Pre-evaluation of humoral immune response of Bactrian camels by the quantification of Th2 cytokines using real-time PCR
2020-11-11XinyuYuYuanWuJiarongZhangJirimutuAzhatiZulipikaerJinChen
Xinyu Yu, Yuan Wu, Jiarong Zhang, Jirimutu, Azhati Zulipikaer, Jin Chen,5,6,✉
1Center for Global Health, School of Public Health, Nanjing Medical University, Nanjing, Jiangsu 211166, China;2Department of Medical Oncology, Jiangsu Cancer Hospital, Jiangsu Institute of Cancer Research, the Affiliated Cancer Hospital of Nanjing Medical University, Nanjing, Jiangsu 210009, China;3Key Laboratory of Dairy Biotechnology and Bioengineering, Ministry of Education, College of Food Science and Engineering, Inner Mongolia Agricultural University, Hohhot, Inner Mongolia 010018, China;4Xinjiang Academy of Animal Science, Urumqi, Xinjiang 830011, China;5The Key Laboratory of Modern Toxicology, Ministry of Education, School of Public Health, 6Key Laboratory of Antibody Technique of National Health Commission, Nanjing Medical University, Nanjing, Jiangsu 211166, China.
Abstract With the increasing immunological studies on camels due to the advantage of their single-chain antibodies for humanizations, it is demanding to develop an easy-to-handle evaluation method of their humoral immune response before proceeding with immunization of foreign antigens that may be toxic to camels. In this study, we quantitatively determined the expression levels of T-helper 2 (Th2) cytokines in peripheral blood lymphocytes obtained from Bactrian camels by real-time PCR. The recorded kinetic profiles resulting from the immunization of ovalbumin (OVA) indicated that after immunization, Th2 cytokines including interleukin (IL) families such as IL-4, IL-10, and IL-13 in the camels were up-regulated by a factor of 1.78, 3.15, and 1.22, respectively, which was validated by traditional enzyme-linked immunosorbent assay (ELISA) methods. Unlike ELISA which requires specific enzyme-labeled antibodies, this established method based on the minimal amount of blood samples holds an advantage in the preliminary evaluation of camel humoral immune response with desirable precision, which is meaningful for biomedical explorations of camel-derived antibodies.
Keywords: Bactrian camels, Th2 cytokines, humoral immune response, real-time PCR
Introduction
Chinese Bactrian camels belong to the Camelidae family that is historically evolved to adapt to harsh living environment conditions[1]. Recently, the heavychain only antibody (HcAb) in camels that is discovered to be naturally devoid of light chains by a chance observation, has gained increasing attention among researchers due to its versatile biomedical applications in therapeutics and bioanalysis[2-5].Typically, several rounds of immunizations in camels are conducted to produce HcAb with sufficient affinity and specificity. However, these frequent immunizations of foreign substances of antigens may cause damage to the immune system of camels, which results in both animal death and economic losses.Enzyme-linked immunosorbent assay (ELISA) is used to estimate antibody titers after immunization with sufficient precision[6-7]. However, unlike other model animals such as mice, Bactrian camels are evolved to live in the arid desert which may hamper further biomedical exploration. Therefore, commercial labeled monoclonal antibodies of camels are not available possibly due to the high economic cost and limited resources. Generally, the lack of enzymelabeled antibodies of Bactrian camels greatly hampers the use of ELISA in evaluating their immune response. Due to the high economic costs as well as the huge size of Bactrian camels, it is thereby urgent to develop an easy-to-handle evaluation method to prejudge the immune response before the antibody production.
Cytokines belong to low-molecular-weight proteins that are essential for the immune regulation and inflammatory responses of the body[8-13]. When activated by the stimulation of antigens during the immune response, T-helper (Th) cells can differentiate into two subgroups with specified functions followed by the secretion of a series of cytokines[14-15].Generally, Those Th1 cells can stimulate stronger cellular immunity while the Th2 cells are more prone to evoke humoral immune[16-17]. Specifically, those cytokines secreted by Th1 cells, known as Th1 cytokines, are more involved in the cell-mediated immunity to protect the body from intracellular pathogens. Meanwhile, Th2 cytokines are more related to the humoral immune response by mediating the production of antibody when facing extracellular substances. Therefore, Th2 cytokines including interleukin (IL) families such as IL-4, IL-10, and IL-13 offer a powerful tool for the evaluation of humoral immune response, which is useful for developing immune-based diagnostic methods. Moreover,previous reports have revealed that cytokine responses play an important role in the pathogenesis of some diseases, in which changed cytokine profiles have been identified[17-21]. For example, cytokine responses have been examined in camels or llamas to obtain more systematic information on their immune regulation[20-24]. Nevertheless, the cytokine profiles related to the humoral immune response have never been applied in Bactrian camels so far.
Real-time PCR has been widely used in modern molecular biological studies due to its feasibility for automation with high-throughput[25-26]. Without the need of enzyme-labeled antibodies, real-time PCR has become readily used to quantify the expression level of cytokines in laboratories. Particularly, with realtime PCR amplification, the detection of target products can be completed in a single step, which greatly saves time especially in the large sample analysis.
Since generating HcAb is closely related to humoral immune responses of camel and the lack of commercial enzyme-labeled antibody, we set out to develop a convenient and accurate method in this study to evaluate the immune efficacy of Bactrian camels by monitoring the expression level of Th2 cytokines with real-time PCR assay. As shown inFig. 1,after immunization, both the lymphocyte and serum were collected for real-time PCR analysis and ELISA.The correlation between the two methods was judged by the regression analysis. The goal of such preevaluation before the production of HcAb in camels will serve a complementary method for those laboratories without specific antibodies to evaluate the immune response, which is meaningful for the biotechnical development of unique Bactrian camels in middle Asia.
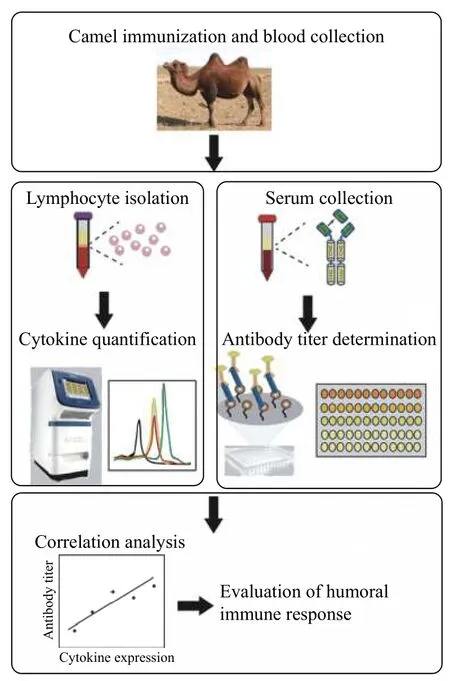
Fig. 1 Study design of established method for pre-evaluation of Bactrian camel immune response.
Materials and methods
Camel immunization and blood collection
The camel immunization was performed according to the protocols with modifications. Healthy male Alxa Bactrian camels raised in the feeding base in Alxa Zuoqi (~3 years old, ~700 kg) were immunized 5 times at weekly intervals with freshly prepared immunogens. 1.0 mg of model antigen, ovalbumin(OVA) was mixed with Freund's complete adjuvant(Sigma-Aldrich, St. Louis, USA) for the first immunization, and with Freund's incomplete adjuvant(Sigma-Aldrich) for the next 4 ones. The prepared immunogens were subcutaneously injected around the bow lymph node in the neck. Blood samples were collected from the jugular vein before the first immunization and 1 day after each week's immunogen administration. Half of the collected blood samples were allowed for clotting at room temperature (RT)for 2 hours. Then the samples were further centrifuged at 5 000 g for 5 minutes at RT to collect the supernatant, and the recovered serum samples were stored at -20 °C for further ELISA to evaluate the serum conversion according to the antibody titers.Another half of the blood samples were collected into the ethylenediaminetetraacetic acid (EDTA)-coated anticoagulant tube and gently inverted to prevent coagulation. Then the samples were further centrifuged at 5 000 g for 15 minutes at RT and the recovered plasma was stored at -20 °C for extraction of total RNA from the peripheral blood lymphocytes(PBLs).
Preparation of llama PBLs and total RNA isolation
PBLs were isolated via a density-gradient centrifugation method by Ficoll (GE Healthcare,Sweden) according to the manufacture's instruction.The total RNA isolation was immediately performed using the Trizol reagent (Invitrogen, USA) according to the manufactory's instruction. The isolated PBLs was added by 1.0 mL Trizol reagent and then after keeping in RT for 5 minutes, by 200 μL pre-cooled chloroform. Then the mixture was shaken and centrifuged at 12 000 g at 4 °C for 15 minutes and the aqueous phase was carefully collected and transferred to a new tube. Subsequently, 500 μL pro-cooled isopropanol was added into the tube and incubated for 20 minutes at -20 °C. After centrifugation at 12 000 g at 4 °C for 10 minutes, the supernatant was carefully discarded and the pellet was washed with 1.0 mL 75%ethanol. The isolated total RNA was re-dissolved by RNase-free water and the quality was verified by Nanodrop 2000 (Thermo Fisher Scientific, Waltham,USA). The isolated RNA was immediately stored at-80 °C before use.
Reverse transcription and real-time PCR assay
An aliquot of the obtained total RNA (~500 ng) was reversely transcribed by HiScript II reverse transcriptase (Vazyme Biotechnology, China) in a 10 μL reaction volume according to the manufacturer's instructions. The obtained cDNA was analyzed immediately or stored at -20 °C. Primers used for the quantitation of cDNA specific for camel Th2 cytokines (IL-4, IL-10, and IL-13) were designed according to the sequences from the Genebank database and listed in Table 1. β-actin was used as the internal control. Real-time PCR analysis was performed on the StepOnePlus system (Applied Biosystems, Austin, USA) using ChamQ SYBR qPCR Master Mix (Vazyme Biotechnology) to quantify the cytokine expression level. Briefly, 1 μL of cDNA was added into the 19 μL reaction mixture including 10 μL ChamQ SYBR qPCR Master Mix, 0.4 μL of each primer (to the final concentration of 10 μmol/L), 0.4 μL of ROX reference dye and 7.8 μL of deionized water. The real-time PCR parameters were listed in Table 2. Expression levels were defined through the threshold cycle and the fold changes were calculated according to the equation of 2-△△ct. Each sample was analyzed in triplicate.

Table 1 Primers for real-time PCR
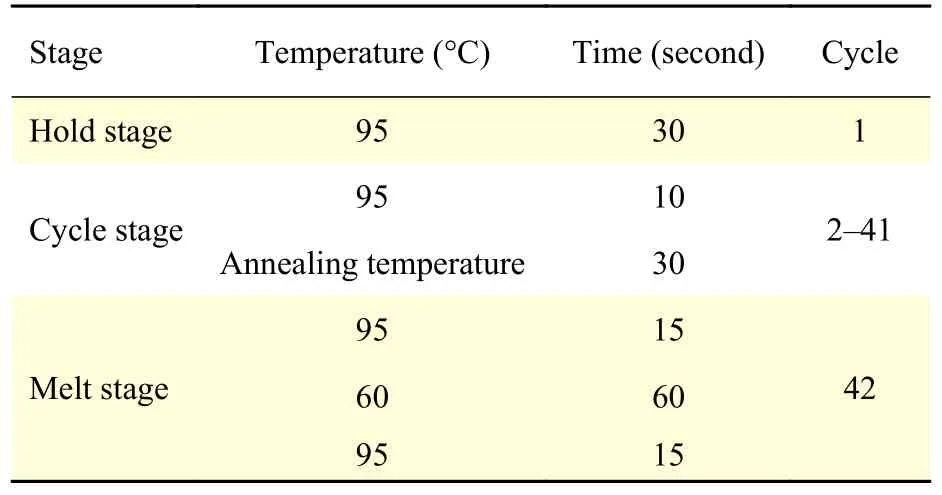
Table 2 Parameters of real-time PCR and melt curve
Antibody titer detection by ELISA
To validate the quantification of the expression levels of Th2 cytokines is in accordance with the antibody titer so as to evaluate the precision and versatility of the established method, the antibody titer of immunoglobulin G (IgG) was measured by traditional ELISA. The horseradish peroxidase (HRP)labeled goat-anti-camel IgG was prepared by Prof.Jirimutu's group of Inner Mongolia Agricultural University. A 96-well plate was firstly coated with 100 μL OVA solution (10 μg/mL in PBS buffer) and the plate was then incubated at 4 °C overnight. After washing the unbounded OVA with PBST (PBS buffer containing 0.05% tween 20), the plate was blocked by 2% BSA in PBST buffer at RT for 2 hours. The antisera were serially diluted by 1% BSA in PBS buffer and added into each well at RT, then followed by 10 washes with PBST buffer. Then the plate was incubated with HRP labeled goat-anti-camel IgG at 37°C for 1 hour. The plate was washed with PBST buffer for 10 times to remove the unbounded conjugate thoroughly and the antibody binding was evaluated by adding 3,3',5,5'-tetramethylbenzidine(Beyotime, China) into each well. The reaction was terminated by adding 2 mol/L H2SO4and the optical density (OD) value was recorded by a microplate reader at 450 nm (Tecan, Switzerland). The antibody titer was calculated according to the reciprocal of the dilution which has the OD values larger than 0.1 standard deviations above background levels obtained from PBS blank at the same dilutions.
Statistical analysis
All the data are shown as mean±standard deviation,and the statistical analysis and regression analysis were performed by SPSS 17.0 software and the significance level was set as 0.05.
Results
The whole research design was shown inFig. 1in which real-time PCR and ELISA had been performed to pre-evaluate the immune response of Bactrian camels. Firstly, the unimodal melt curve (Fig. 2) for amplification of the respective gene was obtained demonstrating the good specificity and sensitivity of the developed method by the use of selected primers.Accordingly, as quantified by real-time PCR, it was found that the expression levels of mRNA of IL-4, IL-10, and IL-13 of the camels may increase by a factor of 0.78, 2.15, and 0.22 after the immunization,respectively. In the cases of either IL-4 or IL-10, the up-regulation displayed a significant difference with thePvalue less than 0.05 at the time interval of week 2 and 3, respectively. The kinetics profile of each Th2 cytokine was also recorded. Notably, the expression levels of all three cytokines exhibited a timedependent increase in the up-regulation. As shown inFig. 3A-C, the expression level of each cytokine was observed to increase after the boost immunization,suggesting the activation of B cells and the evoked humoral immune response.
Next, to examine the accuracy of the proposed method, the antibody titer for IgG was analyzed by the traditional method of ELISA. According to the kinetic profile of antibody titer (Fig. 3D), it was clear that the resulting antibody titer significantly increased(P<0.05) after the initial immunization. Moreover, the increasing titer had also validated the effective generation of antibody after immunization with OVA.To validate the reliability of the method, further regression analysis was conducted to examine the correlation between expression level and antibody titer. As shown inFig. 4, linear correlation plots were obtained for IL-4, IL-10, and IL-13, of which the correlation coefficients were found to be 0.88, 0.83,and 0.89, respectively.

Fig. 2 The melt curve for the amplification of IL-4, IL-10, IL-13, and β-actin.

Fig. 3 Expression profile of Th2 cytokines from real-time PCR and antibody titer from ELISA at different weeks. A-C: Relative expression levels of IL-4, IL-10, and IL-13 after immunization, respectively. Data were standardized by 2-△△ct. D: The kinetic profile of antibody titer after immunization. *P<0.05; **P<0.01.
Discussion
Despite accumulating studies on cytokines of human and model animals such as murine during past decades[27-28], the cloning and quantification of camel cytokines are lacking. With growing interest in HcAb as a unique product of Camelidae, the critical evaluation of the immune responses of camels is of significant importance for practical uses. To obtain HcAb with satisfactory affinity and specificity, the administration of immunization is the key step that the appropriate quality control is demanding.Conventionally, the measurement of antibody titer by ELISA is straightforward which has become a standard method for evaluating immune responses[7].However, the lack of commercial enzyme-labeled antibody has greatly limited the exploration of camelderived antibody production due to the following two consequences. On one hand, the insufficient immune administration will add technical as well as financial burden on the downstream procedures such as phage display screening of HcAb. On the other hand,excessive immunization may cause adverse effects to camels possibly due to the increased toxicity of adjuvants of high dosage used. Therefore, it is requesting for convenient and reliable pre-evaluation of immune responses of camels before the antibody production.
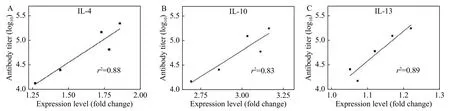
Fig. 4 The correlation analysis between results from real-time PCR and ELISA. The expression levels for IL-4, IL-10, and IL-13 correlated with antibody titer, respectively.
It has been noted that previous studies have been conducted for quantification of expression levels of both Th1 and Th2 cytokine by real-time PCR to estimate the immune response to intracellular bacteria[20]. Although bacterial infection involves mainly cell-mediated immunity, these studies suggest the possibility to evaluate the humoral immune response by real-time PCR. Particularly, cytokines secreted by Th2 cells were shown to be able to stimulate B cell proliferation which is in favor of the production of antibodies[29-30]. Therefore, monitoring the expression levels of these Th2 cytokines can serve as a molecular probe to evaluate the immune efficiency. The immune responses to soluble antigens are strictly thymic-dependent in which the generation of antibodies mainly depends on the participation of antigen-presenting cells and Th cells[31]. Then those antigens can be recognized and captured by B cells specifically and presented to Th cells. With the help of cytokines secreted by Th cells, the naïve B cells are further transformed into plasmocytes followed by the production of antigen-specific antibodies. As a soluble model antigen, OVA mainly stimulates the humoral immune response by producing antibodies and many studies have validated that IL-4 and IL-10 play a critical role in the generation of protective antibodies[32-33]. Being secreted mainly by activated Th2 cells, IL-4 acted as the growth or differentiation factor and participated in the activation, augmentation,and differentiation of B cell[30-34]. Additionally, the increased concentration of IL-4 promotes the activation and proliferation of Th2 cells favorable for producing specific antibodies. Meanwhile, IL-10 was shown to either stimulate the B cell activation or extend the survival periods of B cells[35]. Studies also revealed that among all Th2 cytokines, IL-10 is the most relevant to the secretion of inflammatory cytokines[33-36]. And Th2 cytokine IL-13 is capable to induce B cell proliferation and differentiation once produced by activated Th2 cells[37]. Based on these reports, we hypothesize that the quantification of several Th2 cytokines may be useful for evaluating the immunization responses of Camel. Therefore, in this study, Th2 cytokines related to humoral immunity were chosen as evaluation probes to conduct the realtime PCR assay.
Previous studies have revealed that OVA or other soluble antigens are capable to induce B cell activation in which Th2 cytokines are released[38-39].Therefore, our experimental observations have validated the significantly increased expression levels of mRNAs, especially for IL-4 and IL-10 during the immunization process. The monitoring of Th2 cytokine expression will be important for the molecular understanding of the humoral immune responses in camels, especially during the production of heavy-chain only antibodies. Meanwhile, recording the kinetic profile of Th2 cytokines is also useful for the study of both immune responses and infectious diseases in camels[20-23].
The satisfactory correlation coefficients may suggest the desirable precision of constructed realtime PCR method in the pre-evaluation of the evoked immune responses. As for IL-13, even though the expression level was not significantly up-regulated(P>0.05), the obtained correlation coefficient from two methods was also as high as 0.89, which also indicated a possible candidate to assess immune responses. Taken together, the consistency of the time-dependent up-regulation of expression of Th2 cytokines and the antibody titers has demonstrated the feasibility of the developed method to evaluate the immune response of Bactrian camels. Furthermore,owing to the advantages of real-time PCR platform such as high throughput and sensitivity, the constructed method enables the detection of trace or large-scale samples favorable for the dynamic monitoring of cytokines associated with immune responses[23].
The method described in this study has been successfully used in quantifying Th2 cytokine cDNAs with sufficient accuracy. Meanwhile, without the need of enzyme-labeled antibodies, our developed method is readily available for laboratories to research Bactrian camels. In the long run, considering the infeasibility to directly detect the level of Th2 cytokines, further experiments should be done to quantify their expression by the external standard method once adequate antibodies are ready[40-41].
In conclusion, the present study has firstly developed a high throughput and reliable method to pre-evaluate the immune response of Bactrian camels by quantifying the expression of Th2 cytokines via real-time PCR. The satisfactory correlation coefficients were obtained between the results obtained from realtime PCR and those of standard ELISA, indicating a desirable precision of our developed method.Therefore, the proposed method is suitable for analyzing trace and large-scale samples on a real-time mode, which may further contribute to the molecular understanding of immune responses. Particularly,without the need of enzyme-labeled antibodies[42-43],the developed method will readily available for laboratories to conduct biomedical studies on Bactrian camels and so on. During a long run, further investigations should be conducted to study the concentration effect of these cytokines on antibodies,which will help develop commercial detection kits as well as the conservation of precious Bactrian camels.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (U1703118), Natural Science Foundation of Jiangsu Province (No.BK20181364), Natural Science Foundation of Jiangsu Higher Education Institutions of China (No.19KJA310003), Scientific Research Foundation of Jiangsu health and Health Committee (No.H2018087), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Jiangsu Shuangchuang Program,Open Funds of the State Key Laboratory for Chemo/Biosensing and Chemometrics (2016015),Open project of the National Laboratory of Biomacromolecules (2017kf05), the cooperative project between Southeast University and Nanjing Medical University (2018DN0004) and Jiangsu Specially-Appointed Professor project, China.
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Comparison of the modified Wiltse's approach with spinal minimally invasive system and traditional approach for the therapy of thoracolumbar fracture
- Exposure to environmental bisphenol A inhibits HTR-8/SVneo cell migration and invasion
- Cumulative live birth rates of in vitro fertilization/intracytoplasmic sperm injection after multiple complete cycles in China
- Cofilin participates in regulating alpha-epithelial sodium channel by interaction with 14-3-3 isoforms
- TIR/BB-loop mimetic AS-1 protects vascular endothelial cells from injury induced by hypoxia/reoxygenation
- Postprandial dyslipidemia in insulin resistant states in adolescent populations
