--吡喃葡萄糖苷。
化合物19:淡黄色无定形粉末;ESI-MS/: 179 [M-H]−;1H-NMR (500 MHz, CD3OD): 7.53 (1H, d,= 15.9 Hz, H-7), 7.03 (1H, d,= 2.0 Hz, H-2), 6.93 (1H, dd,= 8.2, 2.0 Hz, H-6), 6.78 (1H, d,= 8.2 Hz, H-5), 6.22 (1H, d,= 15.9 Hz, H-8);13C-NMR (125 MHz, CD3OD): 171.1 (C-9), 149.4 (C-4), 147.0 (C-3), 146.8 (C-7), 127.8 (C-1), 122.8 (C-6), 116.5 (C-5), 115.6 (C-8), 115.1 (C-2)。以上数据与文献报道基本一致[29],故鉴定化合物19为咖啡酸。
化合物20:淡黄色无定形粉末;ESI-MS/: 377 [M+Na]+;1H-NMR (500 MHz, CD3OD): 7.56 (1H, d,= 15.9 Hz, H-7′), 7.04 (1H, d,= 1.1 Hz, H-2′), 6.93 (1H, d,= 8.3 Hz, H-6′), 6.77 (1H, d,= 8.3 Hz, H-5′), 6.28 (1H, d,= 15.9 Hz, H-8′), 5.37 (1H, brs, H-5), 4.18 (1H, brs, H-3), 3.71 (1H, d,= 7.5 Hz, H-4), 2.25~2.01 (4H, m, H-2, 6);13C-NMR (125 MHz, CD3OD): 168.8 (C-9′), 149.6 (C-4′), 147.1 (C-7′), 146.8 (C-3′), 127.8 (C-1′), 123.0 (C-6′), 116.5 (C-5′), 115.3 (C-8′), 115.2 (C-2′)。该化合物采用HPLC方法在相同条件下与绿原酸对照品进行比对,保留时间一致,且氢谱数据与文献报道基本一致[30],故鉴定化合物20为绿原酸。
5 讨论
本实验从密蒙花水提取物中分离并鉴定了20个化合物,包括1个新的环烯醚萜苷类化合物(1)以及2个已知环烯醚萜类化合物(2、3),7个黄酮类化合物(4~10),2个生物碱类化合物(11、12)和8个其他类型化合物(13~20)。其中,有6个化合物为醉鱼草属中首次分离得到,6个化合物为密蒙花中首次分离得到。密蒙花作为一味传统的防治眼疾的中药,具有1000多年临床用药历史,时至今日仍是中医药防治眼部疾病的常规用药,但截至目前,国内外有关密蒙花防治眼部疾病的研究多集中在提取物层面,对其治疗眼疾的药效物质尚不清楚。因此,开展密蒙花中单体化合物防治眼疾的现代药理研究是十分必要的,值得深入研究。
利益冲突 所有作者均声明不存在利益冲突
[1] 中国科学院中国植物志编辑委员会. 中国植物志-第八卷 [M]. 北京: 科学出版社, 1992: 277-279.
[2] 中国药典 [S]. 一部. 2020: 343.
[3] Matsuda H, Cai H, Kubo M,. Study on anti-cataract drugs from natural sources. II. Effects ofonaldose reductase activity [J]., 1995, 18(3): 463-466.
[4] Zhang H Y, PanJX. Phenylpropanoid glycosides and flavonoid glycosides isolated from buds ofMaxim. [J]., 1996, 5(2): 105-110.
[5] Guo H Z, Koike K, Li W,. Saponins from the flower buds of[J]., 2004, 67(1): 10-13.
[6] Tai B H, Nhiem N X, Quang T H,. ChemInform abstract: A new iridoid and effect on the rat aortic vascular smooth muscle cell proliferation of isolated compounds from[J]., 2011, 21(11): 3462-3466.
[7] Lee C, Lee S, Park S Y. A new monoterpene from the flower buds of[J]., 2013, 19(4): 355-359.
[8] Park T W, Lee C, LeeW,. Chemical constituents fromand their inhibitory effects on nitric oxide production [J]., 2016, 22(2): 129-133.
[9] Xie G Y, Xu Q H, Li R,. Chemical profiles and quality evaluation offlowers by HPLC-DAD and HPLC-Q-TOF-MS/MS [J]., 2019, 164: 283-295.
[10] Tai B H, Jung B Y, Cuong N M,. Total peroxynitrite scavenging capacity of phenylethanoid and flavonoid glycosides from the flowers of[J]., 2009, 32(12): 1952-1956.
[11] Sheng G Q, Zhang J R, Pu X P,. Protective effect of verbascoside on 1-methyl-4-phenylpyridinium Ion-induced neurotoxicity in PC12 cells [J]., 2002, 451(2): 119-124.
[12] Gao Y Y, Zeng P, Jia C L,. Two new phenols from[J]., 2017, 19(1): 28-34.
[13] Tatli İ İ, Akdemir Z Ş. 6--α--rhamnopyranosylcatalpol derivative iridoids from[J]., 2003, 27(6): 765-772.
[14] El-Domiaty M M, Wink M, Aal M M A,. Antihepatotoxic activity and chemical constituents oflour [J]., 2009, 64(1/2): 11-19.
[15] Zhang Q, Zhou Q Q, Huo C H,. Phenolic components of the aerial parts of[J]., 2019, 55(2): 337-339.
[16] Abu-Gharbieh E, Shehab N G. Therapeutic potentials ofvar.-Maire leaves and its isolated compounds [J]., 2017, 17: 218.
[17] Petrović S D, Gorunović M S, Wray V,. A taraxasterol derivative and phenolic compounds fromgymnocephalum [J]., 1999, 50(2): 293-296.
[18] Huynh L, Tran H, Bacher M,. Iridoids and flavonoids fromWall. [J]., 2016, 5(3): 245-249.
[19] Yamasaki T, Masuoka C, Nohara T,. A new phenylethanoid glycoside from the fruits ofThunb. var.Rehd [J]., 2007, 61(3): 318-322.
[20] Wagner H, Danninger H, Seligmann O,. Synthesis of glucosiduronic acids in the flavonoid series. V. Synthesis of a naturally occurring flavonoid diglucuronide (apigenin 4′,7-di--β--glucuronide) and of chrysoeriol 7-mono--β--glucuronide [J]., 1973, 106(8): 2536- 2541.
[21] Huang Y, de Bruyne T, Apers S,. Flavonoid glucuronides from-[J]., 1999, 52(8): 1701-1703.
[22] de Novais L M R, de Arueira C C O, Ferreira L F,. 4′-Hydroxy-6,7-methylenedioxy-3-methoxyflavone: A novel flavonoid fromegleri with potential inhibitory activity against cathepsins B and L [J]., 2019, 132: 26-29.
[23] Peng Y, Li J F, Huang R M,. Chemical constituents of the South China sea starfish[J]., 2019, 55(6): 1190-1191.
[24] Hong S S, Choi C W, Choi Y H,. Coixlachryside A: A new lignan glycoside from the roots ofL. var.Stapf. [J]., 2016, 17: 152-157.
[25] 陈天翱, 陈封政. 西红花中苦藏花素的分离及抑菌研究 [J]. 生物化工, 2018, 4(5): 8-10.
[26] Youn U J, Lee J, Nam J W,. Identification of a new isomer of dihydrophaseic acid 3′--β--glucopyranoside from[J]., 2011, 32(11): 4083-4085.
[27] 程智, 王伦, 陈斌, 等. 苍耳子的化学成分 [J]. 应用与环境生物学报, 2011, 17(3): 350-352.
[28] Dang J, Wen H X, Wang W D,. Isolation and identification of water-soluble components ofleaves [J]., 2019, 55(1): 138-140.
[29] Shin S H, Lee S R, Lee E,. Caffeic acid phenethyl ester from the twigs ofinhibits malignant cell transformation by inducing c-fos degradation [J]., 2017, 80(7): 2124-2130.
[30] Hu W C, Zhou J, Shen T,. Target-guided isolation of three main antioxidants from(Fort.) Carr. leaves using HSCCC [J]., 2019, 24(10): 1907.
A new iridoid glycoside from
LONG Ze-hai1, WANG Qi-yao1, LI Bo2, 3, ZHANG Yong2, 3, JIA Qi1, LI Yi-ming1, ZHU Wei-liang2, 3
1. School of Pharmacy, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China 2. Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China 3. University of Chinese Academy of Sciences, Beijing 100049, China
To study the chemical constituents from water extract of the air-dried flower buds of(Loganiaceae).The compounds were isolated and purified by reflux-extraction, solvent extraction and column chromatography (CC), and their chemical structures were elucidated on the basis of 1D-NMR, 2D-NMR, and MS data.Twenty compounds were isolated from the water extract ofand identified as 6α-hydroxy-8β-hydroxymethyl- 1β,5α,6β,7β,9α-pentahydro-7(8)-epoxy-2-oxaind-3-ene-1--α--rhamnopyranosyl-(1→6)-β--glucopyranoside (1), 6--methylcatalpol (2), catalpol (3), linarin (4), apigenin-7--rutinoside (5), luteolin-7--rutinoside (6), neobudofficide (7), apigenin-7--galacturonide (8), apigenin-7,4′-di--glucuronide (9), apigenin-7--α-rhamnopyranosyl-(1→2)-β-glucuronide (10), adenosine (11), guanosine (12), vanillic acid (13), crocusatin M (14), picrocrocin (15), dihydrophaseic acid-3′--β--glucopyranoside (16), dihydrophaseic acid sodium salt-3′--β--glucopyranoside (17), ()-sinapic acid-4--β--glucopyranoside (18), caffeic acid (19), and chlorogenic acid (20).Compound 1 is a new iridoid glycoside, named 6′--α--rhamnopyranosyl-catalpol, and compounds 9, 10, 15—18 are isolated fromgenus for the first time. Furthermore, 3, 8, 11, 12, 19 and 20 are isolated from the flower buds offor the first time.
(Buddleia auct.) Linn.;Maxim.; iridoid glycoside; 6′--α--rhamnopyranosyl-catalpol; apigenin-7,4′-di--glucuronide; picrocrocin
R284.1
A
0253 - 2670(2021)01 - 0035 - 10
10.7501/j.issn.0253-2670.2021.01.006
2020-11-24
国家自然科学基金资助项目(81603270);上海市扬帆计划(16YF1414100)
龙泽海(1995—),男,硕士在读,研究方向为中药化学成分。E-mail: 1808825032@qq.com
贾 琦,女,副教授。Tel: (021)51322207 E-mail: q_jia@126.com
张 勇,男,助理研究员。E-mail: zhangyong109@simm.ac.cn
[责任编辑 王文倩]
