Experimental study on methane dissolved in surfactant-alkane system
2020-12-20ZhinHungMingliChenJingjingWngYinghuZhngLinghuZhngHuiWngYukunGoYupengZhou
Zhin Hung ,Mingli Chen ,Jingjing Wng,Yinghu Zhng ,Linghu Zhng ,Hui Wng ,Yukun Go,Yupeng Zhou
a State Key Laboratory of High-Efficient Mining and Safety of Metal Mines,University of Science and Technology-Beijing,Ministry of Education,Beijing 100083,China
b Work Safety Key Lab on Prevention and Control of Gas and Roof Disasters for Southern Coal Mines,Hunan University of Science and Technology,Xiangtan 411201,China
c State Key Laboratory Cultivation Base for Gas Geology and Gas Control,Henan Polytechnic University,Jiaozuo 454000,China
d Beijing Key Laboratory of Operation Safety of Gas,Heating and Underground Pipelines,Beijing Research Center of Urban System Engineering,Beijing 100035,China
ABSTRACT A surfactant-polyalkane system is investigated using chemical reagents to dissolve methane and control coal seam gas from low-energy,high-efficiency,safety,and environmental protection perspectives.At different temperatures and pressures,a high-temperature and high-pressure reactor,gas chromatograph,and other related experimental equipment were used to perform methane dissolution experiments,and a single surfactant sodium oleate (NaOA) and n-hexane demonstrated superior results.The single-factor experiments of temperature,pressure,and NaOA addition were performed and fitted via a response surface analysis.The optimal conditions for methane solubility were as follows:temperature of 34°C,pressure of 4.5 MPa,NaOA addition of 85 g/L,and time of 1 h.The optimal effect of the surfactant-polyalkane system in dissolving methane was achieved with 32.31 mL/100 mL.Meanwhile,the change in the surface structure of coal before and after washing with the system was compared using scanning electron microscopy.The results indicated that the gas after washing with the surfactant-polyalkane system dissolved,and the surface pore structure of the coal changed.Moreover,the specific surface area and pore size of the coal surface increased after washing.Hence,the desorption of gas from the coal surface into the system becomes easy,thereby reducing the gas content in the coal sample.
Keywords:Surfactant Polyalkane solution Methane
1.Introduction
Coal is the main power source and an important industrial raw material in China,but coal and gas outbursts and gas explosions also constitute major disasters in global mines [1–3].The danger of coal and gas outbursts in China also increases with the coal mining depth increments [4,5].Regional anti-burst measures mainly include mining the protective layer and pre-extracting coal seam gas [6,7].Meanwhile,local anti-burst measures mostly use shallow-hole gas drainage and coal seam shallow-hole water injection [8].The excavation face generally involves advanced drilling and deep-hole loosening,blasting,and hydraulic punching [9].The basic concept of these methods is reducing the gas content and pressure of coal seam to avoid problems caused by excessive gas concentration or pressure,such as coal seam outbursts,gas outbursts,and gas explosion.However,the actual effects are not satisfactory.
This study has investigated a method for managing coal seam gas from chemical reagent perspective,which differs from traditional approaches.Surfactants are amphipathic compounds composed of two different properties of the hydrophilic and hydrophobic groups in a molecule,with emulsification,solubilization,dispersion,wetting,foaming,and other functions[10].Among these functions,solubilization plays a vital role in biological and industrial processes [11–14].Fu et al.investigated the solubility of methane in the alkaline surfactant polymer(ASP)flooding solution of alkalis,surfactants,and polymer solutions.The results demonstrated that the ternary composite flooding fluid has a stronger ability to dissolve methane than pure water and salt solutions at the same temperature and pressure[15].The methane solubilization data,which are recommended by the IUPAC and regarded by many researchers as accurate,indicate the effects of methane dissolution; these effects are related to temperature,pressure,and solvent structure with a total of 23 pure solvents in hydrocarbons[16,17].Yang et al.examined the influence of soluble organic matter on the pore structure and adsorption characteristics of methane in coal.The result indicated that the soluble organic matter in coal occupies part of the coal pores,thereby affecting the coal pore structure [18].Böttger et al.studied the solubility of methane in n-octanol,n-dodecane,and n-hexane at different temperatures and pressures [19,20].However,these studies only focused on methane dissolution via a single chemical reagent.Hence,the solubilization effect cannot be effectively improved.
Therefore,based on the solubilizing methane mechanism of surfactants and multi-alkane solutions,this study performs primaries for two solution types with the ability to dissolve methane.The two systems are compounded to obtain an optimized surfactant-polyalkane system to achieve improved gas dissolution by using a chemical solvent.By observing and analyzing the changes of the coal surface structure before and after washing with the surfactant-alkane system,the effects of the compounding system on the gas dissolution in the coal are verified.
2.Experiment
2.1.Principles of the experiment
(1) Solubilization of methane by surfactant:The molecular structure of the surfactant is amphipathic,with a longchain hydrophobic group at one end and a hydrophilic group at the other.When the surfactant concentration in the solution exceeds the critical micelle concentration,the surfactant forms a colloidal group with a peripheral hydrophilic and hydrophobic core.The colloidal group core provides a hydrophobic environment for several organic materials that are insoluble or slightly soluble in water.Therefore,the surfactant can increase the solubility of methane.
(2) Dissolution of methane by using the polyalkane solution:According to the theory that similarities can be easily soluble with one another,polarity is similar to dissolve,and the structural similarity has a stronger effect on solubility than polarity.Solutes composed of non-polar molecules are easily soluble in solvents with non-polar molecules and insoluble in solvents with polar molecules.A polyalkane solution has similar structure with that of methane,and they both have non-polar organic molecules.Hence,a polyalkane solution can dissolve methane.
(3) Principle of evaluating methane solubility:In this study,the solution was pretreated using a headspace sampling technology to separate the solution from the methane.The methane content was analyzed via gas chromatography to obtain the methane solubility.The main process involves resolving the gas dissolved in the solution by increasing the temperature and reaching the dissolution balance in two gas–liquid phases.The top gas was sampled for the chromatographic analysis to obtain the methane solubility in the solution.During this process,a static headspace analysis method was used for headspace analysis,and the matrix effects were eliminated via multiple headspace extraction techniques.
2.2.Materials and instruments
The experimental materials used include sodium oleate(NaOA),sodium dodecyl sulfate (SDS),dodecyl dimethyl betaine (BS-12),polysorbate (Tween 80),octylphenol ethoxylate (TX-100),polyoxyethylene castor oil (EL-20),n-hexane,cyclohexane,ndodecane,n-pentadecane,n-hexadecane,and cyclohexyl methyl dimethoxy silane.
The experimental instruments used in the experiment include an SLM miniature,high-pressure reactor,Agilent 6820 gas chromatograph,ultrasonic liquid processors,Agilent 6820 headspace sampler,Quanta 450 FEG scanning electron microscope (SEM),3H-2000PS2 static capacity instrument of specific surface area,and aperture analyzer.
2.3.Experimental layout
(1) Dissolving methane with a single surfactant or polyalkane solution:Methane dissolution contrast experiments were performed using the surfactant concentration,with multialkane type,temperature,and pressure as variables.The effects of different surfactant concentrations,multi-alkaline solution types,temperatures,and pressures on the methane solubility were investigated.The optimum surfactants and multi-alkane solutions,as well as their respective reaction conditions and concentrations,were determined.
(2) Dissolving methane with the surfactant-alkane system:The optimal surfactant and polyalkane solution were selected as the main raw materials of the surfactant-alkane system.The effects of surfactant addition,temperature,and pressure on the methane solubility were investigated.A response surface analysis was performed to obtain the optimum ratio and conditions for the surfactant-alkane system.
(3) Effect of surfactant-alkane system on the surface structure characteristics of coal:The surface structure of the coal was scanned using an electron microscope and compared with the surface SEM to determine changes in the structure before and after washing with the surfactant-alkane system.Surface area and pore size analyzers were used to analyze quantitatively the coal surface before and after coal impregnation to measure changes in pore volume and specific surface area.
2.4.Experimental procedure and content
The experiments involved dissolving the methane,testing the methane solubility,scanning the electron microscope,and testing the pore volume and specific surface area.The procedure and content are outlined as follows.
(1) Experiment of dissolving the methane:The reaction solution was initially poured into a reaction vessel.Then,a magnetic stir bar was added,and finally the vessel was sealed.If the reactor is airtight,nitrogen gas is continuously supplied for approximately 5 min to discharge the air in the reaction vessel.Methane was introduced into the reactor at a pressure of approximately 0.4 MPa.After the nitrogen in the reactor was discharged,the methane pressure was increased to that required for the experiment.The test was started after setting the temperature,time,and stirring rate.After the experiment,the reaction solution was collected and sealed.
(2) Experiment of testing the methane solubility:The collected reaction solution was sealed in a headspace vial at 80 °C for 20 min to achieve a dissolution equilibrium between the methane and solution,and the methane dissolved in the solution was resolved.Thereafter,the gas chromatograph was turned on and the gas flow was adjusted in accordance with the experimental requirements.The temperature started to increase after the detector,vaporization chamber,and oven temperatures on the host control panel were set.Finally,fire occurred when the temperature achieved 100 °C.Hence,the samples were injected.
(3) Experiment of SEM:First,the coal sample was cut and reinforced,an electron microscope for the coal sample was prepared,and the prepared coal sample was polished.Meanwhile,resin glue was applied for the reinforcement at the coal sample edge to prevent coal body breakage.Second,coal sample was immersed in a beaker containing a surfactant solution of a certain concentration through a surfactant-alkane system for 2 h.Third,it was dried in a vacuum drying oven,removed from the oven,and sprayed with carbon to enhance its conductivity.Finally,the impregnated coal sample was placed in an SEM for scanning.
(4) Experiment of testing the pore volume and specific surface area:First,the coal samples were sieved and two sets of 60–80 mesh coal samples were weighed in which each group has approximately 100 g when immersed and dried.Second,500 mg samples were weighed,a sample tube was installed,and the samples were purged and degassed.Third,the software data processing system was opened,the liquid nitrogen cup was placed on the lifting bracket after pouring liquid nitrogen into the cup,and liquid nitrogen adsorption was started after the zero adjustment.When the adsorption equilibrium becomes zero,the cup of liquid nitrogen was dropped and then desorped by hot water at 30 °C.Finally,the data were saved after the test.
3.Results and analysis
3.1.Dissolving methane in a single surfactant or polyalkane solution
In this stage,the methane dissolution at different concentrations,temperatures,and pressures was studied for different surfactants and polyalkane solutions.
3.1.1.Effect of surfactant concentration,temperature,and pressure on methane solubility
Experiments on dissolving methane in the surfactants of NaOA,SDS,BS-12,Tween 80,TX-100,and EL-20 were conducted with concentration in the range of 25–150 g/L,temperature of 30–50 °C,and pressure of 0.5–2.5 MPa.The experimental conditions are summarized in Table 1,whereas the results are illustrated in Figs.1–3.
As shown in Figs.1–3,a clear correlation is suggested in which with the increase in the surfactant concentration and reaction pressure,the methane solubility steadily rises and with the rise in temperature,the methane solubility decreases.As shown in Fig.1,NaOA demonstrated the greatest effect on dissolving methane,with a solubility of 24.725 mL/100 mL at a concentration of 150 g/L.As illustrated in Fig.2,NaOA had the highest ability in dissolving methane as the temperature rise,with a solubility of 24.45 mL/100 mL at a temperature of 30 °C.As shown in Fig.3,NaOA has the highest methane solubility among the six surfactants,with a solubility of 24.35 mL/100 mL at a pressure of 2.5 MPa.The ability of dissolving methane follows the order of NaOA > SDS > TX-100 > BS-12 > EL-20 > Tween 80.
3.1.2.Relationship between methane solubility and polyalkane type
Six kind of polyalkanes,including the n-hexane,dodecane,pentadecane,hexadecane,cyclohexane,and cyclohexyl methyl dimethoxy silane,were used to dissolve methane at temperature of 30–50 °C and pressure of 0.5–4.0 MPa.The conditions of the multi-alkane substance solution methane experiments are summarized in Table 2,whereas the results are illustrated in Figs.4 and 5.
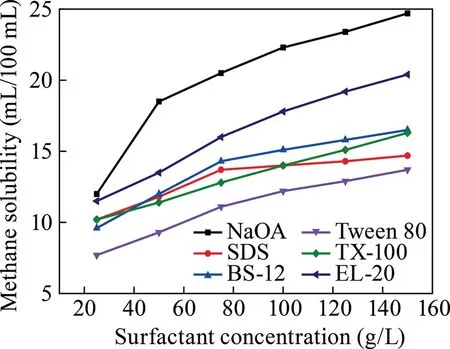
Fig.1.Relationship between surfactant concentration and methane solubility among different surfactants.
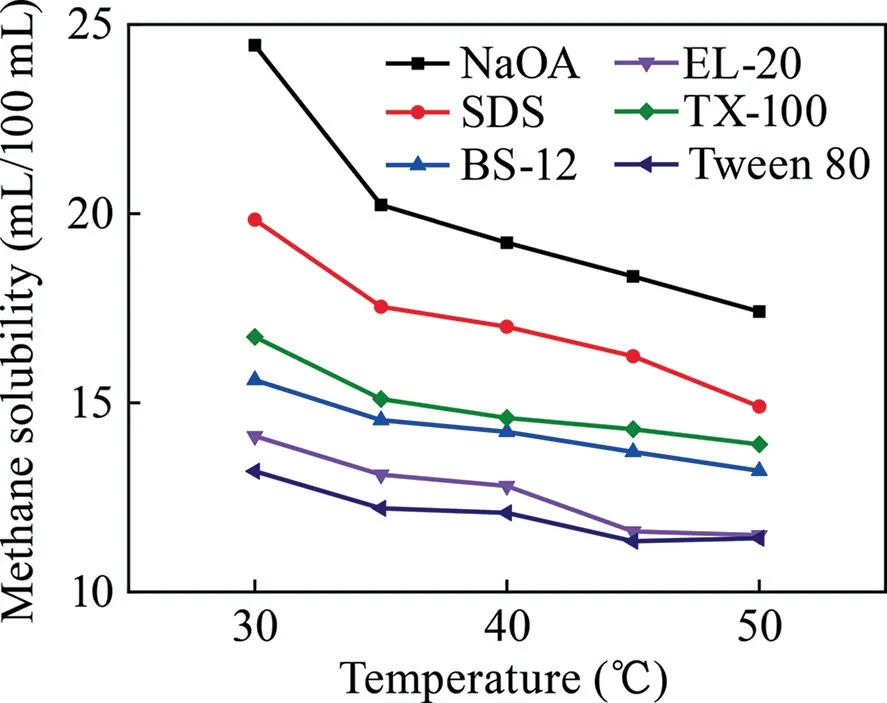
Fig.2.Relationship between temperature and methane solubility among different surfactants.
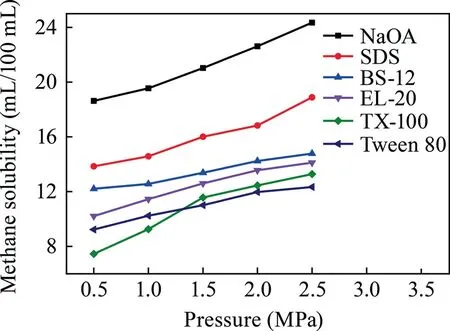
Fig.3.Relationship between pressure and methane solubility among different surfactants.
As illustrated in Figs.4 and 5,a trend exists in which the solubility of methane gradually decreases with an increasing temperature and rises with an increasing pressure.In Fig.4,when the temperature is 30 °C,the methane solubility of six surfactants reached the optimal results,respectively.The methane solubility in n-hexane at the temperature of 30 °C was superior,equal to 10.23 mL/100 mL.When the pressure is 0.5 MPa,these six polyalkanes all exhibited unsatisfactory solubility.With the increase in pressure,not only the solubility effects but also the solubility distances increased.With the addition of carbon atoms,the methane solubility in the polyalkane solution consequently decreased at the same pressure.The n-hexane exhibited superiorsolubility of approximately 15.75 mL/100 mL at a pressure of 4 MPa.As mentioned previously,the ability of dissolving methane follows the order of n-hexane > cyclohexane > n-dodecane > n-pe ntadecane > n-hexadecane > cyclohexyl methyl dimethoxy silane.

Table 1Conditions of dissolving methane at different surfactant concentrations,temperatures,and pressures.

Table 2Conditions of dissolving methane with polyalkane types.
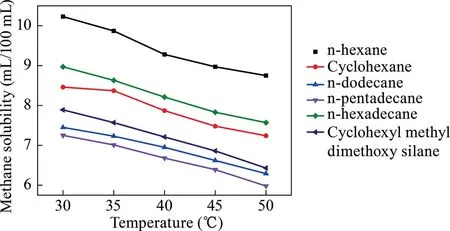
Fig.4.Relationship between temperature and methane solubility among different polyalkanes.
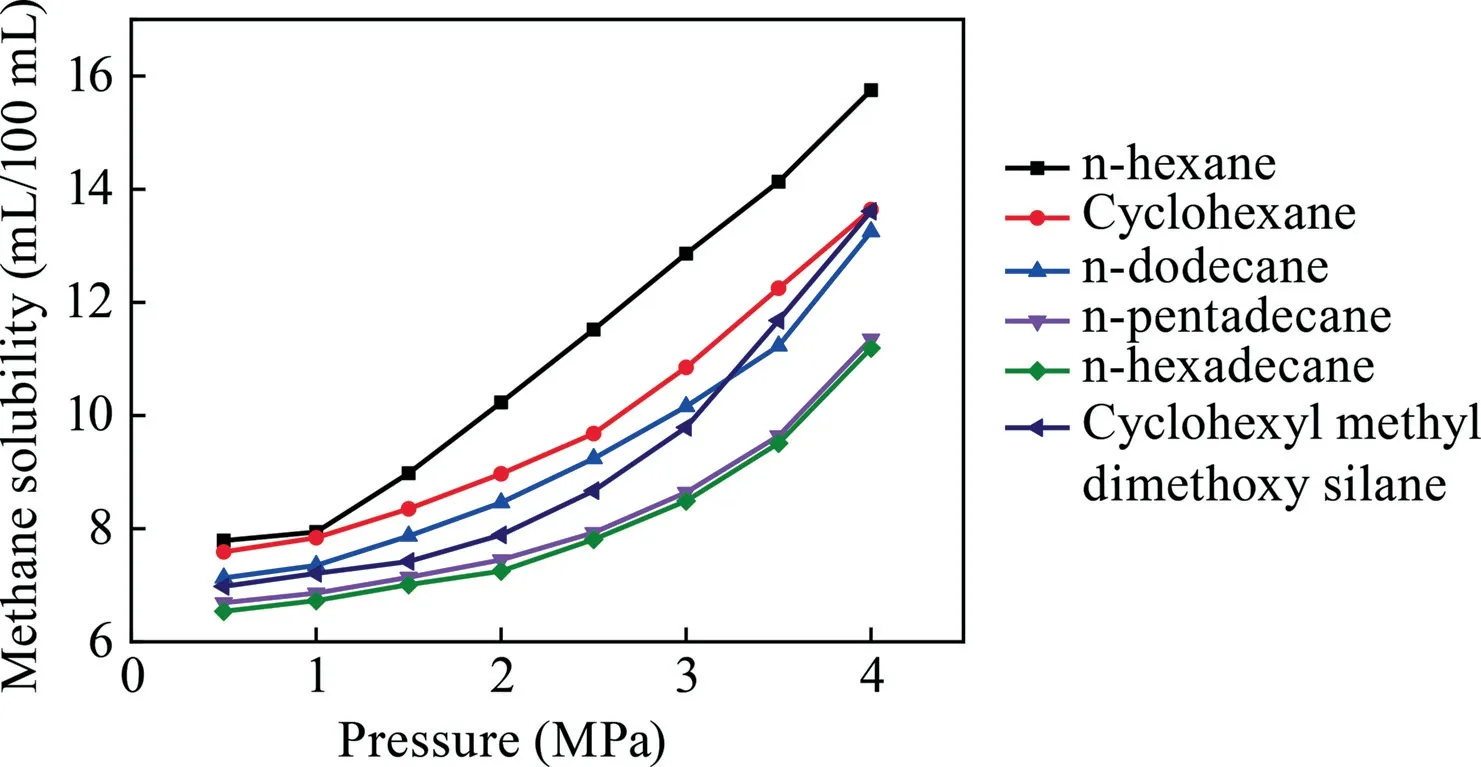
Fig.5.Relationship between pressure and methane solubility among different polyalkanes.
3.1.3.Surfactant release of methane
The law of releasing the methane in a certain period after dissolving it with different surfactants was investigated.The surfactant solution after dissolving with methane was placed at room temperature,and part of the solution was removed from the solution after a certain period to analyze the methane content.The release of methane by the surfactant was investigated as a function of a set time.The result is illustrated in Fig.6.
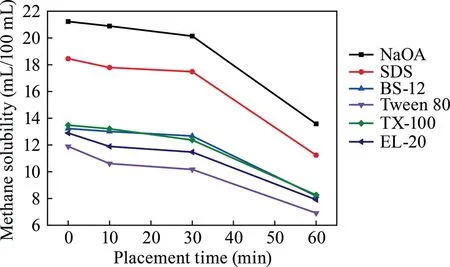
Fig.6.Relationship between placement time and methane solubility among different surfactants.
As shown in Fig.6,the solution in which the surfactant is dissolved in methane is exposed at room temperature,and the methane dissolution by the surfactant causes the release of methane from the solution over time.After the methane concentration was absorbed by the surfactant solution,the methane concentration decreased in 0–10 min and remained stable within 10–30 min.After 30 min,the methane solubility changed significantly and the release rate increased.The NaOA solubility at 10 min was 98.4% compared with the start of the experiment and decreased to 94.9% after 30 min and 64.0% at 60 min.
3.2.Dissolving methane experiments in the surfactant-alkane system
Based on the investigation of a single surfactant and multialkane solution,the surfactant with the optimal methane dissolution effect under certain conditions was selected.NaOA and the multi-alkane solvent n-hexane solution were used as the main raw materials of the surfactant-alkane system,and the effects of the surfactant addition amount,reaction temperature,and reaction pressure were experimentally investigated.Meanwhile,the response surface analysis method was used to obtain the optimal ratio and conditions for the surfactant-alkane system.
3.2.1.Study on methane dissolving effect of the surfactant-alkane system
A certain amount of solid NaOA powder was weighed into an nhexane solution and ultrasonically dispersed using an ultrasonic disperser for 10 min to form a uniform surfactant-alkane system.The decomposition of methane occurred with concentration in the range of 25–150 g/L,temperature of 30–50 °C,and pressure of 0.5–3.0 MPa.The experimental conditions are displayed in Table 3.The relationship between the amount of surfactant added and dissolved methane is illustrated in Figs.7–9.
As shown in Fig.7,with the increase in the amount of surfactant addition,the methane solubility of the methane significantly improves.When the added amount was 75 g/L,the maximum solubility was 29.57 mL/100 mL.When the surfactant was continuously added,the methane dissolution began to decrease.
As illustrated in Fig.8,the solubility of the surfactant-alkane system gradually decreased as the temperature increased.Meanwhile,the solubility decreased rapidly when the temperature was within the range of 35–40 °C.When the temperature was 30 °C,the surfactant-alkane system dissolved the methane to a maximum of 28.78 mL/100 mL.
As shown in Fig.9,the methane solubility gradually increases with the increase in pressure in the surfactant-alkane system.When the pressure was 2.5 MPa,the surfactant-alkane system dissolved the methane up to 29.45 mL/100 mL.
3.2.2.Analysis and optimization of dissolved methane test
The response surface methodology was used for factor optimization,such as adjusting the amount of surfactant added,experimental temperature,pressure,and time during the reaction process.Furthermore,the surface data and contours were drawn from the data,the degree of influence among various factors was determined,and the experimental conditions that fully satisfy the requirements were obtained.

Table 3Experimental conditions of the surfactant-alkane system in dissolving methane.
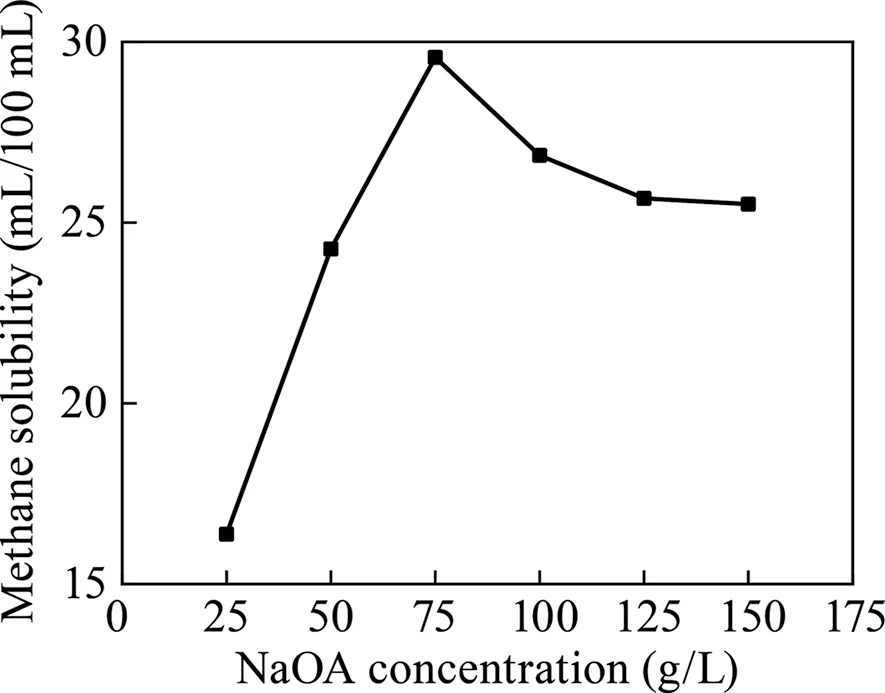
Fig.7.Relationship between NaOA surfactant addition and methane solubility.
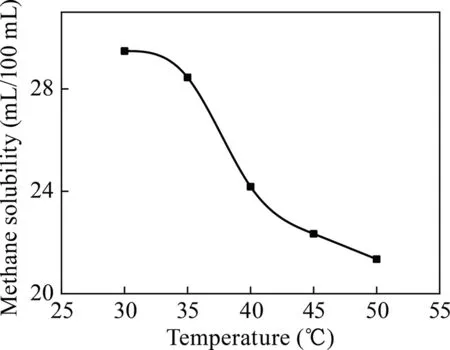
Fig.8.Relationship between temperature and dissolved methane (surfactant:NaOA).
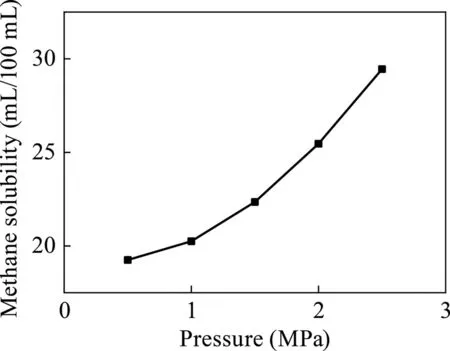
Fig.9.Relationship between pressure and dissolution of methane (surfactant:NaOA).
The study included four variables,namely,surfactant addition(SA),experimental temperature (T),experimental pressure (P),and experimental time (t).Their levels were selected as shown in Table 4.By using the methane solubility as the response value(Y),the design experimental points included 24 factorial and three zero points.The experimental results are displayed in Table 5.
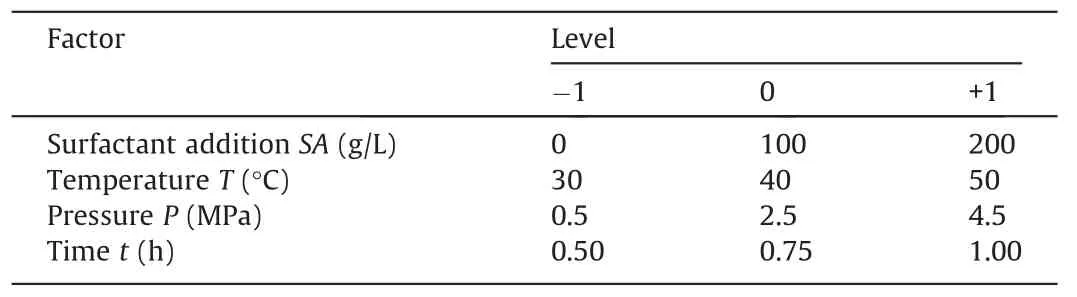
Table 4Four variables in the response surface analysis.
The experimental data were analyzed using the Design-Expert software.A total of 24 types of factorial experiments and three types of zero reals were obtained.The quadratic multinomial regression equations for the methane solubility for the four independent variables were obtained by fitting

On the basis of this quadratic multinomial regression equation,a binary factor analysis was performed to obtain the response surface curves among the factors,as illustrated in Figs.10–15.
The curves and contours depicted in Figs.10–15 can intuitively reflect the influences of various factors on the solubility of methane.If the contour exhibits an ellipse,the interaction of two factors is significant.Meanwhile,if the contour is circle,the interaction is not significant.As shown in Fig.10b,12b,13b,and 14b,the contours are elliptical,thereby indicating that the interaction of the two factors is significant.As shown in Fig.11b,the contours of the two factors affecting the dissolution of methane present a circular contour,thus indicating that the interaction of the two factors in Fig.11b is not significant.The optimal conditions of methane solubility obtained via the response surface fitting were as follows:temperature of 34°C,pressure of 4.5 MPa,NaOA dosage of 85 g/L,and time of 1 h.The optimal effect of the surfactantalkane system in dissolving the methane was 32.31 mL/100 mL.Compared with the previous dissolving methane effect of a singlesurfactant,the effect of dissolving methane at a certain temperature and pressure is better than that of a single surfactant.
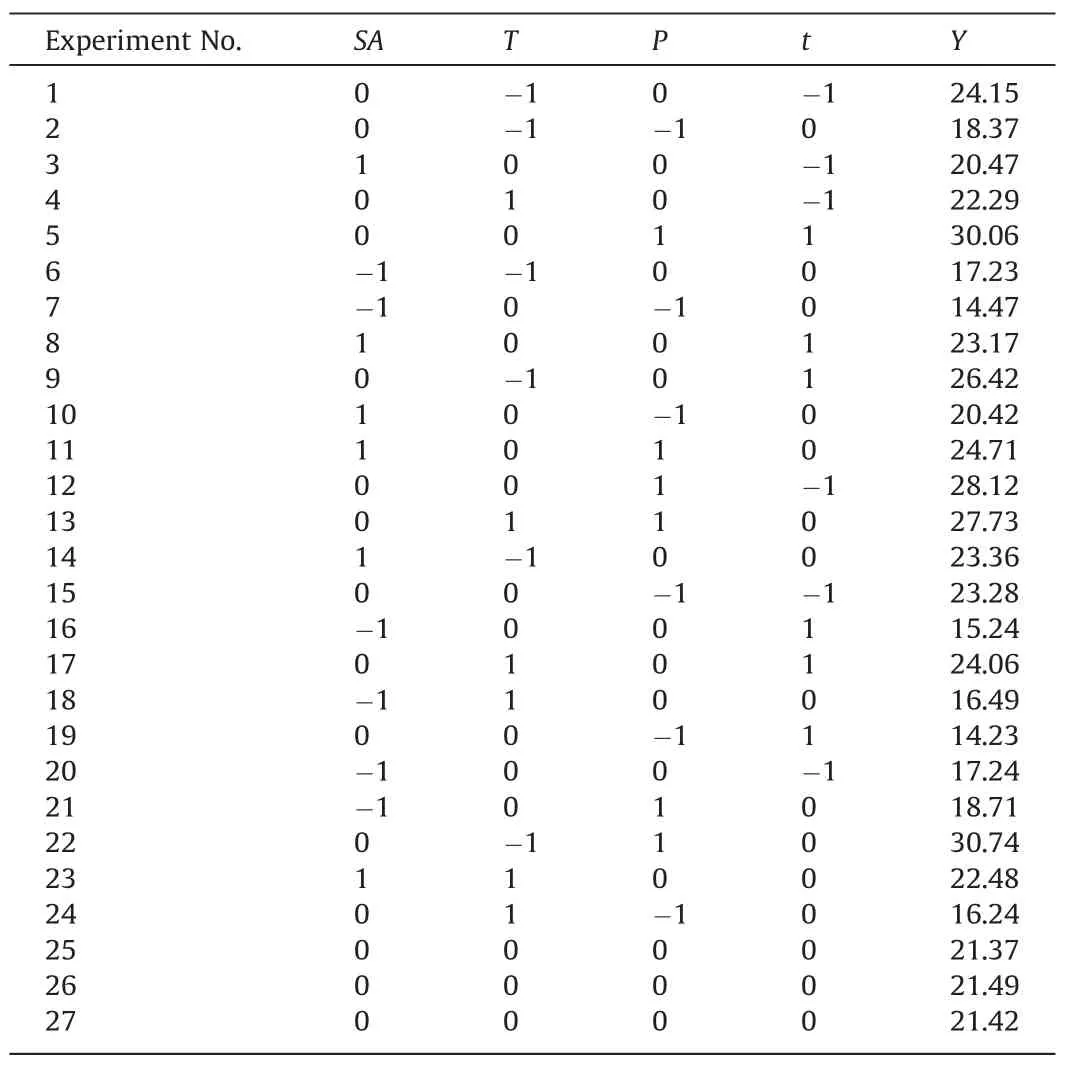
Table 5Factor combinations and test results of the response surface analysis.
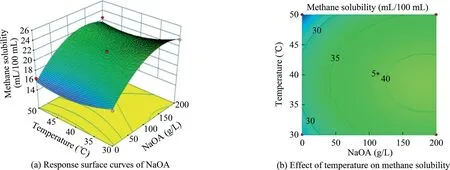
Fig.10.Response surface curves of the effect of NaOA additions amount and temperature on methane solubility.
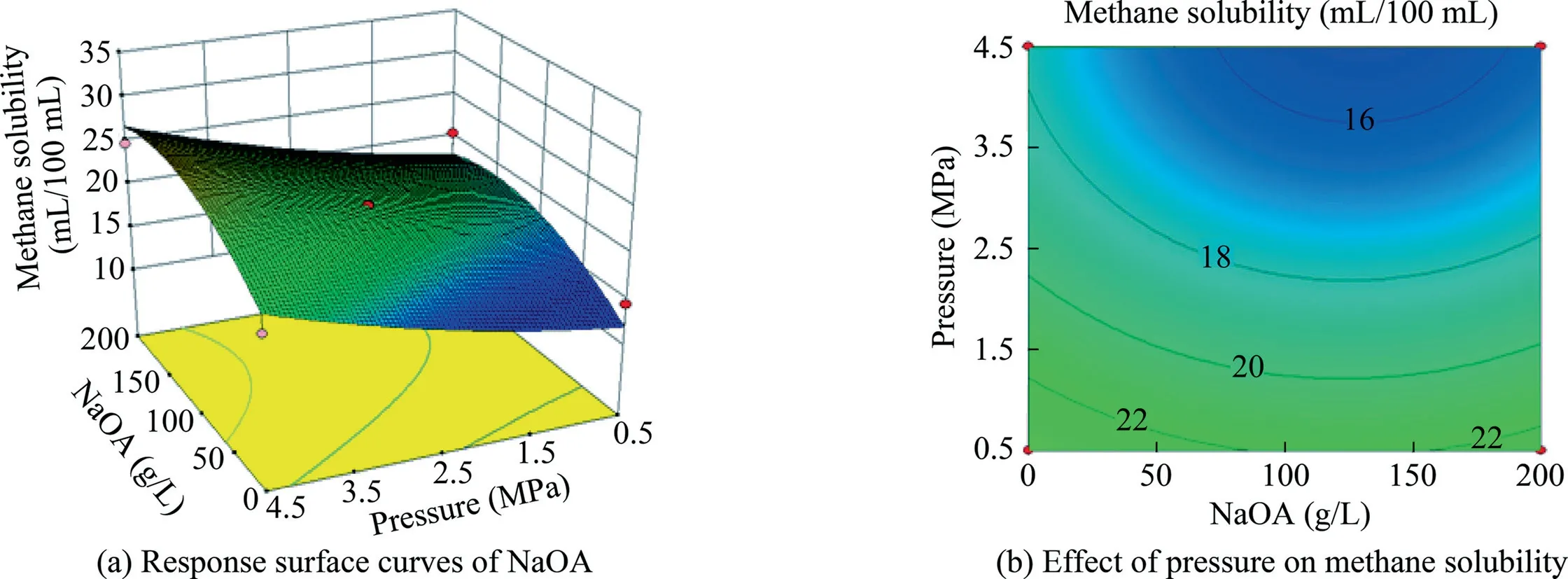
Fig.11.Response surface curves of the effect of NaOA addition and pressure on methane solubility.
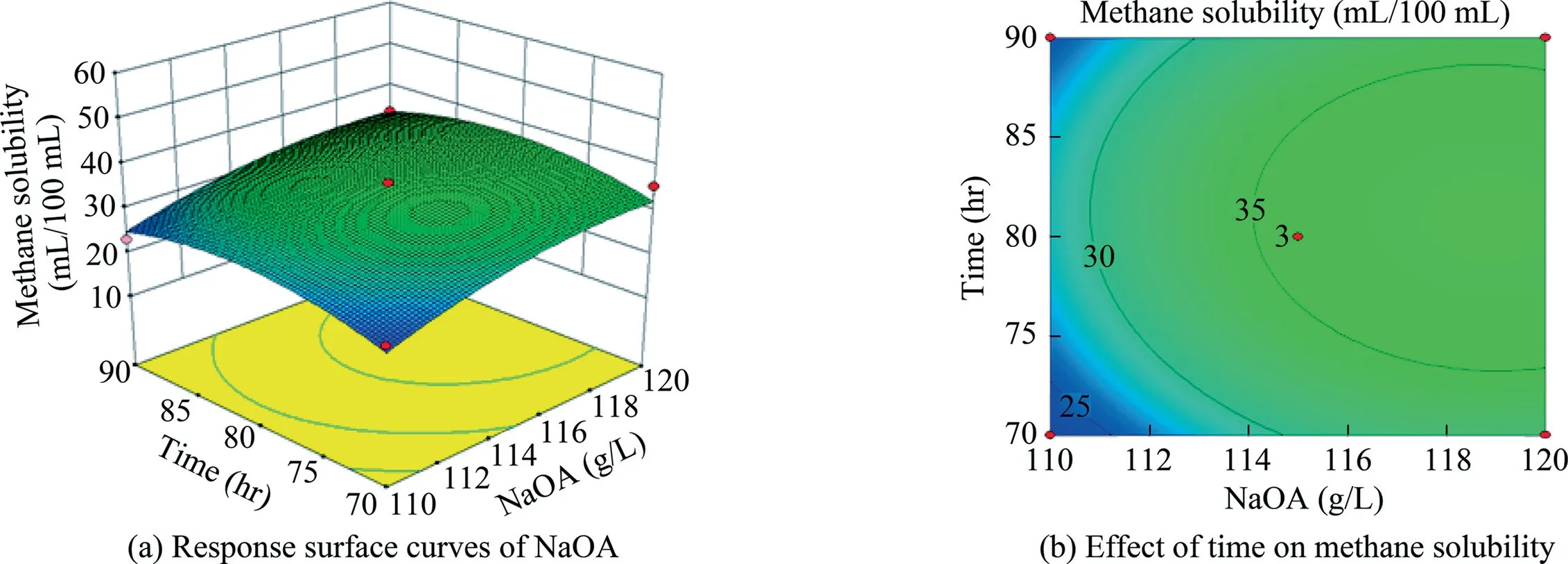
Fig.12.Response surface curves of the effect of NaOA addition and time on methane solubility.
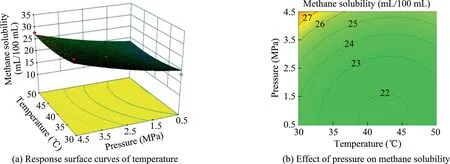
Fig.13.Response surface curves of the effect of temperature and pressure on methane solubility.
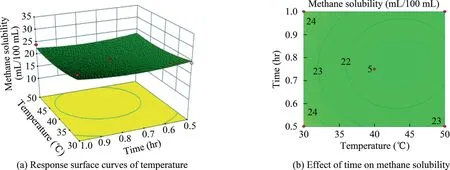
Fig.14.Response surface curves of the effect of temperature and time on methane solubility.
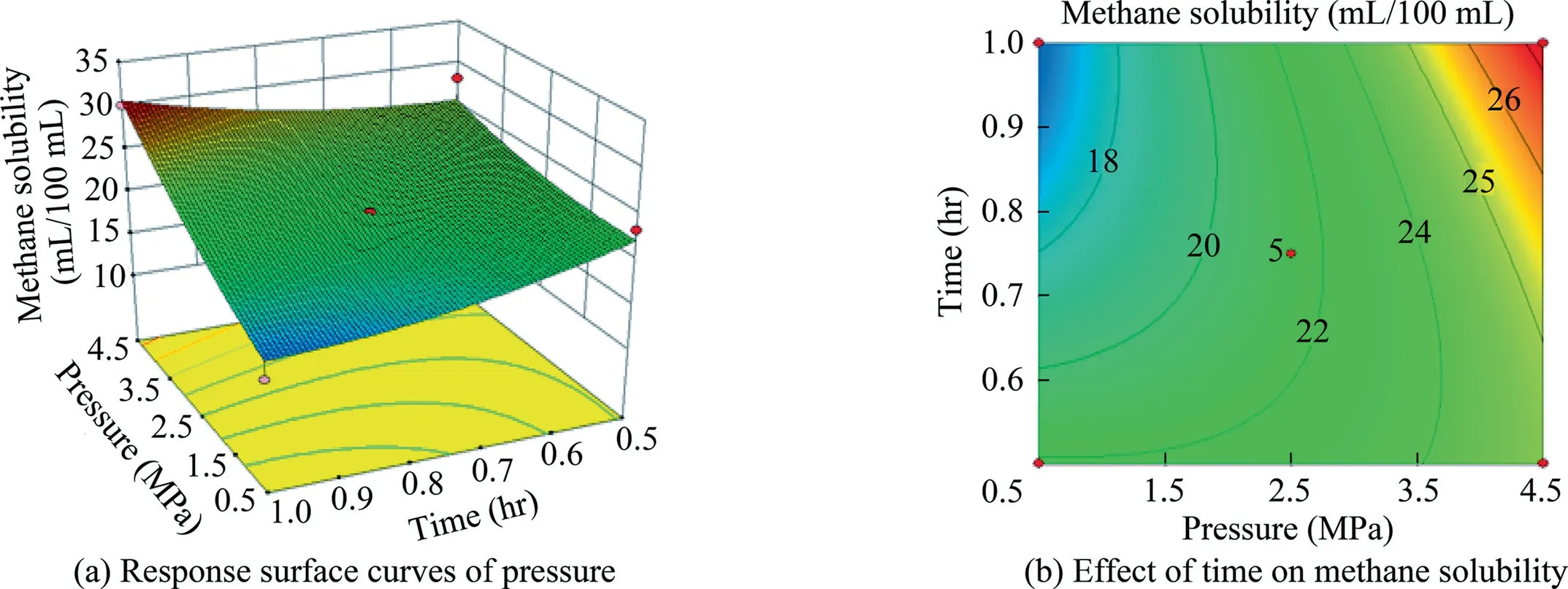
Fig.15.Response surface curves of the effect of pressure and time on methane solubility.
3.2.3.Surfactant-alkane system release methane experiment
A comparative experiment was carried out under the same experimental conditions and a single surfactant release methane experiment,and the methane release rule of the surfactantalkane system after being left at different times is shown in Fig.16.
As illustrated in Fig.16,the decrease ratio of dissolved methane of NaOA is 1.6%in 10 min,5.1%in 30 min,and 36.0%in 60 min.The decrease ratio of dissolved methane of surfactant-alkane system is 0.73% in 10 min,2.8% in 30 min,and 19.0% in 60 min.Compared with the single surfactant,the surfactant-alkane system exhibited superior stability after absorbing the methane under normal temperature conditions,and its stability was approximately twice that of a single surfactant within 60 min.
3.3.Effect of the surfactant-alkane system on the structural characteristics of coal surface
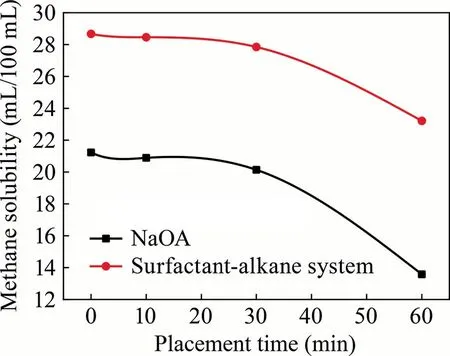
Fig.16.Comparison of methane solubility over time in NaOA and surfactant-alkane systems.
The environmental SEM was used to observe the coal surface structure via electron microscopy scanning to determine the structural changes of the coal.The influence of the surfactant-alkane system on the coal structure was directly obtained.The specific surface area and pore size were analyzed.The instrument performed a quantitative analysis on the coal surface before and after coal impregnation and measured the changes in the pore volume and specific surface area.Hence,relevant conclusions regarding the influence of the surfactant-alkane system on the coal structure were indirectly achieved.
3.3.1.SEM contrast experiment
In this experiment,15#coal collected from No.2 Mine of Shanxi Yangquan Yangmei Group of China was utilized as the experimental object.The coal sample was cut and reinforced to form an electron microscope coal sample and then polished.It was immersed for 2 h in the optimal surfactant-alkane system solution.After being dried in a vacuum drying oven,carbon was sprayed onto the sample to enhance its conductivity.Thereafter,the coal sample was placed in the SEM.The steps involves in the scanning and photographing the surface microstructure of the coal body are as follows.
(1) Coal samples not impregnated with surfactant-alkane system:In the microscopic surface scan of the coal sample,pores,which are mainly divided into two types of different shapes and arrangements can be observed.The first type is isolated pores,that is,an isolated irregular pore exists on the coal surface and no other surrounding interconnected pore exists,as shown in Fig.17.The second type is connected pores,that is,other interconnected pores exist around the larger pores,as shown in Fig.18.In Fig.18,the structure of pores on the surface of the coal sample unwashed with the surfactant-alkane system is clear.Moreover,the structure and distribution of the pores,the relationship between pores,and spread of isolated pores are clearly observed.The direction generally points to another isolated hole.
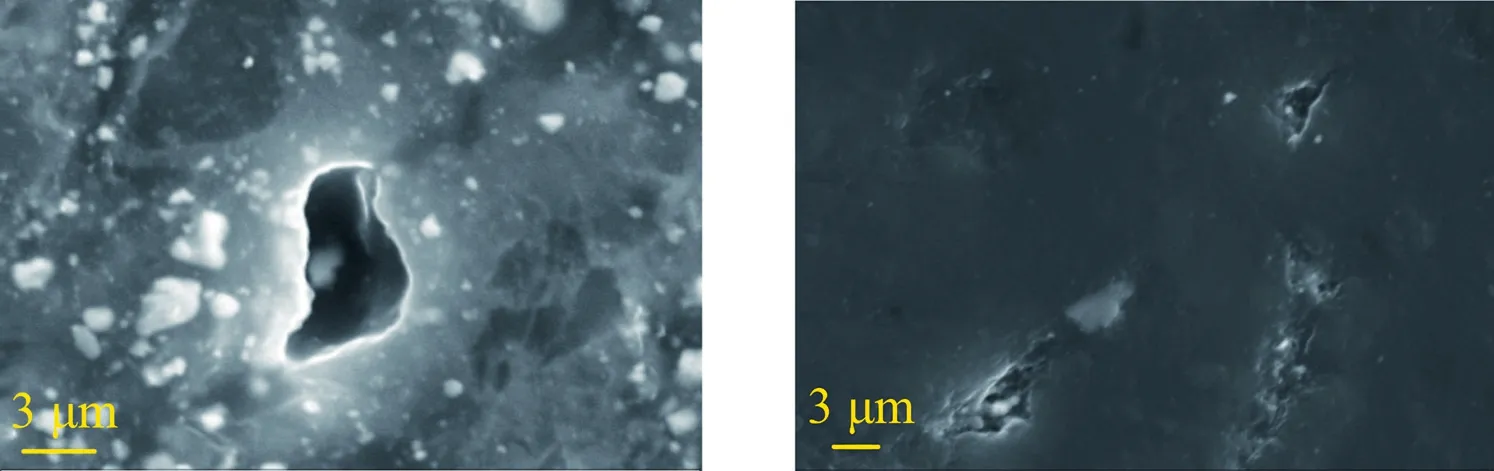
Fig.17.SEM of unwashed surfactant-alkane systems (isolated wells).

Fig.18.SEM of pulse holes unwashed with surfactant-alkane system.
(2) Coal samples impregnated with surfactant-alkane system:When the working distance of the electron microscope was 200 μm,it was found that the surface structure of the coal sample impregnated with surfactant-alkane system changed,and the previous pore structure ceased to exist,as shown in Fig.19.When the working distance of electron microscope is further enlarged to 10 μm,it can be observed that the surface structure of coal sample is no longer smooth and becomes uneven,as shown in Fig.20.
The effect of the system on coal was investigated through the SEM of the coal sample surface after the surfactant-alkane system was impregnated with coal.Thus,the following conclusions were obtained.
(1) After the connected pores,isolated pores and fissure pores on the surface of coal samples were washed with the surfactant-polyalkane solution,the original fissure structure disappeared.Therefore,the organic matter constituting the coal sample surface,including the adsorbed gas,was dissolved into the surfactant-alkane system.
(2) After washing with the surfactant-polyalkane solution,the surface of the coal body exhibited a wavy scale structure,proving the changes in the surface pores and coal body fracture structure.
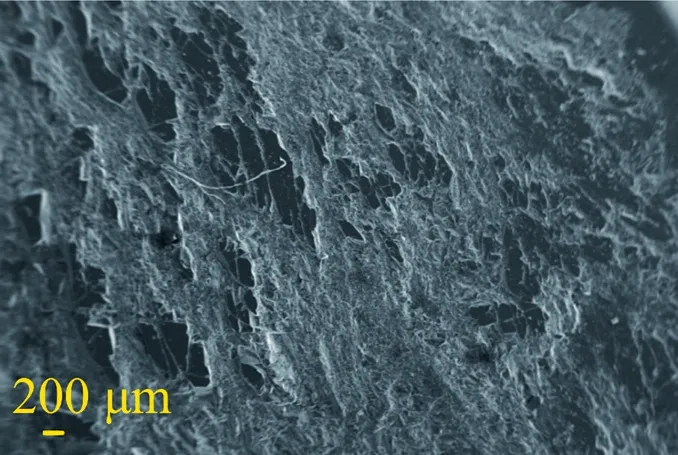
Fig.19.SEM of the coal sample with surfactant-alkane system at low magnification.
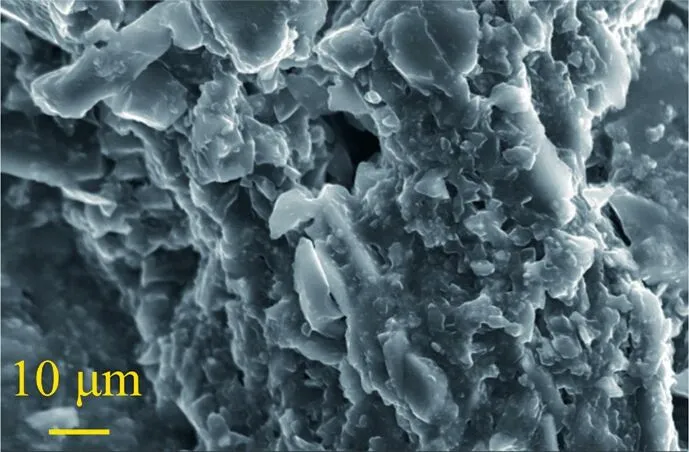
Fig.20.SEM sampling of coal sample with high surfactant-alkane washing.
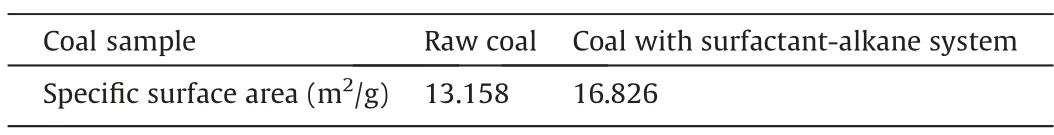
Table 6Results of determining the surface area of coal samples before and after immersion.
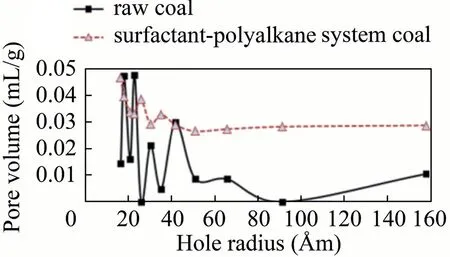
Fig.21.Pore volume of coal with surfactant-alkane system and raw coal samples versus hole radius.
3.3.2.Comparison of specific surface area,pore size,and pore volume before and after the coal sample impregnation
A specific surface area and pore size analyzer was used to compare the specific surface area,pore size,and pore volume of the coal sample before and after dipping in liquid nitrogen.The specific surface area measurement results are displayed in Table 6,and the pore diameter and volume measurement results are illustrated in Fig.21.
As shown in Table 6,the specific surface area of the raw coal sample is 13.158 m2/g,whereas that of coal with the surfactantalkane system is 16.826 m2/g,demonstrating an increase of 27.88% compared with the raw coal.
As illustrated in Fig.21,the pore sizes of the raw coal samples are mainly distributed between 15 and 40 Åm.Volumes of the pores with sizes of 18.492 and 23.301 Åm are the largest,reaching 0.04728 and 0.04756 mL/g,respectively.In addition,pore sizes of the coal samples treated with the surfactant-alkane system are evenly distributed between 15 and 160 Åm.The volume of the pore with a size of 16.534 Åm is largest,reaching 0.04662 mL/g.
The results of the specific surface area and the pore size analyzer indicated that with the same pore size,the pore volume of the coal sample exhibited a significant improvement compared with the original coal sample.Hence,raw coal with the same pore size was obtained.The voids with a pore volume of 0 exhibited a large increase in the pore volume after treatment,indicating that the influence of the coal samples has broadened the smaller pores or created new pores.
4.Conclusions
In this study,the surfactant and multi-alkane solutions were investigated at various temperatures and pressures.The response surface was used to analyze the optimal compounding conditions.Meanwhile,the surfactant-alkane system was applied to the coal surface.The effect of the structure was studied and the following conclusions were obtained.
(1) The NaOA and n-hexane solutions were selected as raw materials for the surfactant-polyalkane system.The results demonstrated that the surfactant-alkane system after reconstitution exhibits higher methane solubility than the single surfactant.Through the response surface fitting,when the optimal conditions for the methane solubility are specifically adjusted (e.g.temperature of 34 °C,pressure of 4.5 MPa,NaOA addition of 85 g/L,and time of 1 h),the optimal effect of the surfactant-alkane system in dissolving the methane is 32.31 mL/100 mL.
(2) When the methane-dissolved surfactant solution is left open at room temperature,methane is gradually released from the solution.Unlike the single surfactant,the surfactantalkane system exhibited superior stability against the gradual release of methane at room temperature.
(3) When comparing the changes in the pore and fracture structures of different coal samples before and after washing,the surface of the coal body impregnated with the surfactantalkane system exhibited a wavy scale structure distribution,indicating the changes in pore surface and fracture structure of the coal body.The coal sample with smooth surface after washing was clearly eroded.Meanwhile,the specific surface area and pore diameter increased significantly,and the specific surface area increased by 27.88% compared with the raw coal.The pore diameter changed from 15 to 40 to 15–160 Åm,making it easier for the gas to be desorbed from the surface of the coal sample and dissolved into the surfactation-alkane system,thus reducing the gas content in the coal sample.
Acknowledgements
The authors appreciate the financial support of project No.FRFIC-20-01 and No.FRF-IC-19-013 provided by the Fundamental Research Funds for the Central Universities,China project No.51974015,No.51904292,and No.51474017 provided by the National Natural Science Foundation of China,China project No.2018YFC0810600 provided by the National Key Research and Development Program of China,China project No.2017CXNL02 provided by the Fundamental Research Funds for the Central Universities (China University of Mining and Technology),China project No.BK20180655 provided by the Natural Science Foundation of Jiangsu Province,China project No.WS2018B03 provided by the State Key Laboratory Cultivation Base for Gas Geology and Gas Control (Henan Polytechnic University),China and project No.E21724 provided by the Work Safety Key Lab on Prevention and Control of Gas and Roof Disasters for Southern Coal Mines of China (Hunan University of Science and Technology),China.
杂志排行
矿业科学技术学报的其它文章
- Performance evaluation of near real-time condition monitoring in haul trucks
- Correlating common breakage modes with impact breakage and ball milling of cement clinker and chromite
- Key technologies and engineering practices for soft-rock protective seam mining
- Effect of the distributor plugging ways on fluidization quality and particle stratification in air dense medium fluidized bed
- Numerical simulation to determine the gas explosion risk in longwall goaf areas:A case study of Xutuan Colliery
- Effect of slurry conditioning on flocculant-aided filtration of coal tailings studied by low-field nuclear magnetic resonance and X-ray micro-tomography
