Winter Hardiness Physiological Response with Dehydration in Winter Wheat
2020-11-03QinPengZhaoLieWangXiaonanLiTongFuLianshuangWangTaoLiZhuofuandLiuXin
Qin Peng, Zhao Lie, Wang Xiao-nan, Li Tong, Fu Lian-shuang, Wang Tao, Li Zhuo-fu, and Liu Xin
Key Laboratory of Germplasm Enhancement, Physiology and Ecology of Food Crops in Cold Region, Ministry of Education, Northeast Agricultural University, Harbin 150030, China
Abstract: Freezing stress presents a severe threat for winter wheat (Triticum aestivum L.) during overwintering.Dehydration plays a significant role in plant cold-hardiness.In this study, dehydration-related traits were investigated between highly and tender freezingtolerant winter wheat cultivars during the overwintering period.Accompanied by a decrease in temperature, the water content in crowns of highly freezing-tolerant winter wheat was significantly lower compared to that of tender freezing-tolerant winter wheat(control).The ratio of free and bound water content had slight changes in highly freezing-tolerant winter wheat, though there were wide fluctuations in the control.The transcript levels of dehydration-related genes were more expressed in the highly freezing-tolerant winter wheat than those in the control under freezing stress.The plant growth also showed significant differences between the two winter wheat cultivars.Based on these results, the study proposed that the highly freezing-tolerant winter wheat produces higher expression of dehydrins under freezing stress, causing additional dehydration in the tissue to avoid cell death caused by the formation of ice crystals.Furthermore, winter hardy cultivar also reduced the percentage of free water content that inhibited plant growth, and regulated the water composition for plant survival under freezing stress.
Key words: freezing, dehydration, water content, winter wheat
Introduction
The overwintering ability of crops is one of the most important features and affects the agronomic performance associated with the quality and economics of winter crop plants.Freezing stress represents a severe threat for plants throughout the overwintering period.However, the freezing tolerance of the winter crops is a complex physiological process that involves photosynthesis, carbohydrate metabolism, reactive oxygen species scavenging and dehydration (Janmohammadiet al., 2015).Dehydration is a key physiological process for plants to cope with freezing stress that can be induced by numerous environmental factors, such as low temperature, drought and salinity.Dehydration helps to avoid the ice crystallization that destroyed cell membrane structures (Houdeet al., 2004).In addition,the water content and the component ratio (free and bound water content) of temperature-sensitive tissues also have a clear relationship with plant freezing tolerance (Houdeet al., 2004).
Previous studies focusing on plant cell dehydration have reported a close relationship between the level of tissue dehydration in plant and plant cold tolerance(Danyluket al., 1994; Tommasiniet al., 2008; Kosováet al., 2014).Dehydrins belong to a large family of late embryogenesis abundant proteins and fall into four groups (Kn, SKn, YnSKm and KnS) in common wheat(Kosováet al., 2014).The main group of dehydrins(DHNgenes) is classified as the YnSKmtype, and they are induced by various environmental factors,such as low temperature, drought and salinity (Choiet al., 1999).Dehydrins share the same K-segment(EKGIMDKIKEKLPG), and the number of copies of the K-segment associates with its protective effects under dehydration stress (Driraet al., 2013).DHN5 belongs to Kn type dehydrins and is induced by cold,drought and plant hormone ABA (Tommasiniet al.,2008; Wanget al., 2014).DHN5 predominantly accumulates in the epidermis, crown and leaves which are the primary regions of ice nuclei formation and are thus susceptible to freezing (Kosováet al., 2011).Besides responding to the cold and drought stress,DHN5 protein has shown to preserveβ-glucosidase and glucose oxidase enzyme activitiesin vitrounder heat stress, and the K-segment is indispensable for the enhanced stability of both enzymes under various stress conditions (Briniet al., 2010).
The Kn type wheat cold-specific protein (WCS120)is the most abundant dehydrin in the cold-acclimated tissues of wheat under freezing stress (Kosováet al.,2007).WCS120 is rapidly induced by low temperatures, and WCS120 mRNA is quickly degraded when the cold stress release, indicating its instability under normal temperatures and its important role in cold tolerance (Shenet al., 2003).TheWCS120 gene is induced more in winter hardy wheat compared to tender spring wheat (Ganeshanet al., 2009).In addition, the WCS120 protein accumulation is positively correlated with the cold tolerance in susceptible tissue between mild and high-freezing tolerant winter wheat (Ganeshanet al., 2009; Kosováet al.,2012a).Therefore, the higher level of gene expression contributes to the cold tolerance in winter wheat,and WCS120 protein can be served as a molecular marker for frost tolerance inGramineae(Houdeet al.,1992).Wheat cold-regulated 39 (WCOR39) dehydrin accumulates in the leaves, roots and the crown tissues in winter wheat under cold stress, and is rapidly induced by low temperatures and returns to a relatively low level as the temperature rise.In addition, the transcripts of WCOR39 responds to the exogenous application of ABA and water stress (Guoet al.,1992).
Aside from the dehydrins, the dehydration responsive element binding (TaDREB1) protein gene,which belongs to the AP2/EREBP transcription factors, also responds to the low temperature stress,and plays a significant role in plant cold hardiness(Shenet al., 2003).The TaDREB1 protein contains a conserved DNA-binding domain of 58-60 amino acids, and specifically binds downstream genes to regulate gene expression and enhance the levels of plant cold hardiness (Liuet al., 1998; Itoet al., 2006;Agarwalet al., 2007; Gaoet al., 2009).In winter wheat, TaDREB is rapidly induced to a high level by low temperature, salinity and drought, whereas the expression of TaDREB is relatively low in spring wheat.Furthermore, expression of the marker geneWCS120 closely aligns with the expression patterns of TaDREB, suggesting that TaDREB may activate transcription of theWCS120 gene (Shenet al.,2003).
Dongnongdongmai 1 (D1) is a highly freezingtolerant winter-hardy wheat cultivar and can tolerate extremely low temperatures (Zenget al., 2011).In this study, dehydration-related physiological traits between D1 and the less winter-hardy wheat cultivar Jimai 22 (J22) are investigated throughout the whole overwintering period.Furthermore, the transcript levels of dehydration-related genes, which shows significant expression differences in previous RNA sequencing studies (Xie and Li, 2015), are compared between the two winter wheat cultivars.Finally, the close relationship among the water content, the component ratio (free and bound water content) and freezing tolerance in highly freezing-tolerant winterhardy wheat cultivars under natural freezing stress is discussed.
Materials and Methods
Plant materials and field experiment
The winter wheat cultivar Dongnongdongmai 1 (D1),which was the only winter wheat variety planted in high latitude in China, was selected for assessing related traits of overwintering.The elite winter wheat cultivar Jimai 22 (J22) was used as a control, which was from the Shandong Academy of Agricultural Science (China).From Sep.2013 to Mar.2014, these cultivars were grown in a field in Harbin (45°70' N,126°60' E), China to assess the winter hardiness of wheat under field conditions.A randomized block design was used with three replications.Each plot contained five rows of 5 m length, with a spacing of 5 cm between plants and 30 cm between rows.More than 200 seeds from each plot were used for the investigation of germination rate after 15 days of seeding.The sampling period began on Nov.11,followed by Nov.23, Dec.7, Dec.18, Jan.6, Jan.30,Feb.19, Mar.5 and Apr.1, lasting for 142 days (Fig.1).Five separate samples were collected for each timepoint in each cultivar.The crown, which was sensitive and important for overwintering, was collected as tissue samples.For indoor treatment, the seedling plants were planted in an artificial climatic chamber with 12 h photoperiod at 20℃, 50% humidity.
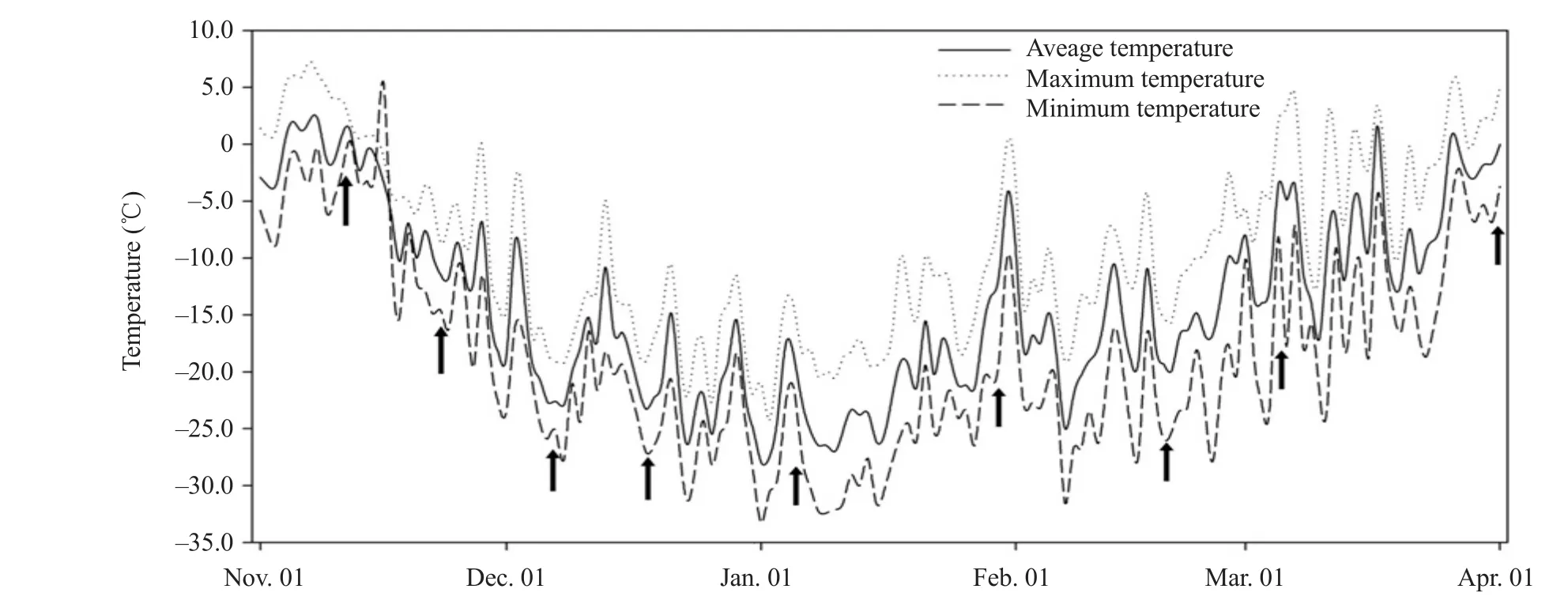
Fig.1 Temperature trends during sampling periods
Electrolyte leakage assays
Electrolyte leakage was measured based on the relative conductivity.Conductivity measurements were taken in the crown consisting of a 1.5 cm segment of the white, nonphotosynthetic tissue between the upper photosynthetic green shoot and the primary root.Each sample consisted of mixed 6-8 plants.The detailed method for the electrolyte leakage assay was used as previously described (Dhillonet al., 2010).
Water content assays
The crown was used to measure the water content.The fresh weights of more than five samples were scored as fresh weight (FW).Then, the samples were suspended in deionized water at 4℃ in darkness for 12 h, and the weights were scored as the turgid weight (TW).Finally, the samples were dried in an oven at 70℃for 48 h and weighed as dry weight (DW).The water content of tissues was calculated with the following formula:WC=(FW-DW)/FW.The relative water content was calculated with the following formula:RWC=(FW-DW)/(TW-DW).The fresh samples were placed in a 65% sucrose solution for 6 h in the dark.Then, the free water content (FWC) was calculated with a two WAJ Abbe refractometer (ShanghaiCSOIF, China).The bound water content (BWC) was calculated asBWC=WC-FWC.
Extraction of the total RNA and qRT-PCR analysis
The total RNA was extracted from the crown using the RNAiso for Polysaccharide-rich Plant Tissue Kit(TaKaRa, Japan).Each sample consisted of mixed RNA collected from 6-8 plants.First-strand cDNA was generated using Easy Script First-Strand cDNA Synthesis Super Mix (Transgen, China).The transcript levels of the wheat genes,WCS120, dehydrin gene 5 (TaDHN5), dehydration response element-binding protein (TaDREB) and WCOR39 were quantified using a Roche LightCycler®480 system with Trans Start Top Green qPCR Super Mix (Transgen, China) and three technical replicates for each sample were tested as the method described (Liuet al., 2016).The wheat glyceraldehyde-3-phosphate dehydrogenase (GAPDH)gene was used as an internal control to determine the relative expression levels ofTaDHN5,WCOR39,WCS120 andTaDREB.The gene-specific primers used for qRT-PCR are listed in Table 1.Data analyses were conducted using the 2(-ΔΔCt) method (Livak and Schmittgen, 2001).
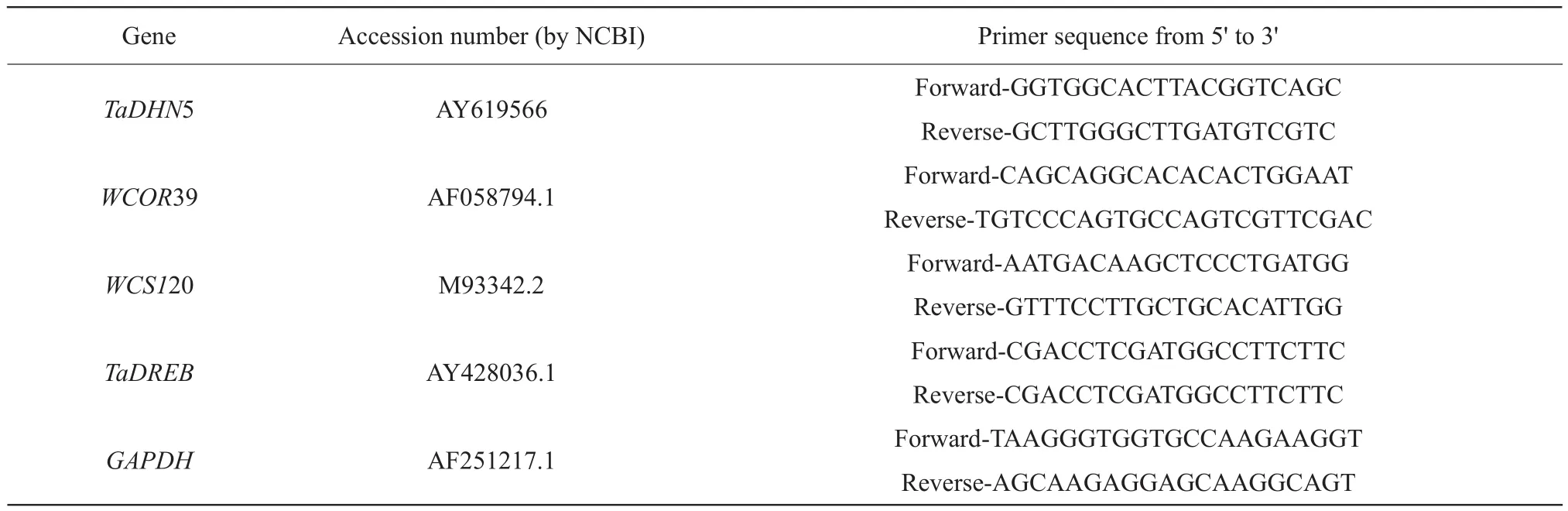
Table 1 Gene-specific primers used for qRT-PCR
Results
Differences in cold hardiness between D1 and J22
To examine the differences in cold hardiness between the two varieties, the survival rates after the overwintering stage were investigated.The germination rate showed little differences between D1 and J22 with 92.33% and 93.67%, respectively (Fig.2A).As shown in Fig.2B and 2C, the survival rate in D1 was more than 80%, whereas the rate for J22 was approximately 30%.Previous studies proved that electrolyte leakage was a key trait for freezing damage (Gilmouret al.,1988; Thalhammeret al., 2014).Therefore, electrolyte leakage based on the relative conductivity was measured during the frost period.With the decrease of temperature, the relative conductivity significantly increased in both D1 and J22.Accompanied by an increase in temperature, the relative conductivity between D1 and J22 sharply decreased.However, the level of the relative conductivity in D1 was associated with a decrease compared to J22, during the whole overwintering period, indicating that D1 suffered less damage than J22 under frost stress and showed better cold hardiness (Fig.2B).
Relationship between water content and cold hardiness under cold stress
During the entire overwintering period for wheat in high-latitude regions, the water content of the winter-hardy wheat D1 and negative control wheatJ22 was examined.Accompanied by a decrease in temperature, the water content showed a downward trend in the crown tissue (Fig.3A), which indicated that a lower temperature would induce dehydration in wheat tissues.Furthermore, the water content in wheat D1 was significantly lower compared to wheat J22 throughout the whole winter period (Nov.11-Feb.19 lasting for 101 days).The results suggested that the more water content in tissues might have a negative effect on wheat cold hardiness.
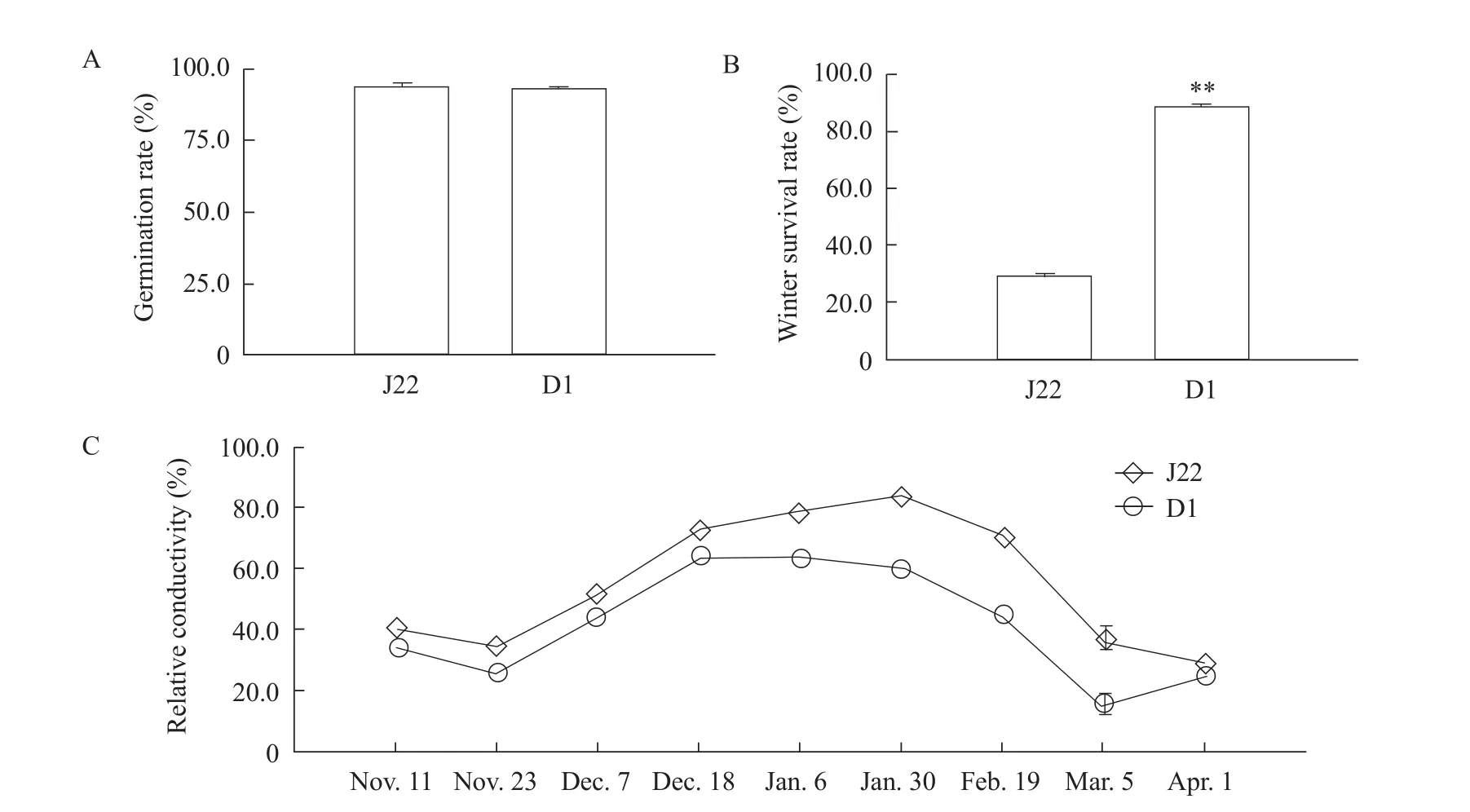
Fig.2 Freezing performance of D1 and J22 under field conditions (Harbin, China, 2014)
The relative water content of J22 significantly decreased with a temperature decrease from Nov.23 to Dec.18 (lasting for 26 days), and the content of J22 was higher than that of D1 (Fig.3B).With comparison with D1, the relative water content of J22 had a clear fluctuation with the temperature increase from Jan.6 to Apr.1 (lasting for 86 days), and it was significantly lower than that of D1 before the period, finally approached the same level as that of D1 in the spring(Apr.1).These results indicated that stable relative water content was favorable to plant cold tolerance during the overwintering period and significant variations in the relative water content reduced plant cold hardiness.
Previous study showed that high percentages of free water content had negative effects on plant cold hardiness (Kosováet al., 2014).Therefore, the ratio of free and bound water content in the two varieties were also examined (Fig.3C).In the crown, the ratio of free and bound water content firstly decreased and then increased with temperature recovery.In D1, the trend in the ratio of free and bound water content was relatively gentle (Fig.3C).In J22, the ratio of free and bound water content was higher compared to the content in D1 from Nov.11 to Dec.18 lasting for 38 days.In addition, wide fluctuations in J22 between Jan.6 and Apr.1 were observed and the ratio of free and bound water content in J22 was lower than that in D1 after the frost period.Taken together, these data indicated that dehydration in wheat was a key feature for mediating freezing stress, and the level of dehydration illustrated the differences in plant cold hardiness between D1 and J22 under freezing stress.
Seedling growth characteristics of D1 and J22
Under the field conditions, D1 presented a prostrategrowth compared to an erect growth in J22 (Fig.4A),and the plant height of highly freezing-tolerant winter wheat D1 was significantly lower than that of tender winter wheat J22 after freezing stress (Fig.4B).At the beginning of freezing period in Harbin (Nov.11),the shoot apex just developed into the elongation stage in D1, but into distinctive one ridge stage in J22,indicating D1 developed slow comparing to J22 in this period under freezing stress (Fig.4C).However,in an artificial climatic chamber, D1 and J22 showed no significant differences in plant height and seedling morphology (Fig.4B, D).These results indicated that low temperature induced the differences of seedling growth characteristics between D1 and J22.

Fig.3 Water contents and compositions in crown tissues during overwintering periods
Quantitative real-time (qRT)-PCR assays for expression of dehydrin genes
In previous transcriptome profile assay, the genes involved in plant dehydration (WCS120,WCOR39,TaDHN5 andTaDREB) showed significantly different expression patterns between the two wheat cultivars under frost stress (Xie and Li, 2015).Therefore, the transcript levels of these genes in winter wheat D1 and J22 during the whole overwintering period were monitored (Fig.5).
The transcript abundance ofWCS120 was nearly the same between D1 and J22 after cold acclimation(Nov.5).With the temperature decrease (Nov.20-Dec.20 lasting for 31 days), the expression ofWCS120 gene sharply increased in winter-hardy wheat D1 but decreased in control wheat J22, although both cultivars eventually had a similar level of gene expression(Fig.5A).Expression ofWCOR39 showed significant differences between D1 and J22, during the wholeoverwintering period.In J22, the expression level ofWCOR39 had no significant changes from Nov.5 to Jan.5; only small increases ofWCOR39 expression level occurred on Jan.20 (Fig.5B).However, in D1,the transcript abundance ofWCOR39 increased as the temperature fell (Nov.5-Dec.20 lasting for 47 days), and expression of this gene was approximately four fold higher compared to J22 on Dec.20, then significantly decreased (Jan.5-Jan.20 lasting for 16 days).The expression level ofWCOR39 gene was higher in D1 compared to that in J22, during the whole sampling period (Fig.5B).

Fig.4 Plant growth status under different environments

Fig.5 Quantitative real-time (qRT)-PCR analysis of dehydration-related genes during overwintering periods
The expression level of the dehydrin geneTaDHN5 in D1 was significantly higher compared to that in J22 on Nov.20 and Dec.5, and there were no significant differences between the two varieties in other periods (Fig.5C).With the temperature decrease,the transcript abundance ofTaDREBgene changed on a very small scale in J22.However, the gene was dramatically induced (Dec.5-Jan.5 lasting for 32 days), and the expression level was higher compared to J22 (Fig.5D).
These gene expression patterns suggested low temperatures induced expression of genes that were involved in the dehydration of winter plants.With the temperature decrease, there were substantial differences between highly freezing-tolerant winterwheat and the negative control in the expression levels of dehydration-related genes.The differences in the expression patterns during the frost period may had a relationship with plant cold hardiness.
Discussion
Several recent studies had shown that plant dehydration played an active role in plant freezing tolerance(Kosováet al., 2007; Ganeshanet al., 2009; Kosováet al., 2014).In this study, significant differences in the water contents and compositions in temperaturesensitive tissues between the winter hardy winter wheat D1 and the tender winter wheat J22 were observed through the overwintering period.In D1, the water content in the crown of D1 was lower compared to that in J22 with the decrease of temperature, which indicated that more water contents in the crown tissue were unfavorable for freezing tolerance, during the overwintering period, and the result conformed to the previous studies (Graether and Boddington,2014; Kosováet al., 2014).Accompanied with the temperature increase, there was a rapid increase of water content in D1 compared to that in J22 after the overwinter period, indicated that adequate water benefited plant recovery after freezing stress in winter wheat.Furthermore, the relative water content in D1 varied slightly, during the whole sampling period.However, in J22, relative water contents had the wide fluctuations.These results suggested that wide fluctuations in the relative water contents worked against plant freezing tolerance, and winter wheat with strong freezing-tolerance could maintain relatively stable water contents.
Except for the variances in the water contents, the compositions of the water in the crown also exhibited differences between D1 and J22.During the whole overwintering period, the ratio of free and bound water content in D1 first showed a downward trend then a sharp upward trend.Unlike D1, obvious fluctuations in the ratio of free and bound water content were observed in J22, especially during the period of Dec.18 - Feb.19, in which free water induced the formation of ice crystals at low temperatures.The results indicated that a relatively low percentage of free water content might benefit plant cold hardiness by avoiding cell death from ice crystals formation, and this finding was consistent with previous studies that high percentages of free water content had negative effects on plant cold hardiness (Graether and Boddington,2014; Kosováet al.,2014).A relatively high percentage of free water content would be beneficial for plant recovery after freezing stress.Furthermore, a relatively high percentage of bound water content might help the cells maintain structural integrity under freezing stress and supply water storage for plant recovery after the frost period.In this study, freezing stress inducing differential plant height and seedling morphology was observed between D1 and J22.The winter hardy D1 growth was sensitive to low temperature and developed slower than less hardy wheat J22 after freezing stress,indicating that the faster growth and development at the seedling stage played a negative effect on wheat cold hardiness.
The expression of dehydration-related genes also exhibited significant differences between the highly and low freezing-tolerant winter wheat under freezing stress.Previous studies showed thatWCS120 gene was more induced in winter hardy wheat compared totender spring wheat and could be served as a molecular marker for frost tolerance in the wheat (Houdeet al.,1992; Kosováet al., 2011; Kosováet al., 2012b).In the freezing tolerant wheat D1, the expression ofWCS120 gene was significantly higher compared to tender winter wheat J22 under freezing stress.In addition,the team observed that the dehydration-related genesTaDREBandWCOR39 were also induced more in D1 compared to that in J22 from Dec.5 to Jan.5,which had the lowest temperature of the winter.In addition, the overexpressionDREB-likegenes isolated fromBrassica napus, rice and maize can increase the cold tolerance in non-host plants (Jagloet al., 2001;Dubouzetet al., 2003; Qinet al., 2004).These results suggested thatTaDREBandWCOR39 genes coupled withWCS120 involved in keeping stable and adequate relative water content, and could be used as molecular markers for highly freezing tolerance selection in winter wheat, and the overexpression of these genes by transgenic technology in winter wheat might be efficient for improving plant freezing tolerance.The expression level of the dehydrin geneTaDHN5 in D1 was significantly higher compared to J22 on Nov.20 and Dec.5, and there were no significant differences between the two varieties in other periods.The result indicated that differential expression of dehydrin geneTaDHN5 between D1 and J22 might be induced by lower temperature than other detected genes, andTaDHN5 could be possibly served as an efficient molecular marker for highly freezing-tolerant wheat breeding.
Conclusions
In this study, the water content in crowns of highly freezing-tolerant winter wheat was significantly lower compared to that of tender freezing-tolerant winter wheat, and the transcript levels of dehydration-related genes were more expressed in the highly freezingtolerant winter wheat than those in the control under freezing stress.Based on the results, a connection between dehydration and plant freezing tolerance was proposed.Freezing stress induced the higher expression of dehydrins in the plant tissues, which caused the more dehydration to avoid cell damage.Meanwhile, the highly freezing-tolerant winter plants maintained a relatively low ratio of free and bound water with small fluctuations in the tissues to keep the basic activities needed for cell metabolism.Finally,tissue dehydration and the low percentage of free water content both showed negative effects on plant growth, but an active process for plant survival under freezing stress, and these findings proved the previous opinions.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Effects of Drought Stress and Re-watering on Osmotic Adjustment Ability and Yield of Soybean
- Effects of Constant High Temperature on Survival, Development and Reproduction of Aphis glycines Matsumura
- Fine Genetic Mapping of Dwarf Trait in Cucumber (Cucumis sativus L.)Using a RIL Population
- Evolution of Heavy Metal Speciation During a Large-scale Sewage Sludge Composting
- Generation of a Canine-origin Neutralizing scFv Against Canine Parvovirus
- Study on Relationship Between Differential Proteins of Bacillus cereus LBR-4 and Its Salt Tolerance Mechanism
