Cofilin participates in regulating alpha-epithelial sodium channel by interaction with 14-3-3 isoforms
2020-11-11AshfaqAhmadShahBukhariXueZhangMinLiAnranZhaoHaoDongXiubinLiang
Ashfaq-Ahmad-Shah Bukhari, Xue Zhang, Min Li, Anran Zhao, Hao Dong, Xiubin Liang,3,✉
1Department of Pathophysiology, 2Department of Pathology, Nanjing Medical University, Nanjing, Jiangsu 211166,China;3Department of Nephrology, the Affiliated Sir Run Run Hospital of Nanjing Medical University, Nanjing, Jiangsu 211166, China.
Abstract Renal epithelial sodium channel (ENaC) plays a crucial role in maintaining homeostasis and sodium absorption.While insulin participates in controlling sodium transport across the renal epithelium, the underlying molecular mechanism remain unclear. In this study, we found that insulin increased the expression and function of alphaepithelial sodium channel (α-ENaC) as well as phosphorylation of cofilin, a family of actin-binding proteins which disassembles actin filaments, in mouse cortical collecting duct (mpkCCDc14) cells. The wild-type (WT)cofilin and its constitutively phosphorylated form (S3D), but not its constitutively non-phosphorylable form(S3A), contributed to the elevated expression on α-ENaC. Overexpression of 14-3-3ε, β, or γ increased the expression of α-ENaC and cofilin phosphorylation, which was blunted by knockdown of 14-3-3ε, β, or γ.Moreover, it was found that insulin increased the interaction between cofilin and 14-3-3 isoforms, which indicated relevance of 14-3-3 isoforms with cofilin. Furthermore, LIMK1/SSH1 pathway was involved in regulation of cofilin and α-ENaC expression by insulin. The results from this work indicate that cofilin participates in the regulation of α-ENaC by interaction with 14-3-3 isoforms.
Keywords: cofilin, ENaC, insulin, 14-3-3, LIMK1, SSH1
Introduction
Sodium transport is mainly facilitated and regulated by selective sodium channels on the apical membranes to maintain the homeostasis that can preserve the normal levels of fluids[1-2]. The precise control of epithelial sodium channels (ENaC) maintains the extracellular volume, sodium balance, and blood pressure[3].
Dysfunction of ENaC results in many clinical disorders in humans, including salt wasting(pseudohypoaldosteronism)[4-5]and salt-sensitive hypertension, which confirm the physiological need of these channels in salt and water balance[6]. ENaC is the rate-limiting step for sodium absorption in connecting tubules, late distal tubule, and entire collecting duct of distal nephron. Therefore, proper regulation of ENaC is required in homeostasis, longterm blood pressure control, and renal sodium excretion[7]. Besides that, ENaC is present in the lungs[8], brain[9], and colon[10].
Three homologous subunits, α, β, and γ ENaC are identified[11-12]. Each subunit consists of two membrane-spanning domains, an intracellular N and C terminus and the extracellular loop[13]. Distal nephron principal cells present β- and γ- subunits of ENaC during sodium repletion, but the low expression of the α-ENaC subunit, because α-subunits of ENaC is essential for the assembly of functional sodium channels that can traffic to the apical surface[14].Hormones such as insulin, aldosterone, and vasopressin highly regulate ENaC expression in the kidney[15]. Acute regulation of ENaC activity mainly caused by vasopressin, insulin and prostaglandins.Insulin is one of the causative factors for hypertension consequent from renal Na+retention by activation of ENaC. It has been reported that insulin induces the migration of ENaC from the cytoplasm to the apical and lateral membrane in A6 cells[16], and that insulin excites ENaC activity in mouse cortical collecting duct (mpkCCDc14) cells[17].
Cofilin is a family of actin-binding proteins that maintains the function and polarity of proximal tubular epithelial cell. It has an essential role in actin rearrangement and dynamics by depolymerizing and severing the actin filaments at their acanthus ends[18].Cofilin activities can be reversibly regulated by phosphorylation at serine-3 (Ser3) residue by LIM kinases (LIMKs) and dephosphorylation by Slingshot(SSH) phosphatase[19-20]
The 14-3-3 proteins are a family of highly preserved regulatory molecules that usually bind to phosphorylated residues within their protein targets.They play essential roles in a broad range of cellular processes, including cell cycling, signal transduction,membrane trafficking, and protein biogenesis[21-22].The 14-3-3 have seven mammalian isoforms (β, ε, γ,η, θ, σ, and ζ), each encoded by a distinct gene,function as hetero- or homo-dimers of different isoforms[23].
The cortical collecting tubule and collecting ducts regulate the final excretion of sodium from kidneys.We hypothesized that cofilin, the major regulator of actin polymerization/depolymerisation, plays roles in ENaC regulation in this region. Therefore, we evaluated the impact of insulin on ENaC, 14-3-3, and cofilin in mpkCCDc14cells, and revealed that cofilin participates in the regulation of α-ENaC by interaction with 14-3-3 isoforms in this study.
Materials and methods
Antibodies and plasmids
The antibodies specific for cofilin, phosphorylated cofilin, LIMK1 and phosphorylated LIMK1 and SSH1 were purchased from Cell Signaling Technology(UK). The antibodies for 14-3-3 isoforms (β, ε and γ)were purchased from Santa Cruz Biotechnology(USA). GAPDH antibody was obtained from Sigma-Aldrich (USA). The rabbit IgG and secondary antibodies against rabbit and mouse were purchased from Thermo Fisher Scientific (USA). Plasmids of cofilin (wild-type [WT], constitutively phosphorylated form [S3D], constitutively non-phosphorylable form[S3A]) and 14-3-3 isoforms (ε, β and γ) were gifts from Dr. Xuerong Wang (Nanjing Medical University, China). 14-3-3 shRNAs including isoforms ε, β, and γ were purchased from Dharmacon(USA). LIMK1 and SSH1 siRNA were purchased from GenePharma (China).
Cell culture and transient transfection
mpkCCDc14cells were kindly provided by M. Bens and A. Vandewalle (INSERM, France). As previously described by Liang et al, the modified medium was used to seed the cells in a plastic flask[24]. The modified medium was composed of equal volumes of DMEM and F12, plus 5 mg/L transferrin, 50 nmol/L dexamethasone, 1 nmol/L triiodothyronine, 60 nmol/L sodium selenate and 2% fetal bovine serum (FBS).The pH of this solution was 7.4. Cells were maintained in 95% air atmosphere, 5% CO2at 37 °C.In vitro transfections of the mpkCCDc14cells with plasmids were performed using Lipofectamine-3000(Invitrogen, USA) according to the manufacturer's instructions. The expression of target genes was determined by Western blotting at 48 hours after transfection.
Antibody transfection
According to manufacturer's instructions, Pro-Jec Pierce Protein Reagent kit (Thermo Fisher Scientific)was used to deliver the anti-14-3-3ε antibody into the mpkCCDc14cells. In brief, 5 μg of anti-14-3-3ε antibody was prepared in phosphate buffered saline(PBS: 150 mmol/L NaCl, 20 mmol/L Na3PO4, pH 7.4)and hydrated the dried Pierce Reagent with the diluted protein solution and incubated for 5 minutes at room temperature. For 6 well plate, a total of 1 mL of serum-free medium was added to mix the Pierce reagent-protein complex and delivered to the cells.
RNA extraction and quantitative reverse transcription PCR
Triazole reagent (Thermo Fisher Scientific) was used to extract RNA from mpkCCDc14cells. A Kit(Takara, Japan) was used for reverse transcription of RNA. PCR was performed using SYBR Green Master Mix (Applied Biosystems, USA) and the Applied Biosystems Step One Plus Real-Time PCR system.The following sequences of primers were used: α-ENaC forward 5 ′-TGTGTCCAGCTACAAACCAA TG-3′ and reverse 5′-CATCATGCCCACTTCGTAAC A-3 ′; Cofilin forward 5 ′-CAGACAAGGACTGCC GCTAT-3′ and reverse 5′-TTGCTCTTGAGGGGTG CATT- 3′; GAPDH forward 5′-GCAAGTGCTTCT AGGCGGAC-3′ and reverse 5′-AAGAAAGGGTG TAAAACGCAGC-3′.
Western blotting analysis
Equal amounts of proteins taken from respective polarized mpkCCDc14cells were resolved by 10%SDS-PAGE on gels. The unbound sites were blocked with 5% non-fat milk in Tris buffer saline (TBS)containing 0.1% Tween-20 for 1-2 hours at room temperature. These blots were incubated with appropriate primary antibodies in required dilution(anti-α-ENaC, 1:5000; anti-cofilin, 1:500; antiphosphorylated cofilin, 1:500; anti-phosphorylated LIMK1, 1:1000; anti-LIMK1, 1:1000; anti-SSH1,1:1000; anti-14-3-3β, 1:2000; anti-14-3-3γ, 1:2000;anti-14-3-3ε, 1:2000; anti-GAPDH, 1:8000), and then incubated with horseradish peroxidase (HRP)conjugated secondary antibodies (anti-Rabbit, 1:8000;anti-Mouse, 1:8000) for 1 -2 hours at room temperature on rotating shaker. Enhanced chemiluminescence (ECL) was used to detect the immuno-recognition signals, and the Image Quant ECL system (PerkinElmer life Sciences, USA) was used to obtain the signals. Data were quantified with Image Lab software.
Immunofluorescence staining
Cell stationary liquid (Beyotime, China) was used to fix the mpkCCDc14cells cultured on filter support for 20 minutes at room temperature. Permeabilization was done with 0.3% Triton X-100 in PBS for 5 minutes. After washing with PBS, 5% goat serum prepared in PBS was used to incubate the cells for 1 hour. The specified antibodies against α-ENaC(1:200) were used to incubate the cells overnight at 4 °C and interacted with cyanine FITC donkey antirabbit IgG (Jackson Immuno Research Laboratories,USA) for 1 hour at 37 °C. These mpkCCDc14cells were mounted with Vecta Shield Mounting Medium(Vector Laboratories, USA) and all images were obtained by Carl Zeiss LSM 10 microscope equipped with a digital camera (USA) and processed using Photoshop software (Adobe Systems Incorporated,USA).
Co-immunoprecipitation assays
For co-immunoprecipitation studies, lysates were collected from mpkCCDc14cells and lysed with NP40 lysis buffer containing 10 mmol/L Tris-HCl (pH 7.4)and 10 mmol/L NaCl. The protein concentrations of the lysates were determined by BCA, and then 0.5 mg protein was incubated with specific antibodies of target gene overnight at 4 °C before incubation with 100 μL of protein A beads for 2-6 hours. The immunocomplexes were washed with NP40, degenerated with SDS loading buffer, and subjected to Western blotting. IgG antibody was used as a control for the immunoprecipitation in an amount equal to primary precipitating antibody.
Surface protein biotinylation
To perform sample biotinylation, the mpkCCDc14cells were incubated for 20 minutes with 0.5 mg/mL SS-Biotin (Thermo Fisher Scientific) in borate buffer(85 mmol/L NaCl, 4 mmol/L KCl, 15 mmol/L Na2B4O7, pH 9) at 4 °C after washing 5 times with ice-cold PBS (containing Mg+and Ca2+) on ice. The reaction was stopped by adding a double volume of FBS-containing medium. A cell homogenate was obtained by lysing cells in lysis buffer (0.4%deoxycholic acid, 1% NP-40, 50 mmol/L EGTA, and 10 mmol/L Tris-Cl, pH 7.4) for 10 minutes, and then the lysate was centrifuged at 14 000 g for 5 minutes at 4 °C. The supernatant was collected to assay for protein concentration. For the biotinylated sample,200 μg of protein was mixed with streptavidin(Thermo Fisher Scientific) and incubated overnight at 4 °C with gentle rotation. Samples from the streptavidin beads were incubated with 4× sample buffer containing 10% β-mercaptoethanol for 20 minutes at room temperature after washing with lysis buffer. Finally, samples were separated by SDSPAGE, and blotted as above to determine the density of ENaC at the apical membrane surface of mpkCCDc14cells.
Short circuit currents recordings
The mpkCCDc14cells were grown on filter supports for a high resistance monolayer. The filter supports were mounted in modified Ussing chambers to record short circuit currents (Isc) with an automatic voltage clamp as previously described[25]. The bathing solution buffer consisted of 120 mmol/L NaCl, 25 mmol/L NaHCO3, 3.3 mmol/L KH2PO4, 0.8 mmol/L K2HPO4,1.2 mmol/L MgCl2, 1.2 mmol/L CaCl2, and 10 mmol/L glucose. 10 μmol/L Amiloride was added to the apical bath to determine ENaC mediated transepithelial currents.
Statistical analysis
All the experiments were performed three times.GraphPad Prism 6 (GraphPad Software, USA) was used for statistical analyses. Depending on the number of experimental groups and factors to be compared,statistical analysis was performed with Student'st-test or one-way ANOVA followed by post hoc test (SNK).All data were presented as the mean±standard error of the mean (SEM). Differences were considered statistically significant ifP<0.05.
Results
Insulin regulated α-ENaC expression and cofilin phosphorylation
Western blotting and quantitative reverse transcription PCR (qRT-PCR) were performed to determine the expressions of α-ENaC and cofilin in mpkCCDc14cells treated with insulin at a dose course for 2 hours as previously described[25]. Insulin treatment increased total, apical surface α-ENaC expression and cofilin phosphorylation in a dosedependent manner (Fig. 1A), whereas the expression of total cofilin remained unchanged. Quantitation of α-ENaC expression and cofilin phosphorylation from all experiments were provided inFig. 1BandC. Next,we determined the functional impact of insulin on ENaC-mediated transepithelial Na+absorption by determining the amiloride-sensitiveIscacross the mouse cortical collecting duct (mCCD) epithelia. As shown inFig. 1D, insulin stimulation elicited an approximately 2.5-fold increase in the amiloridesensitiveIscacross mCCD epithelia. qRT-PCR also revealed enhanced α-ENaC mRNA expression in mpkCCDc14cells treated with insulin (Fig. 1E). These functional data and transcriptional level data were in agreement with the biochemical findings obtained above. To further confirm the effect of insulin on α-ENaC expression in mpkCCDc14cells,Immunofluorescence (IF) staining was performed. We found increased expression of α-ENaC induced by insulin (Fig. 1F), and the data were summarized inFig. 1G.
Cofilin increased α-ENaC protein expression
We determined whether cofilin participates in the regulation of α-ENaC by transient transfection of wild-type cofilin (WT cofilin) into mpkCCDc14cells.Wild-type cofilin overexpression significantly increased the expressions of cell surface α-ENaC and total α-ENaC in mpkCCDc14cells (Fig. 2A). The administration of insulin (100 nmol/L, 2 hours) further increased the expression of α-ENaC protein as compared to the cells transfected with WT cofilin only. Quantification data of α-ENaC expression were provided inFig. 2B.
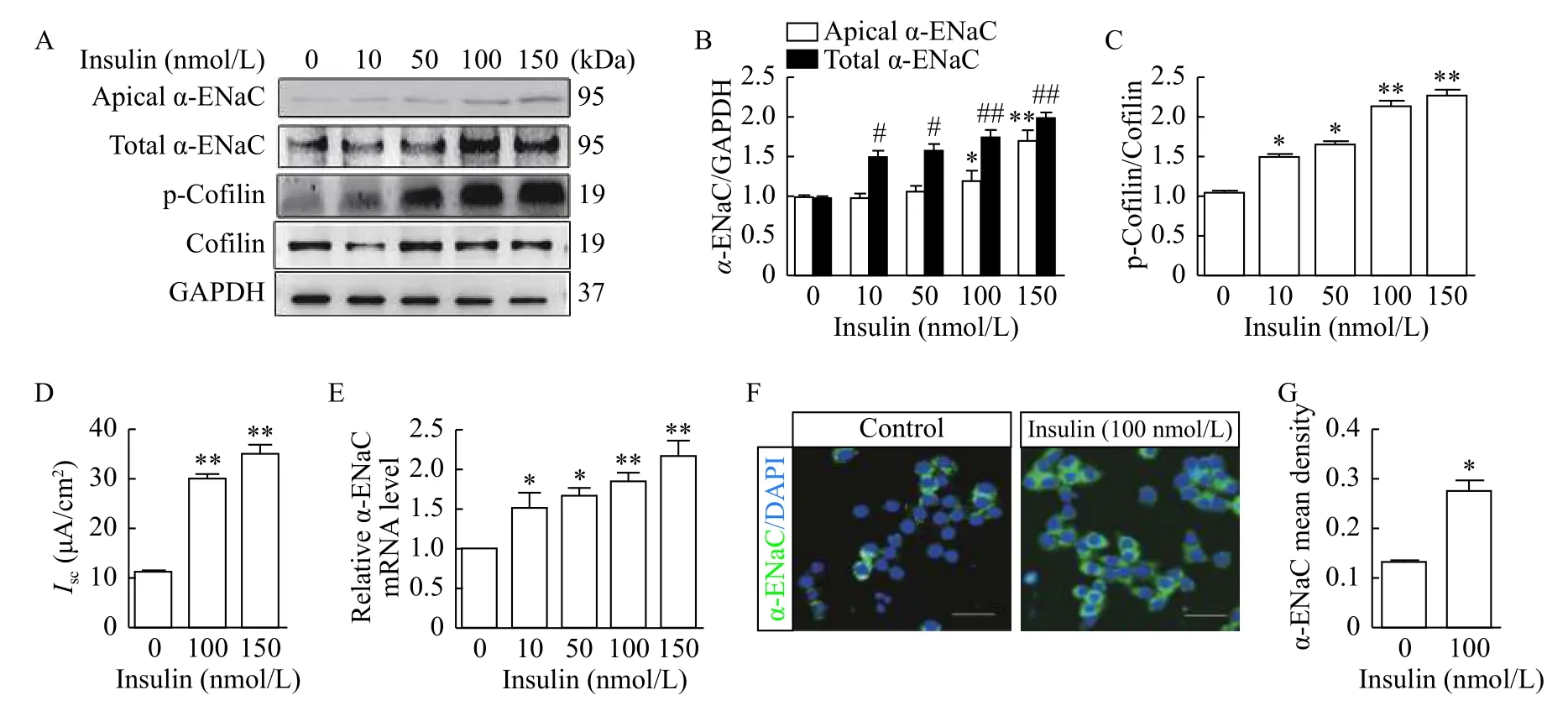
Fig. 1 Insulin regulated α-ENaC expression and cofilin phosphorylation in mpkCCDc14 cells. A: Western blotting analysis of apical α-ENaC, total α-ENaC, p-cofilin and cofilin expressions in mpkCCDc14 cells treated with different dose of insulin. B: Quantification of apical α-ENaC and total α-ENaC expressions normalized to GAPDH shown in A. *Compared with control group of apical α-ENaC; #compared with control group of total α-ENaC. C: Relative quantification of p-cofilin expression shown in A. D: Amiloride-sensitive Isc was monitored across mpkCCDc14 epithelia during insulin stimulation. E: mRNA expression level of α-ENaC in mpkCCDc14 cells treated with different dose of insulin. F: Immunofluorescence staining of α-ENaC expression in mpkCCDc14 cells treated with indicated concentrations of insulin.G: Quantification of α-ENaC expression shown in F. Data represent mean±SEM from three independent experiments, bars indicate SEM.*P<0.05, **P<0.01, #P<0.05, ##P<0.01.
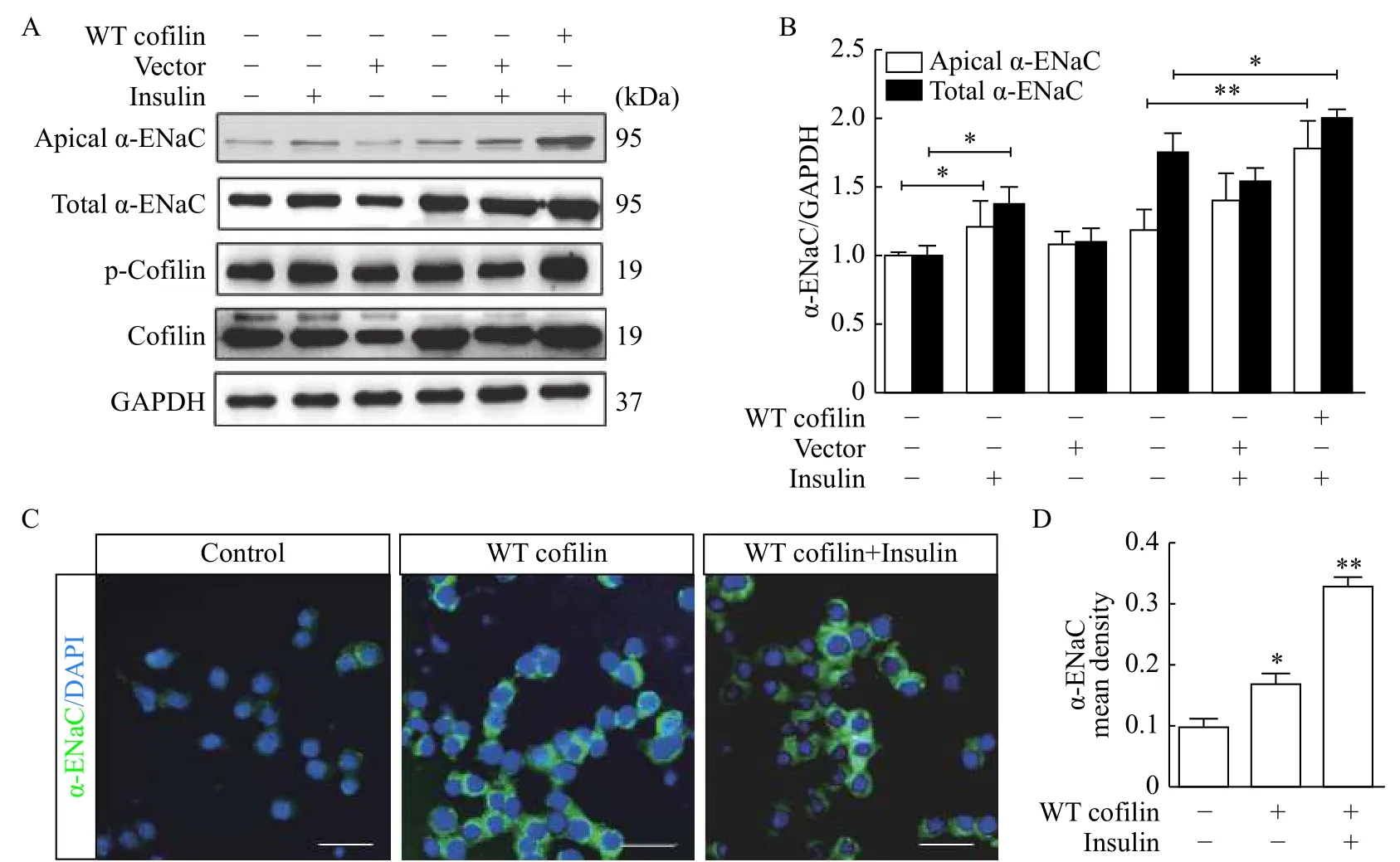
Fig. 2 Cofilin increased α-ENaC protein expression. A: Western blotting analysis of apical α-ENaC, total α-ENaC, p-cofilin and cofilin expressions in mpkCCDc14 cells transfected with wild-type cofilin (WT cofilin) and treated with or without insulin. B: Quantification of α-ENaC protein expression shown in A. *Compared with control group of apical α-ENaC; #compared with control group of total α-ENaC. C: α-ENaC expression determined by immunofluorescence staining. D: Quantification of α-ENaC expression shown in C. *Compared with control group. Data represent mean±SEM from three independent experiments, bars indicate SEM. *P<0.05, **P<0.01, #P<0.05.
To verify the effects of cofilin overexpression on α-ENaC expression, IF staining was used with a rabbit polyclonal antiserum against α-ENaC in polarized mpkCCDc14cells with or without WT cofilin transfection. As shown inFig. 2C, α-ENaC expression was significantly increased in mpkCCDc14cells transfected with WT cofilin, and noticeably additional increased expression was achieved in the presence of insulin, which is consistent with the biochemical data described above. The data are summarized inFig. 2D.
S3D cofilin increased α-ENaC protein expression
S3D cofilin, a constitutively phosphorylated form of cofilin, was transiently transfected into the mpkCCDc14cells. Using Western blotting, S3D cofilin significantly increased the expression of α-ENaC and cofilin phosphorylation in mpkCCDc14cells (Fig. 3A).Addition of insulin (100 nmol/L, 2 hours) in these cells induced extra increasing of α-ENaC and cofilin phosphorylation as compared to the cells transfected with S3D cofilin only. Quantitative data of α-ENaC expression are shown inFig. 3B. When the mpkCCDc14cells were transfected with S3A cofilin, a constitutively non-phosphorylable form of cofilin, it had no effect on expression of α-ENaC (Fig. 3CandD).
14-3-3 isoforms participated in regulation of α-ENaC and cofilin expression
The role of 14-3-3 isoforms in the regulation of α-ENaC and cofilin expression was evaluated. β, ε and γ isoforms of 14-3-3 were included in this experiment because these are the highly expressed isoforms in the mpkCCDc14cells[26]. Overexpression of 14-3-3 isoforms (ε, β, and γ) was performed in mpkCCDc14cells with 14-3-3ε, β and γ under basal condition.Western blotting results revealed the increased expression of α-ENaC and cofilin phosphorylation with transfection of 14-3-3ε (Fig. 4AandB), 14-3-3β(Fig. 4CandD) and 14-3-3γ (Fig. 4EandF).Consistently, knockdown of 14-3-3 isoforms (ε, β, and γ) with transfection of 14-3-3 shRNA decreased the expression of α-ENaC and cofilin phosphorylation(Fig. 4G-L).
Insulin increased the cofilin interaction with 14-3-3 isoforms
We examined the interaction of different isoforms(β, ε, and γ) of 14-3-3 with cofilin in mpkCCDc14cells. The antibodies against the cofilin were assayed as protein complexes in the absence and presence of insulin. Western blotting was applied to check isolated proteins and for different 14-3-3 isoforms.Immunoprecipitation experiment was used to detect the signals of 14-3-3 isoforms where IgG served as control yielding no signals. Under basal conditions,positive signals were detected for binding of cofilin to 14-3-3β, ε, and γ (Fig. 5A). 14-3-3ε showed predominant interaction with cofilin in mpkCCDc14cells under basal conditions. Insulin treatment (100 nmol/L) enhanced the binding of 14-3-3 isoforms with cofilin (Fig. 5A). In the next step, we confirmed the role of 14-3-3ε in the regulation of α-ENaC in a competition experiment using an antibody against 14-3-3ε. The antibody was introducedviathe Pro-Ject Protein Transfection against 14-3-3ε into mpkCCDc14cells. Cell lysates were obtained from mpkCCDc14epithelia under basal and insulin treated conditions after transfection with the 14-3-3ε targeting antibody.As anticipated, the level of α-ENaC expression and cofilin phosphorylation were minimum in basal condition as compared to insulin-stimulated condition,where enhanced expression of α-ENaC and increased cofilin phosphorylation were observed (Fig. 5B).However, pre-transfection of mpkCCDc14cells with an antibody against 14-3-3ε reduced the level of α-ENaC expression and cofilin phosphorylation.Quantitative data of α-ENaC expression from all experiments are shown inFig. 5C.
Reciprocal role of LIMK1 and SSH1 on regulation of cofilin phosphorylation and α-ENaC expression
To ascertain the interaction of cofilin phosphorylation and α-ENaC regulation, we used siRNA to knockdown LIMK1 and SSH1 in the mpkCCDc14cells. Western blotting results showed that cofilin phosphorylation as well as α-ENaC expression decreased by usingLIMK1siRNA(Fig. 6AandB), but increased by usingSSH1siRNA in the mpkCCDc14cells (Fig. 6CandD). This regulation was also influenced by insulin, indicating that the regulation of cofilin and α-ENaC expression by insulin probably relevant to LIMK1/SSH1 pathway.
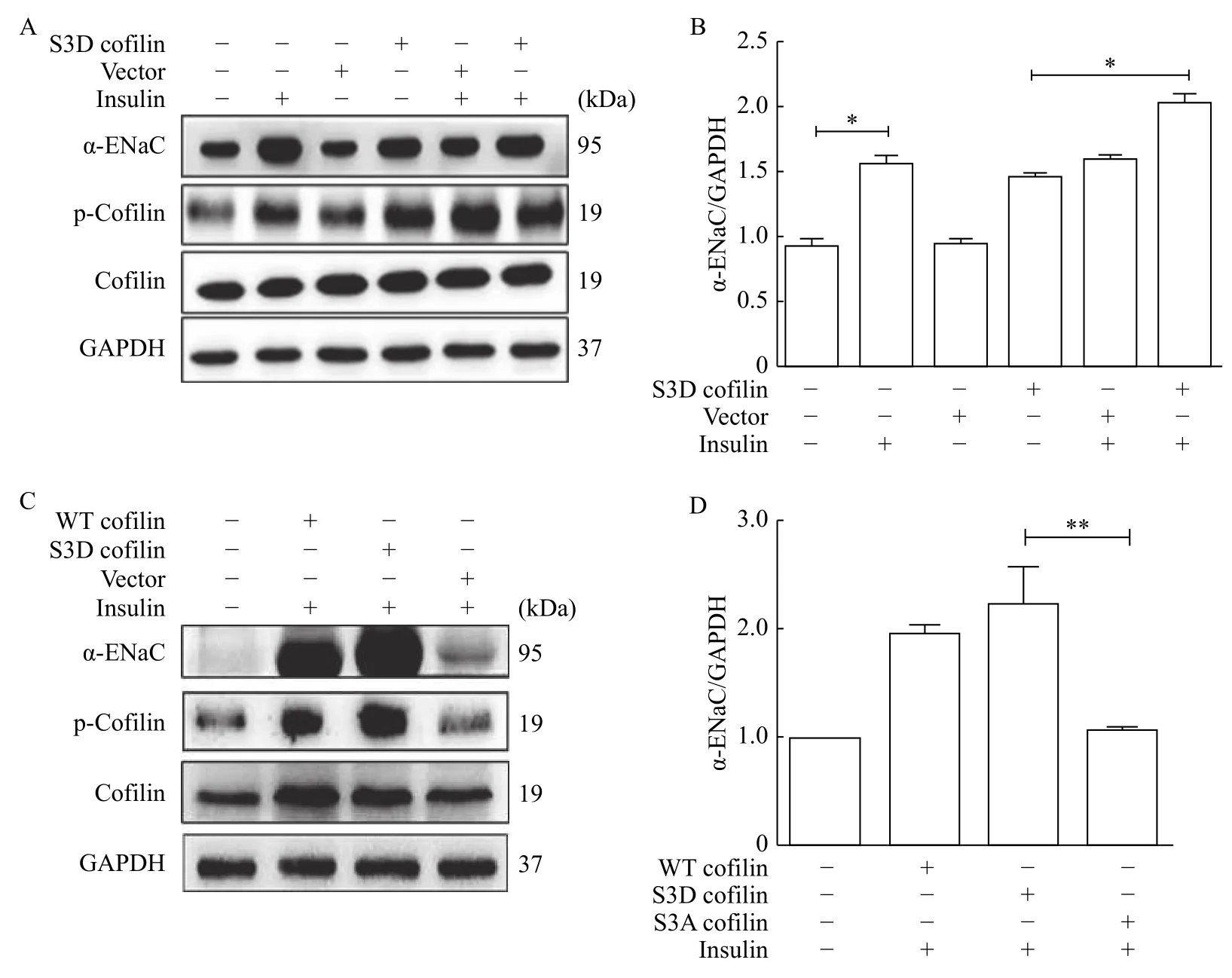
Fig. 3 Cofilin S3D increased α-ENaC protein expression. A: Western blotting analysis of α-ENaC and p-cofilin expressions in mpkCCDc14 cells transfected with S3D cofilin and treated with or without insulin. B: Relative quantification of α-ENaC expression shown in A. C: Western blotting analysis of α-ENaC and p-cofilin expressions in mpkCCDc14 cells transfected with S3D or S3A cofilin and treated with insulin. D: Quantification of α-ENaC expression shown in C. Data represent mean±SEM from three independent experiments, bars indicate SEM. *P<0.05, **P<0.01.
Discussion
In response to endocrine and dietary stimuli,cortical collecting duct plays a critical role to achieve systemic homeostasis by promoting absorption and secretion of water and electrolytes[27]. Multiple studies have provided evidences that there is involvement of numerous hormones and drugs in regulation and trafficking of ENaC. Phosphorylation of proteins plays a key role in mediating the effects of insulin on their target cells[28]. Here, we demonstrate that insulin regulates α-ENaC through cofilin, an actin regulator,providing a novel mechanism underlying this significant physiological process.
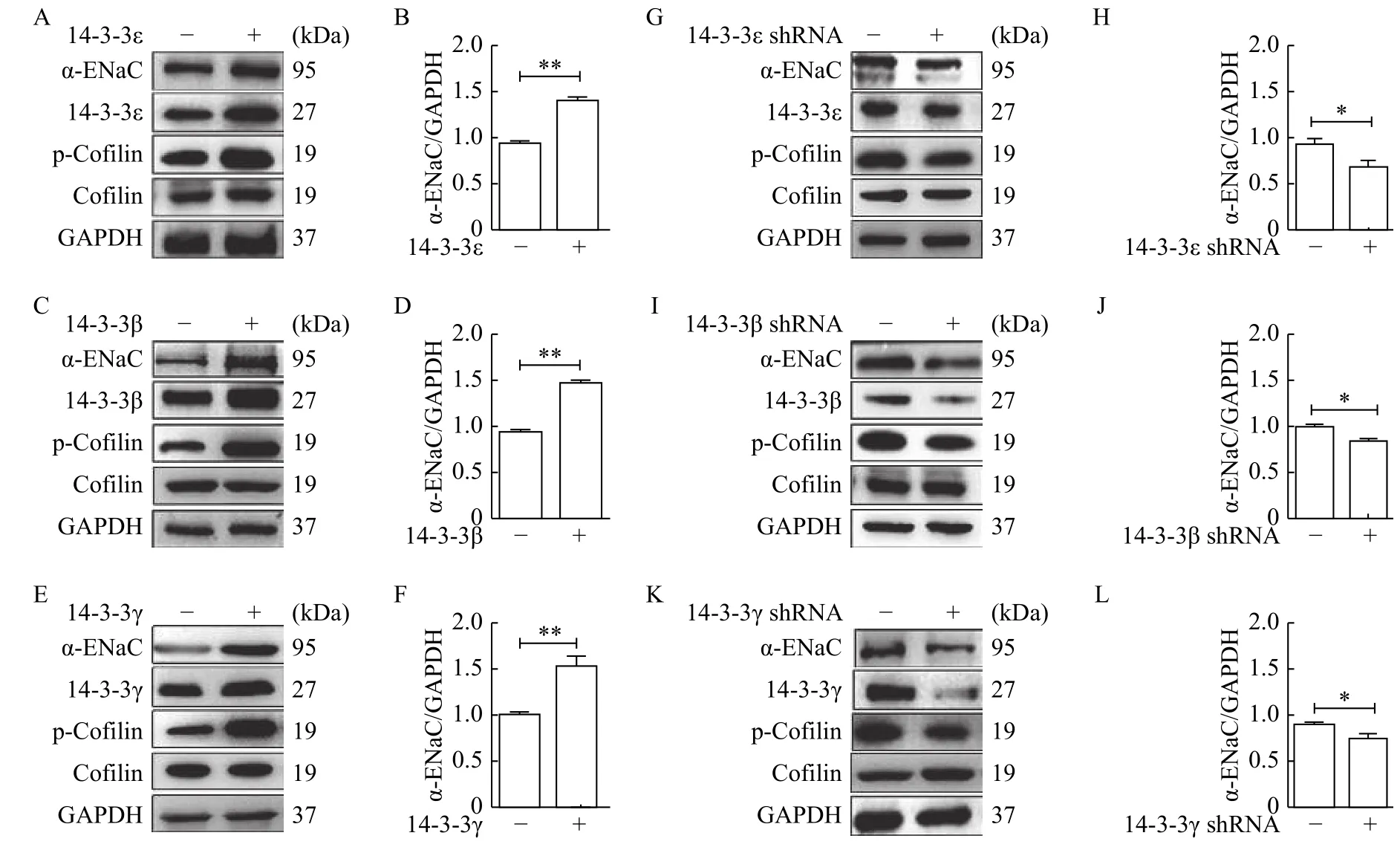
Fig. 4 14-3-3 isoforms participated in regulation of α-ENaC and cofilin expression. A-F: Overexpression of 14-3-3 isoforms (ε, β, and γ) was performed in mpkCCDc14 cells under basal condition. Western blotting analyses were performed to evaluate expressions of α-ENaC and cofilin, and quantification of α-ENaC expression was normalized by GAPDH. 14-3-3ε (A and B), 14-3-3β (C and D), 14-3-3γ (E and F).G-L: shRNA transfections of 14-3-3 isoforms (ε, β, and γ) were used in knockdown experiments, Western blotting analyses were performed to evaluate expression of α-ENaC and cofilin, and quantification of α-ENaC expression was normalized by GAPDH. 14-3-3ε (G and H),14-3-3β (I and J), 14-3-3γ (K and L). Data represent mean±SEM from three independent experiments, bars indicate SEM. *P<0.05, **P<0.01.

Fig. 5 Insulin increased the cofilin interaction with 14-3-3ε. A: Co-immunoprecipitation was performed to test the interaction between cofilin and different 14-3-3 isoforms (β, ε, and γ) in mpkCCDc14 cells. 14-3-3ε showed the predominant interaction with cofilin in mpkCCDc14 cells under basal conditions. B: The levels of α-ENaC and cofilin phosphorylation were observed in the mpkCCDc14 cells pretransfected with an antibody against 14-3-3ε (14-3-3ε [Ab]). C: Relative quantification of α-ENaC protein as shown in B. Data represent mean±SEM from three independent experiments, bars indicate SEM. *P<0.05.
Cofilin is of small size (19 kDa), hence can easily diffuse in and out of the nucleus. However, during stress condition, the large number of cofilins localizes to the nucleus[29]. In different regions of nephron, the principal cells (PC) of the distal convoluted tubule, the connecting tubule, and the cortical, outer and inner medullary collecting duct explicitly express all three subunits of Na+channels[30-33].
We selected α-ENaC for our research project and provided evidence that cofilin is a positive regulatory factor for α-ENaC expression in mCCD epithelia,which is further augmented by insulin. Insulin takes part in the stimulation of ENaC mediated sodium reabsorption in the principal cells of CCD[34]. Our data reveal that insulin influences the stimulation of Na+channels to initiate the process of reabsorption and increases the expression of cofilin protein in mCCD epithelia. Under basal condition, the wild-type and S3D cofilin enhanced the expression of α-ENaC in mpkCCDc14cells, proposing an important role of cofilin in the regulation of α-ENaC and it responded more with the addition of insulin. Moreover, insulin stimulates the phosphorylation of cofilin, resulting in the transport of cofilin from the nucleus, indicating excitatory action of cofilin under basal condition with further enhancement in the presence of insulin.
Our previous study has shown that 14-3-3 proteins play a significant role in the stimulation of transepithelial transport of Na+by binding to a protein that has been phosphorylated in response to steroid hormones[24]. In the present study, 14-3-3 isoforms knockdown decreased the expression of α-ENaC as well as cofilin in mpkCCDc14cells. Our findings also suggest that cofilin has effect on apical expression of α-ENaC. Moreover, over-expressed 14-3-3 isoforms increased the expression of α-ENaC related to cofilin in mpkCCDc14cells. Cofilin cellular function is mainly controlled by dephosphorylationphosphorylation cycle, involving specific phosphatase(slingshot) as well as kinase (LIMK) and scaffold protein 14-3-3 isoforms. The localization, expression and proximity of cofilin are affected by LIMK1, and phosphorylation of cofilin is promoted by 14-3-3 isoforms. However, it is still not clear whether this process interferes phosphorylation or dephosphorylation of cofilin. To confirm the pathway involvement, we also evaluated the role of LIMK1 and SSH1 in the above-mentioned cycle. We found that reduction in slingshot activity led to increased level of phosphorylated cofilin and α-ENaC expression, whereas reduction in LIMK1 activity decreased the cofilin phosphorylation and α-ENaC expression.

Fig. 6 Reciprocal role of LIMK1 and SSH1 on regulation of cofilin phosphorylation and α-ENaC expression. A: Western blotting analyses were performed to evaluate expressions of α-ENaC, LIMK1 and cofilin in mpkCCDc14 cells transfected with LIMK1 siRNA, and treated with or without insulin. B: Quantification of α-ENaC and p-LIMK1 expression was normalized by GAPDH. C: Western blotting analyses were performed to evaluate expressions of α-ENaC, SSH1 and cofilin in mpkCCDc14 cells transfected with SSH1 siRNA, and treated with or without insulin. D: Quantification of α-ENaC and SSH1 expression was normalized by GAPDH. Data represent mean±SEM from three independent experiments, bars indicate SEM. *P<0.05, **P<0.01.
Many pathways may be involved in the trafficking of ENaC as it becomes unchanged or more active in different conditions. Our findings show that cofilin is a marker of α-ENaC insertion sites under the insulin stimulation, modulating the local actin cytoskeleton to assist in vesicle fusion. However, the interactions of many proteins with ENaC are needed to be identified to confirm the role of these proteins in ENaC trafficking. Potentially, the pursuit of this study led to the identification of mediators which regulate ENaC insertion, such as the motor proteins that utilize restructured actin tracts to drive vesicles toward the apical membrane. Yipet alexplained that several nonconventional myosins were identified by 14-3-3 affinity capture, and Myo1c was shown recently to mediate a late step in GLUT4 trafficking, undergoing insulin-dependent phosphorylation accompanied by 14-3-3 binding[35]. Therefore, by confirming the role of cofilin in α-ENaC regulation, we will have a deeper insight into pathophysiological conditions which mainly give birth to abnormalities in the process of regulation of ENaC, and then lead to determine the targets for its remedial manipulation.
Acknowledgments
This work was supported by the National Natural Science Foundation of China to XL (Grant No.81870467 and No. 81670619). AASB, XZ, ML, and XL designed and conducted the study. HD and AZ participated in the data collection and analysis. AASB and XL prepared the manuscript.
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Pre-evaluation of humoral immune response of Bactrian camels by the quantification of Th2 cytokines using real-time PCR
- Comparison of the modified Wiltse's approach with spinal minimally invasive system and traditional approach for the therapy of thoracolumbar fracture
- Exposure to environmental bisphenol A inhibits HTR-8/SVneo cell migration and invasion
- Cumulative live birth rates of in vitro fertilization/intracytoplasmic sperm injection after multiple complete cycles in China
- TIR/BB-loop mimetic AS-1 protects vascular endothelial cells from injury induced by hypoxia/reoxygenation
- Postprandial dyslipidemia in insulin resistant states in adolescent populations
