Generation of a Canine-origin Neutralizing scFv Against Canine Parvovirus
2020-11-03PiXueleiWangYuyangZouYimengGuoXiaochenKangKaiWangMengxiaLiDeshanandRenGuiping
Pi Xue-lei, Wang Yu-yang, Zou Yi-meng, Guo Xiao-chen, Kang Kai, Wang Meng-xia, Li De-shan, , and Ren Gui-ping, *
1 Biopharmaceutical Lab, College of Life Sciences, Northeast Agricultural University, Harbin 150030, China
2 Key Laboratory of Agricultural Biological Functional Gene, Northeast Agricultural University, Harbin 150030, China
Abstract: Canine parvovirus (CPV) is a high morbidity and lethality virus which causes severe enteric disease in dogs.In an attempt to gain canine-origin neutralizing antibodies against CPV, single chain variable fragment (scFv) bacteria display libraries against the protective antigen VP2 were constructed and screened.VP2 specific scFvs were selected following three rounds of screening procedures.Selected scFvs were characterized by FCM and ELISA.Seven scFvs showed high affinity and specific binding to CPV.Moreover, the neutralizing activity of the antibody was preliminarily identified by CPV (100 TCID50) in vitro and scFv-2 showed the neutralizing ability with a titer of 32 768.This study generated the first canine-origin neutralizing scFv against CPV.The neutralizing scFv would be constructed into full-length antibody in the future.This study laid the foundation for the generation of an effective therapeutic reagent with long half-life and no immunological rejection for the prevention and treatment of CPV infection.
Key words: canine parvovirus, VP2 protein, canine-origion antibody library, ScFv
Introduction
Canine parvovirus (CPV-2), belonging to genus Parvovirus and family Parvoviridae, causes fatal gastroenteritis and myocarditis in dogs.Since its discovery in 1978, CPV-2 has spread rapidly across the world and become one of the most serious threats to canine life.In the early 1980s, two mutations of CPV-2 appeared, termed CPV-2a and CPV-2b (Lamm and Rezabek, 2008; Parrishet al., 1988).Comparing with the original type 2 virus, the two variants get changes in antigenic epitopes and can infect feline cells.Currently, CPV-2a and CPV-2b are the major parvovirus species in the world.In 2000, a new variant named CPV-2c was first reported in Italy and subsequently appeared globally (Buonavogliaet al., 2001).
CPV-2 is major spread by infected dogs.The virus excretes out of the body through vomiting, saliva and feces.Dogs of any sexes, ages and breeds can be affected by CPV-2, but puppies between 6 weeks and 6 months of age often suffer the severest infection(Houstonet al., 1996) and pedigrees are reported more susceptible than mutts (Glickmanet al., 1985).Canine parvovirus disease occurs in all four seasons,especially in late autumn and spring.Cold weather,overcrowding, passive or active immune deficiency and complicating diseases greatly aggravate thefatality rate of this disease.For the treatment of CPV-2 infected dogs, traditional hyper immune serum and murine monoclonal antibody reagents are produced.However, the unstable antibody titer, immunological rejection and virus diffusing of hyper immune serum and the immunological rejection of murine monoclonal antibody make the recovery rate of Canine parvovirus disease is only about 50% in clinical.Therefore, there is an urgent need for an effective treatment reagent for CPV-2 infected dogs.Here, the objective of this research was to generate a canine-origin recombinant neutralizing scFv against CPV-2.This study paved the way for the development of canine-origin monoclonal antibody against CPV-2.The canineorigin monoclonal antibody would be a quality controllable and no immunological rejection with long half-life therapeutic reagent, which possessed high clinical value.
Full CPV-2 capsid is assembled from 60 copies of the viral proteins, approximately 10 copies of VP1 and 50 copies of VP2 (Parket al., 2007; Callawayet al., 2017).VP2 is the protective antigen of CPV-2.Several studies verify that three dominant neutralizing antigenic determinants of CPV are founded, one on the N terminal of VP2 and the other two on the threefold spike of the virus capsid which are also located on VP2(Langeveldet al., 1993; Jaet al., 1991; Strassheimet al., 1994).In this research, VP2 was used as the bait-protein to catch neutralizing scFvs.
Bird (Hustonet al., 1988) and Huston (Birdet al.,1988) were the first to generate murine single chain variable fragment (scFv) in 1988.scFv is composed of variable regions of heavy (VH) and light (VL)chains combining by a flexible peptide linker, which can be easily expressed inE.coliand remain the same specificity to bind antigens as the parent antibody.A scFv antibody library from a CPV-immuned dog was built in this study.To obtain neutralizing scFvs,bacteria surface display(BSD) technology (Fig.1)combining with flow cytometry screening was used.The bacterial surface display method was first reported in 2004 (Harveyet al., 2004).At present, BSD is meanly applicated on isolating high affinity antibodies from antibody repotoire (Jeonget al., 2007).

Fig.1 Bacteria display technology
Materials and Methods
Materials
The pT-linker and pBSD vector were used for scFv library display and generated in Biopharmaceutical Lab, College of Life Sciences, Northeast Agricultural University, Harbin, China.FITC labled VP2 was laboratory stock.CPV-2a was laboratory stock.Cat kidney cells (F81) were purchased from ShanghaiCell Library of Chinese Academy of Sciences.E.coli-DH10B andE.coli-BL21 (DE3) were purchased from TransGen Biotech.
Methods
Animals and cDNA synthesis
Beagles immunized with CPV were provided by Dabeinong Company.The splenic tissues were collected when the valence of antibody against CPV reached its peak.Samples were immediately stored in liquid nitrogen.The total RNA was extracted from all the samples using the RNeasy kit.RNA quantity was determined by UV absorbance at 260 and 280 nm and RNA quality was determined by gel electrophoresis.mRNA was isolated by PolyATtract®mRNA Isolation System II (Omega) with Magnetic Stand.The cDNA was synthesized from the mRNA samples using oligo dT and Superscript II (Invitrogen).
Construction of canine ScFv library
Degenerate primers in Table 1 were used for canine VH and VL (λandκ) chains amplification.PCR products of VH and VL were digested byHindⅢ/NheⅠandBamHⅠ/XhoⅠrespectively and inserted into the up and down streams of the peptide-linker(Gly4Ser) 3 gene in pT-linker.Then, VH-linker-VL fragments were generated by the digestion withSfiⅠand inserted into the display vector pBSD.The recombinant plasmids were electroporated into DH10B,according to standard procedures, and the library was termed a canine-pBSD-scFv library.
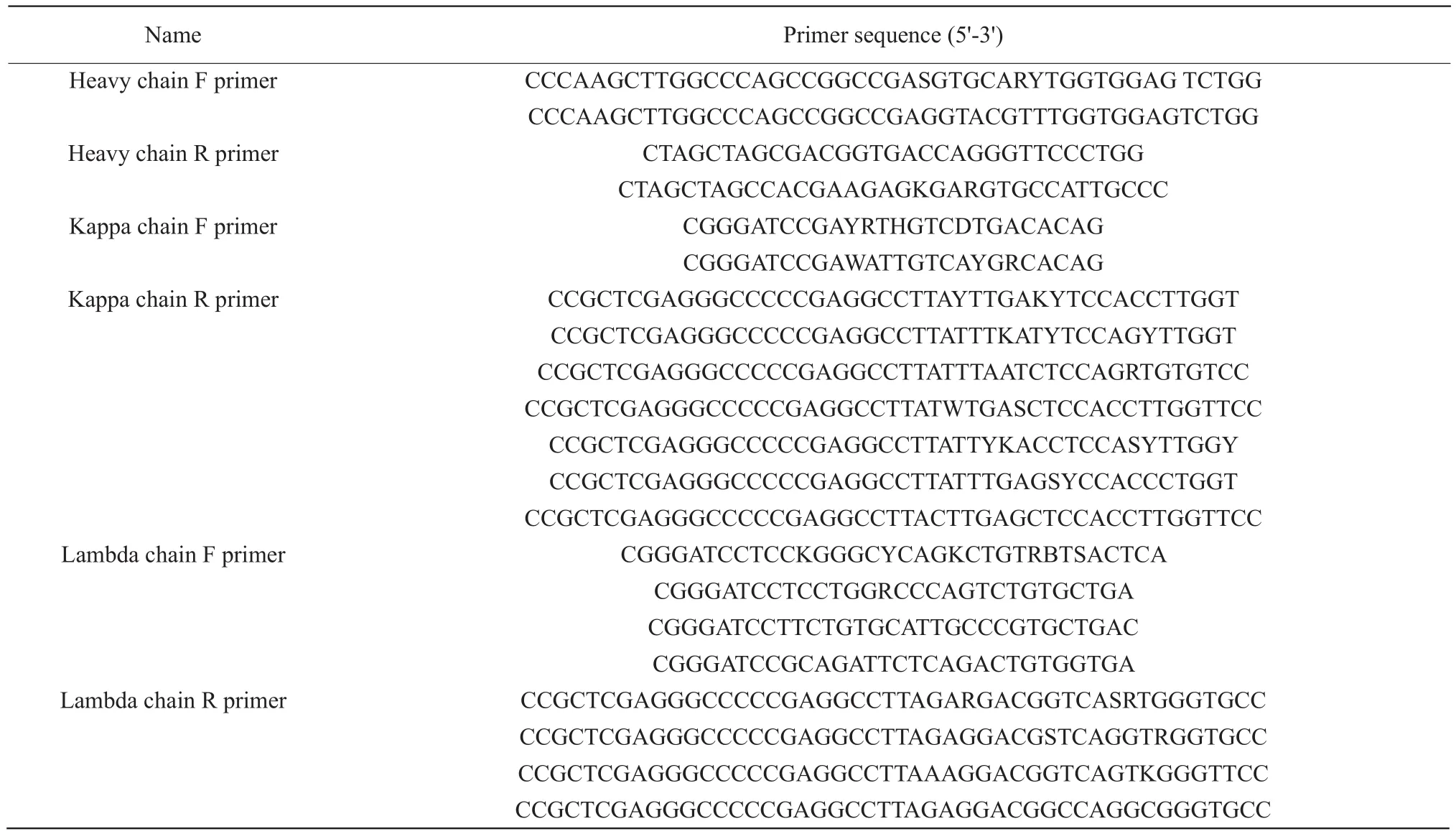
Table 1 Primer sequences
Spheroplasts preparation and canine-pBSD-scFv library screening
All the colonies from the canine-pBSD-scFv repotoire transformedE.coliDH10B were collected and cultured in lysogeny broth (LB).When the culture OD600nmwas between 0.4 and 0.6, addition of 0.25 mmol · L-1IPTG were used for inducing the cells and the cells were cultured at 37℃ for 4 h.The spheroplasts were prepared as previously reported (Jeonget al., 2007).
The spheroplasts were incubated with 1 μL FITCVP2 (2 mg · mL-1) on ice for 1 h.After washing twice with phosphate buffer saline (PBS), the library was sorted by FCM (BD FACSAriaTM IIu Cell Sorter, BD Biosciences).Isolated scFv DNA, rescued by plasmid extraction from the collected cells, was electroporated intoE.coliDH10B and subjected to another round of sorting by FCM.When the positive population reached 80%, colonies were picked up at random and detected by FACS and subjected to DNA sequencing(Invitrogen).
Expression and purification of isolated scFv antibodies
Seven isolated scFv genes were amplified by PCR,then digested byNdeⅠ/XhoⅠand cloned into the expression vector pET-28a (+).The recombinant plasmids were transferred intoE.coliBL21 (DE3).Colonies ofE.coliBL21 (DE3) were grown in LB containing 50 μg · mL-1kanamycin.When the culture OD600nmwas between 0.4 and 0.6, a final concentration of 0.25 mmol · L-1IPTG was added to induce the expression of scFvs with shaking at 37℃ for 4 h.All of the scFvs were expressed as inclusion bodies.The inclusion bodies were harvested by centrifugation at 12 000×g for 30 min and resuspended in washing buffer (2 mol· L-1Urea).After washing two times,the inclusion bodies were dissolved in denaturing buffer (8 mol· L-1Urea) and stored at 4℃ for 16 h.The denatured proteins were droped slowly into the renaturation solution (2 mol · L-1Urea) to a final concentration of 0.7 mg · mL-1with stirring and stored at 4℃ for 24 h.The aggregated proteins were eliminated by centrifugating and the supernatant dialyzed in PBS at 4℃ for 24 h.The purities of refold proteins were analyzed by SDS-PAGE.
ELISA analysis
The 96-well microtiter plates were coated with 100 μL purified scFv antibodies at different concentrations(100, 50, 20, 10, 1 and 0.1 μg · mL-1in NaHCO3/Na2CO3buffer, pH 8.7) at 4℃ overnight.Uncoated samples were removed by washing three times with PBST.Wells were blocked with 5% skimmed milk in PBS at 37℃ for 2 h.After washing, each well was incubated with 50 μL CPV-2a (TCID50=10-4.5/0.1 mL) at 37℃ for 1 h.After washing, the canine anti-CPV poly-clonal antibody (1 : 8 000) was incubated at 37℃ for 1 h, followed by incubation with HRP-sheep anti-dog IgG (H+L) (1 : 7 500) at 37℃ for 1 h.The reactions were detected with TMB solution (100 μL · well-1) for 15 min at 37℃.The development of color product was terminated by the addition of 50 μL 2 mol · L-1H2SO4.OD value was read at 450 nm using a ELISA reader.Three negative controls included control 1 (without CPV-2a, N1), control 2 (without canine anti-CPV polyclonal antibody, N2) and control 3 (scFv incubated with IBDV insread of CPV-2a, N3).
Viral neutralization assays
Each scFv (50 μL) was diluted with different ratios(1, 2, 4, 8, 16, 32, 64, 128, 256, 512, 1 024, 2 048,4 096, 8 192, 16 384, 32 768, 65 536, 131 072, 262 144,524 288) and then incubated with CPV-2a (TCID50=10-4.5/0.1 mL 50 μL) for 1 h at 37℃.The mixtures were added to F81 cells in 96-well cell culture plates and incubated at 37℃ in the presence of 5% CO2for 5 days (Nykkyet al., 2010).The neutralizing ability of each scFv depended on the block of cytopathic effect(CPE).Three controls were included control 1 (cells were treated with CPV-2a, control 2 [cells were treated with DMEM (100 μL)] and control 3 (cells were treated only with scFv).The experiment was repeated three times independently.
Results
Construction of scFv library
The canine-pBSD-scFv library was successfully constructed and confirmed by PCR and enzyme digestion(Fig.2).The genes of canine VH and VL were cloned into the display vector pBSD for screening, which were electro transformed to give rise to 9×108transformants.Six transformants were randomly selected forsequencing.The sequencing results showed that the selected clones were canineIgGgenes with a wide diversity (data not shown).
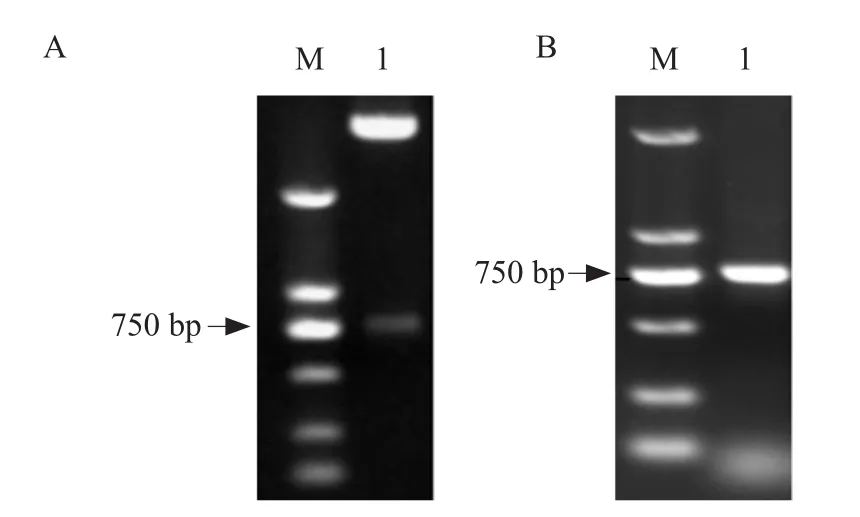
Fig.2 Identification of canine-pBSD-scFv library by PCR and enzyme digestion
Screening of high affinity scFv antibodies by FCM
Colonies with high fluorescence intensities detected by FCM were isolated after four rounds of screening.Following each round of screening, approximately 1%of the highest events were isolated and the scFv library showed a steady increase in VP2-specific binding.In the third round of screening, there was an obvious fluorescence population shift in the fluorescein emission (FITC-A) channel and the positive clones could be enriched up to 83.3% (Fig.3).After that,colonies were randomly isolated and identified by FACS analysis.Seven scFvs (1, 2, 3, 4, 5, 6 and 7) with high fluorescence intensities (Fig.4) were selected.All of them were successfully expressed and used for further neutralization activity detection.

Fig.3 Screening and identification of CPV-specific scFvs by FCM
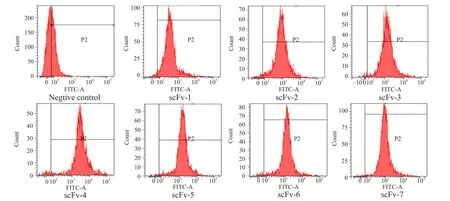
Fig.4 Detecting specificity and affinity of isolated clones by FCM
Expression and purification of scFv antibodies
SelectedscFvgenes were subcloned into the pET-28a (+) vector to express the recombinant proteins.The scFvs were expressed as the inclusion bodies.The purified proteins of seven scFvs were obtained afterdenaturing and refolding.SDS-PAGE analysis showed that the purified scFv proteins were approximately 27 ku and the purity was more than 90% (Fig.5).
Characterization of purified scFv antibodies
ELISA analysis demonstrated that all the seven clones could specifically bind to CPV-2a and the value of OD450nmincreased in a dose dependent manner.The values of the three negative controls were negligible(Fig.6).
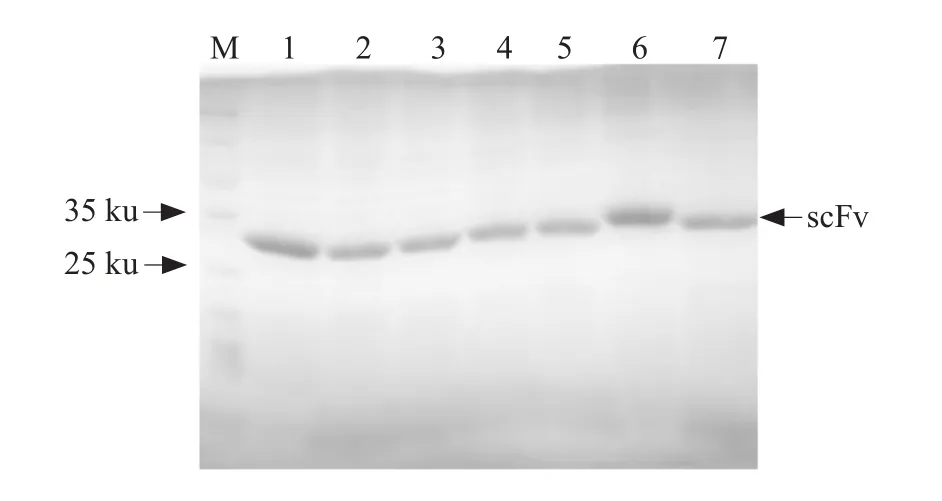
Fig.5 SDS-PAGE analysis of purified scFvs antibodies
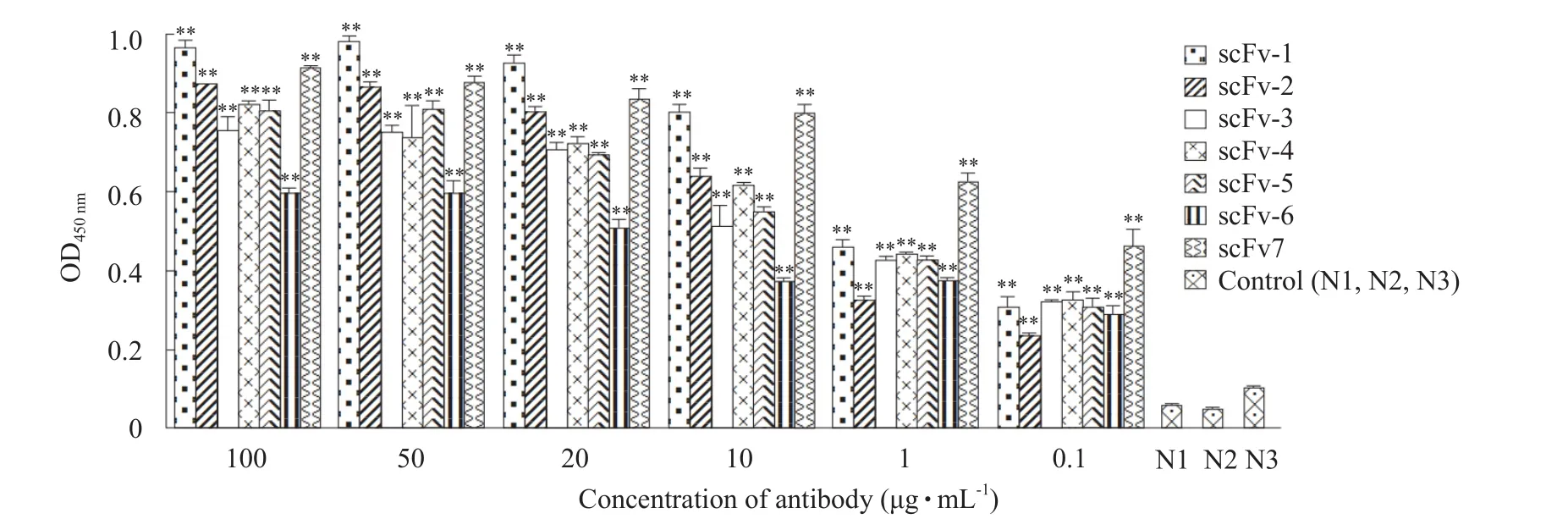
Fig.6 ELISA analysis of purified scFvs against CPV-2a
Neutralizing activity
Seven scFvs were assessed for their ability to block CPV-2a infection.Only one purified scFv was able to neutralize CPV.scFv-2 showed the ability to block CPE and the titer was 32 768, while others showed no neutralizing properties (Table 2).

Table 2 Virus neutralization titers of scFv antibodies against CPV-2a
Discussion
With the rapid development of canine farming and pet industry worldwide, the raising quantity of dogs in the world is increasing.CPV, as a high morbidity and lethality virus, has seriously obstructed the stable and health development of dog raising industry.Currently,prophylactic vaccination for CPV is the most effective practice worldwide.However, the improper preservation in vaccine transport, unreasonable immune procedure, individual difference and especially the exist of maternal antibodies which are acquiredviathe colostrums (Miranda and Thompson, 2016), often lead to immune failure.For the treatment of infected dogs,specific egg yolk antibody solution, hyper immune serum and monoclonal antibody (Mab) were produced(Attyatet al., 2015; Vanet al., 2006).In the clinical therapeutic effect, the recovery rate of Mab was 10%higher than hyper immune serum and the specific egg yolk antibody solution was rarely used.For hyper immune serum, the unstable antibody titer and immune rejection of heterogenous serum made the therapeutic effect uncontrollable.Moreover, a potential risk of virus diffusing cannot be ignored.Although the traditional murine monoclonal antibody had achieved a certain effect on the treatment, the side effects caused by its immune rejection cannot be ignored.Murine monoclonal antibody had a short half-life in caninevivo, so repeated injections are needed.However, repeated injections of murine monoclonal antibodies could lead to allergy and induce antiantibody which greatly reduces the therapeutic effect.Here, a canine-origin neutralizing scFv antibody was developed.In the future, the neutralizing scFv-2 would be constructed as a full-length antibody taking the most obvious advantage of a long half-life and no immune rejection.The treatment effect on dog would be discussed in the future.
In this study, seven scFvs with high affinity to CPV-2a were isolated as the flow cytometry and ELISA results demonstrated.However, only one of them own neutralizing activity.The results were consistent with the fact that the antibody with high affinity do not definitely had neutralizing activity.In the traditional screening process, the isolation of scFvs was based on high affinity.Since the unity of high affinity and neutralization activity couldnot be ensured, the efficiency of screening neutralizing antibodies seems to have been reduced.To solve this problem, the bait-protein for screening might be optimized.In the previous study, a synthetic peptide vaccine composed of the first 21 amino acids of the amino terminus of the protective antigen VP2 of CPV induced protection of dogs against CPV had been reported (Vanet al.,2010).The study proved that exogenous antigen epitopes could bind CPV-2 neutralizing antibodies.If the synthetic peptide or other antigenic epitopes could be expressed and used as the bait to catch neutralizing scFvs in the BSD system, the specificity of screening neutralizing scFvs would increase significantly in theory.
Conclusions
In summary, this study isolated seven scFvs with a high affinity to CPV-2a from a canine-origin bacterium display library.One of them named scFv-2 showed the ability to neutralize CPV-2a with virus neutralization titer of 32 768.
Acknowledgement
Pi Xue-lei and Wang Yu-yang contributed equally to this work and should be considered co-first authors.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Effects of Drought Stress and Re-watering on Osmotic Adjustment Ability and Yield of Soybean
- Winter Hardiness Physiological Response with Dehydration in Winter Wheat
- Effects of Constant High Temperature on Survival, Development and Reproduction of Aphis glycines Matsumura
- Fine Genetic Mapping of Dwarf Trait in Cucumber (Cucumis sativus L.)Using a RIL Population
- Evolution of Heavy Metal Speciation During a Large-scale Sewage Sludge Composting
- Study on Relationship Between Differential Proteins of Bacillus cereus LBR-4 and Its Salt Tolerance Mechanism
