Evolution of Heavy Metal Speciation During a Large-scale Sewage Sludge Composting
2020-11-03DaiHangyuZhangYingandXuXiuhong
Dai Hang-yu, Zhang Ying, and Xu Xiu-hong
College of Resources and Environmental Sciences, Northeast Agricultural University, Harbin 150030, China
Abstract: The evolution of metal species during sludge composting in aerobic reactor has been widely investigated, but little is known in large scale.In this study, the transformations of heavy metals speciation (Zn, Cu, Pb and Cd) in the process of sludge composting were studied in a bunker.Physico-chemical parameters, such as pH, moisture content (MC), organic matter (OM), humic acids (HA) and fulvic acids (FA) contents, were determined to evaluate their impacts on the redistribution of Zn, Cu, Pb and Cd in sludge compost.During the composting process, the contents of Cu, Pb, Zn and Cd in oxidizable and residual forms increased,whereas the contents of the exchangeable Cu, Pb, Zn and Cd decreased, with a slight increase in the total heavy metal contents.The contents of Cu, Zn and Cd in carbonate form and the reducible Cu, Zn and Pb falled; however, the content of Pb in carbonate formed and the reducible Cd increased.The results indicated that heavy metals (Zn, Cu, Pb and Cd) in sewage sludge could be passivated during composting process.The heavy metals speciation in the compost correlated with the physico-chemical parameters.In addition,linear regression analysis indicated that the content of mobile Pb could predict the total content of Pb.The contents of the residue fraction for Pb, Zn and Cd were decreased, but those for Ni and Cr were increased; the Cu residue fraction was almost constant.The contents of the total mobile fractions (including fractions 1-4) for Zn and Pb were significantly increased, but the increase of those for Cu and Ni were not so remarkable.There were significant degrees of correlation between heavy metal fractions and changes of some selected parameters (for example, pH, composting temperature and OM content).Only the content of the total mobile fractions for Cu could be predictable from its total content.For the prediction of the total mobile fractions of Zn, Ni, Cd and Cr, the R2 value was significantly increased by the inclusion of other variables such as pH, temperature and OM content.
Key words: heavy metal, speciation, sewage sludge composting
Introduction
Sewage sludge is a source of organic fertilizer for its rich nitrogen, phosphorus and trace elements.The application of sewage sludge to land is considered as an economically and environmentally acceptable management strategy (Gabhaneet al., 2012).However, the presence of extra heavy metals in the sludge hinders its use in agricultural land (Singh and Kalamdhad, 2013).Composting is a common procedure for sewage sludge treatment, in which labile organic matter (OM) is decomposed into CO2,inorganic nutrients and stable organic materials,and the heavy metal speciation is redistributed from more labile and soluble forms to more stabilized ones depending on temperature, pH and OM content (Amiret al., 2005).Therefore, the assessment of heavy metalspeciations in compost contributes to the evaluation of its bioavailability and suitability for land application(Amiret al., 2005).Heavy metal speciation involves the fractionation of its total content into exchangeable,acid extractable (carbonate bound), reducible (Fe-Mn oxides bound), oxidizable (organic bound) and residual forms.The exchangeable and acid extractable forms are mobile fractions and easily available.The oxidizable and reducible forms will be leached out only under extreme conditions, while the residual fraction is almost inert (Venkateswaranet al., 2007).
The influence of municipal solid waste composting on the concentration, water solubility, phase association of Pb, Cd, Zn and Cu at high concentrations in the starting materials, was studied more recently by Castaldiet al.(2006).The physical,chemical and biological reactions happen during composting, which may lead to pH variation, organic matter mineralization and the humic substances formation.These changes may influence the metals distribution behavior and their speciation in composted sludge (Amiret al., 2005).It is the interaction of the HA with metals that is one of the main factors affecting the partitioning of heavy metals as HA has been shown to have a stronger sorption effect on heavy metals, particularly Cu and Zn.Organic matter transformations during composting could influence the metal distribution through HA.And it is assumed that the transformation of heavy metals is influenced in very complicated way during composting.Although the speciation of heavy metals in sewage sludge has already been studied, less is known about the transformation of metal species during composting,especially, under large-scale composting conditions.
This work aimed to monitor the variation of total concentration and speciation of heavy metals (Zn,Cu, Pb and Cd), during large-scale sewage sludge composting using sequential extraction method (Walteret al., 2006).Moreover, the correlation between physico-chemical parameters and metal speciation transformation in composting process is to be elucidated.
Materials and Methods
Composting materials
Sewage sludge came from Wenchang Wastewater Treatment Plant and rice straw from Xiangfang Farm,Harbin, China.The characteristics of the materials are listed in Table 1.
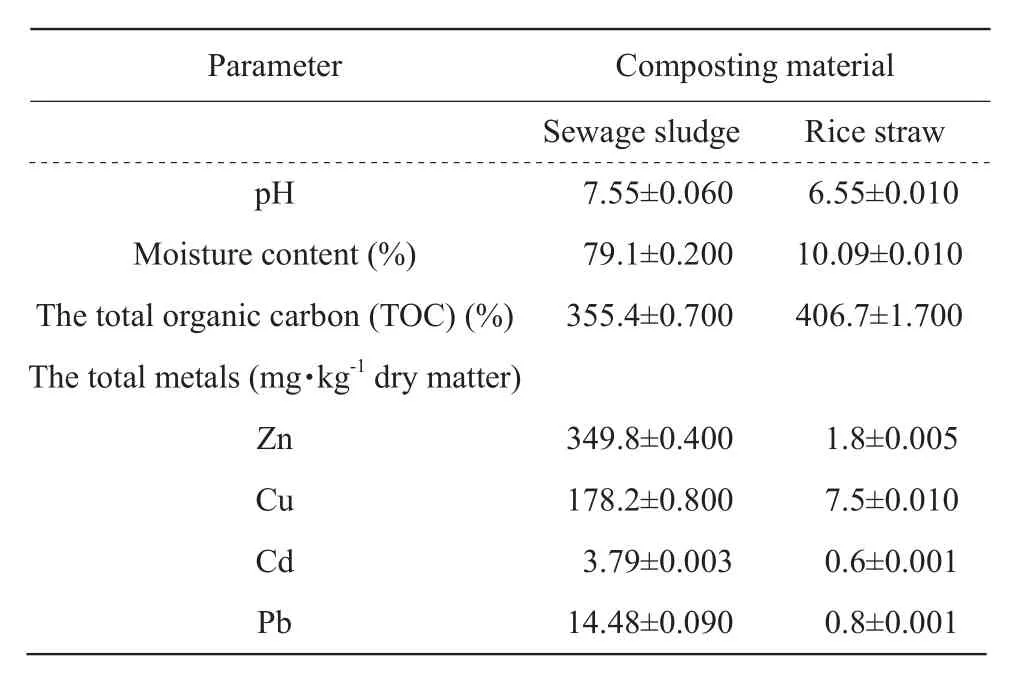
Table 1 Characteristics of composting materials
Composting
A composting bunker (a length of 8 m, breadth of 3 m and height of 2.2 m) was constructed in greenhouse with two underneath ventilation pipes linking to a blower (Fig.1).Mixture of shredded rice straw and sewage sludge was loaded into the bunker manually to keep the structure of the compost.The height of compost mixture was 1.8 m at the beginning and 1.5 m at the end of composting.The airflow was adjusted manually to maintain optimum composting temperature.
Sampling and analysis of physical and chemical parameters
Temperature was monitored using an electronic thermometer placed at three different depths: top(1.3 m from the base), middle (0.9 m from the base)and bottom (0.4 m from the base) throughout the composting period.Samples were collected at threedepths respectively on the 0, 3, 7, 12, 17, 22, 29, 36 and 40th day, and then samples from same day were mixed thoroughly into a homogenized sample and stored at 4℃ for subsequent analysis.
Moisture content was determined by drying the samples in oven at 105℃ for 24 h.pH value (1 : 10 w/v waste: water extract), the total organic matter and humic substances were tested according to Dimitrios'method (Komilis and Kletsas 2012).Soybean seeds were used to determine the Germination Index(Roca-Pérezet al., 2009).The total metals (Zn, Cu,Pb and Cd) were determined by atomic absorption spectrometer (AA-6800 model, Shimadzu-GL, Japan)after 0.2 g sample with 10 mL of H2SO4and HClO4(5 : 1) mixture was incubated in a block digestion system at 300℃ for 2 h.

Fig.1 Composting bunker
Sequential extraction of heavy metal speciation
The conventional method was designed and developed by Tessier (Venkateswaranet al., 2007) for heavy metal speciation into five species, whereas heavy metal mobility decreases in the order from C(1) to C(5).C(1), C(2), C(3) and C(4) were the mobile fractions of heavy metal representing exchangeable, carbonate,reducible and oxidizable metals, respectively.C(5) was the stable fraction of heavy metal which represented residual metal.The method yielded five different solutions: exchangeable (1 mol · L-1MgCl2, pH 7),bound to carbonates (1 mol · L-1NaOAc/HOAc, pH 5),bound to Fe-Mn oxides (0.04 mol · L-1NH2OH · HCl in 25% HOAc), bound to organic matter (0.02 mol · L-1HNO3in 30% H2O2, pH 2; 3.2 mol · L-1NH4OAc in 20% HNO3) and residual (digested with concentrated HNO3+HClO4).The concentrations of Zn, Cu, Pb and Cd were determined using flame atomic absorption spectrophotometry (Zn 213.9 nm, Cu 324.7 nm, Pb 217.0 nm and Cd 228.8 nm).All the biosorption experiments were performed in triplicate and the average values were used in data analysis.
The bioavailability factors (BF) was calculated as the followings:
BF=C(a)/C(t)
Where,C(a) was the sum ofC(t), C(2), C(3) and C(4), andC(t) was the sum ofC(a) and C(5).
Results
Temperature and physico-chemical analysis
The temperature profile during the composting is given in Fig.2.The composting process could be divided into four phases: (1) initial phase (days 1-2);(2) thermophilic phase (days 3-12); (3) mesophilic phase (days 13-29); (4) maturation phase (days 30-40).Variation in temperature during the composting process played a selective role in both the evolution and succession of the microbial communities maximizing the sanitization, biodegradation rate and the microbial diversity.In this work, the temperature of the top was lower than that of the middle and the bottom in the early stage, and then the former was higher with the development of composting process.In the early stage, it was easier for heat to accumulate in the middle and at the bottom, which resulting in higher temperature.After the first turning (on day 13), airflow was increased to prevent anaerobic composting.It was likely that the intensive airflow drove the heat to the upper layer of the pile and led to higher temperature there.
pH of piles increased gradually during the early composting, after 12 days, it decreased to 7.14±0.01 at the end of composting (Table 2).The rise in pH was related to the NH3released, resulting from the degradation of organic matter through microbial activities, while the decrease in pH, which took place later, was caused by ammonium volatilisation.Similar profiles have been observed in other composting processes (Yamamotoet al., 2014).The reduction in pH might also be due to CO2and organic acids produced during microbial metabolism.In general,the compost moisture decreased during the course of composting because of the self-heating process and the aeration.The organic matter in compost was biodegraded and its content gradually decreased from 381.3 to 191.8 g · kg-1.The FA content underwent a very significant decrease from 120.4 to 52.1 g · kg-1whereas HA content increased from 55.2 to 84.9 g · kg-1during the fermentation process.The increased HA represented the humification process and indicated the maturity of compost.Generally,the C/N ratio fell (from 29.6 and decreased to 18.9)over 40 day of composting.The GI was a parameter used to assess phytotoxicity.The results obtained after 40 day of composting showed that GI reached 91.8%.This provided another indicator of compost maturity.
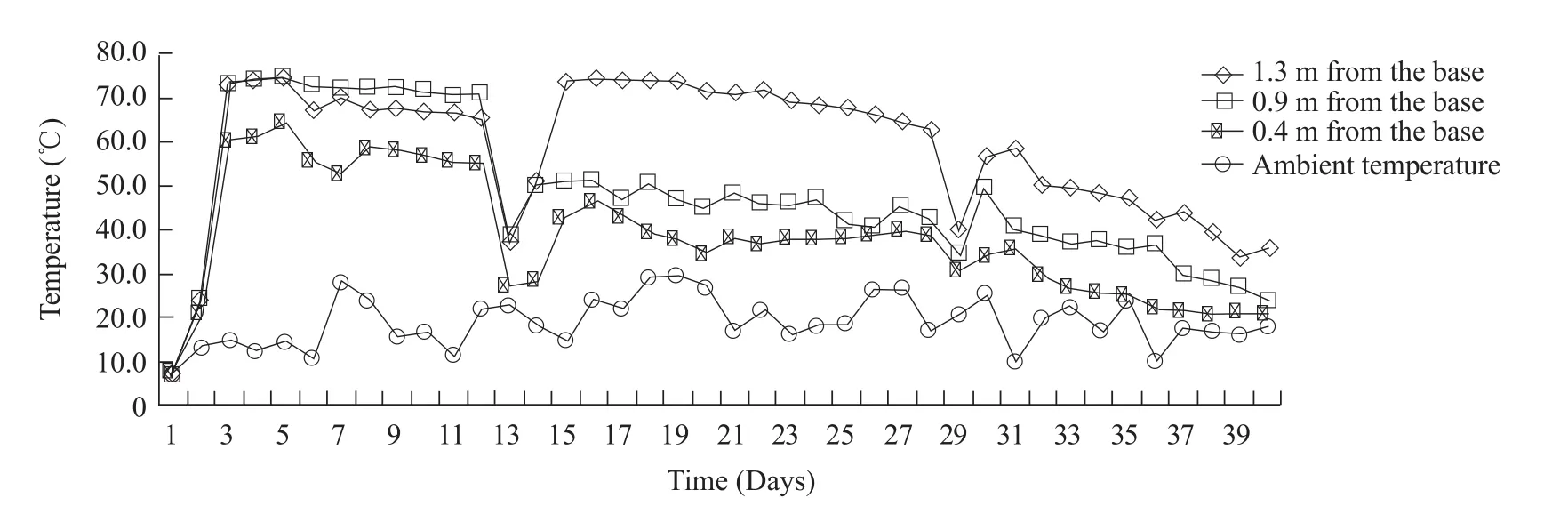
Fig.2 Temperature profile during composting

Table 2 Variation in physico-chemical properties during composting
Evolution of heavy metal speciations during composting
Fig.3 illustrated the total concentration of metals(Zn, Cu, Pb and Cd ) during the composting process.The total concentration of Zn, Cu, Pb and Cd increased slightly, due to weight loss in the course of composting following organic matter decomposition,releasing of CO2and water and mineralization processes (Singh and Kalamdhad, 2013).The order of the total metal contents in the composted sewage sludge was Zn>Cu>Pb>Cd.The percent contribution of exchangeable, car-bonate, reducible, oxidizable and residual fractions of Zn, Cu, Pb and Cd in their total metal contents of composting is presented in Fig.4.
Zinc (Zn)
The fractions of exchangeable Zn and carbonate Zn decreased rapidly during the thermophilic phase of composting and then tended to decrease slowly.Generally, the fractions of reducible Zn increased by 8.8% with the highest percentage (33.4%) occurring at the end of the mesophilic phase (on day 22).The fractions of oxidizable Zn increased from to with a slight decrease observed during the mesophilic phase.The fraction of residual Zn increased rapidly during the thermophilic phase and mesophilic phase of composting and then tended to increase slowly.The results showed that the total fractions of reducible Zn, oxidizable Zn and residual Zn increased during the composting, while those of exchangeable Zn and carbonate Zn decreased, indicating that exchangeable Zn and carbonate Zn were transformed into reducible Zn, oxidizable Zn and residual Zn.The bioavailability factors (BF) of Zn fell from 0.92 (initial) to 0.78(final).

Fig.3 The total metal contents (Zn, Cu, Pb and Cd) during composting

Fig.4 Distribution of metal species in composting
Copper (Cu)
The fractions of exchangeable Cu and reducible Cu showed a declining tendency with a sharp decrease observed during the mesophilic phase.The fractions of carbonate Cu decreased by 0.8% at the end of composting, though an increase of its percentage was found on day 12 (3.9%).The fractions of oxidizable Cu and residual Cu tended to increase during the course of composting, with a rapid rise observed during thermophilic phase for residual Cu.Based on these observations, it was assumed that the movable forms of Cu (exchangeable, carbonate and reducible)were converted into residual and oxidizable Cu, during composting.The bioavailability factors (BF) decreased from 0.57 (initial) to 0.52 (final).
Lead (Pb)
The fractions of exchangeable Pb and reducible Pb tended to decrease during composting.The former showed a rapid decrease during the thermophilic phase, whereas the latter decreased sharply during the maturation phase.The fractions of oxidizable Pb,carbonate Pb and residual Pb showed gradual increase during the whole process.These results indicated that the fractions of exchangeable Pb and reducible Pb were converted into carbonate Pb, oxidizable Pb and residual Pb during composting, which might result in a lower bioavailability of Pb as indicated by the decreased BF (from 0.60 to 0.50 final).
Cadmium (Cd)
The fractions of exchangeable Cd and carbonate Cd decreased by 9.2% and 7.2%, respectively in the course of composting.Whereas, the fractions of reducible Cd, oxidizable Cd and residual Cd increased by, respectively.The results indicated that the fractions of exchangeable Cd and carbonate Cd were transformed into the fractions of reducible Cd,oxidizable Cd and residual Cd.This was supported by the bioavailability factors (BF) which decreased from 0.89 (initial) to 0.86 (final) during the composting process.Over 80% of Cd in the composted sludge was present as the mobile fractions (exchangeable,carbonate, reducible and oxidizable), among which the reducible fraction was dominant.
Influence of pH, moisture content (MC),organic matter (OM), humic acids (HA) and fulvic acids (FA) content on speciation of heavy metals
Correlation between heavy metal fractions and pH,MC, OM, HA and FA contents were explored to determine the influence of these selected variables on the changes of metal speciation during composting(Table 3).
The contents of MC, OM, HA and FA had significant influence on the mobility and bioavailability of heavy metals in composting.There was not close correlation between pH and most of the heavy metals(Zn, Cu, Pb and Cd) speciation in the compost,regardless of carbonate Cu (R=0.967**).It was assumed that the alkaline compost might weaken the effect of pH (Achibaet al., 2009).The increases of exchangeable Zn and carbonate Zn were significantly positively correlated with the decrease of moisture(R=0.800**, 0.895*, respectively); however, the nonmovable forms of heavy metals (Cu residual Cu,oxidizable Pb, residual Pb and oxidizable Cd) were significantly negatively correlated with the decrease of moisture.Moisture was closely correlated with the speciation change of Zn and Pb, with that of Zn being more closely related to moisture than that of Pb; however, moisture seemed to have only slight impact on the speciation of Cu and Cd.The fractions of exchangeable Zn were significantly positively correlated with the decrease of organic matter content(R=1.000**) and residual Zn was significantly negatively correlated (R=-1.000**).The speciation of other heavy metals (Cu, Pb and Cd) were significantly correlated with organic matter.Therefore, the transformation of raw organic matter to stable humic substances during composting process allowed the transformation of heavy metals from exchangeable tostable organic forms.

Table 3 Linear correlation coefficients between heavy metal fractions and pH, MC, OM, HA and FA of sewage sludge during composting
The influence of pH, MC, OM, HA and FA contents on the total mobile fractions of metals (Zn, Cu, Pb and Cd) was illustrated by the stepwise linear regression equations (Table 4).Based on statistical analysis, Pb-C(T) could be predicted by Pb-C(a) (R2=0.643**).For Zn and Pb, the inclusion of the variable OM content led to significant increase ofR2value.
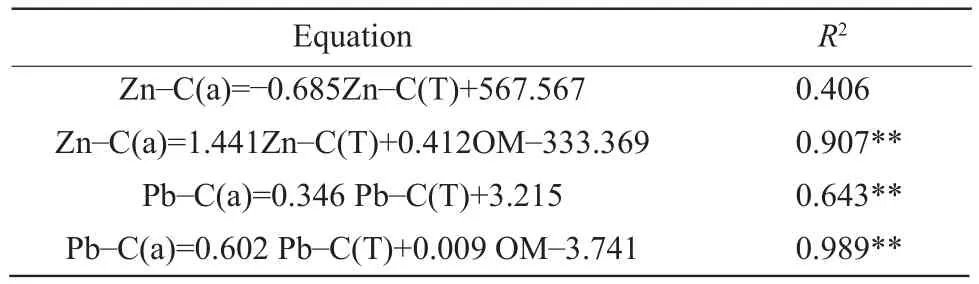
Table 4 Linear regressions for the total mobile fractions of heavy metals in relation to pH, MC, OM, HA, FA and C (T)
Discussion
The variation characteristics of temperature and physico-chemical factors were in accordance with the research of Finsteinet al.(1986), who found that forced aerating influenced the temperatures at different positions.The decrease of BF of Zn was in accordance with that of Amiret al.(2005) who analyzed the speciation of Zn in compost.The reduction of the movable Zn (exchangeable and carbonate) might be explained by the fact that Zn could precipitate with hydroxides, carbonates, phosphates and sulfides presented in compost.In addition, cation exchange andcomplexation by organic ligands during composting were suggested to be the main Zn mobility controlling mechanisms (Kumpieneet al., 2008).The BF of Cu decreased might be attributed to the formation of Cu ion complex with organic functional groups mainly carboxylic, carbonyl and phenolic, so that the ion was immobilized in a rigid inner-sphere complex (Shenget al., 2007).In this research, the fractions of residual Cu and oxidizable Cu were the dominant speciation,during the composting process of sludge.During the composting, the residual Pb was the dominating fraction, which corroborated the results observed by Wong and Selvam (2006).One possible explanation for Cd reduction might be due to the ability of Cd to chemically bond strongly with organic materials(Harounet al., 2007).
The speciation of heavy metals was significantly correlated with HA and FA.This could be explained by the fact that humic substances with a high molecular weight and a low content of acidic functional groups could sorb the ionic metal (Caiet al., 2007).These results confirmed that humification process of organic matter played an important role in reduction in availability of heavy metals in the compost and the bioavailability decreased over the period of composting and maturity.This behavior had been attributed to the formation of stable metal-humus complexes and during the composting process (Tandyet al., 2009).For Zn, Pb and Cd, their total contents also played an important role in the distribution of the speciation.
Conclusions
The compost was mature after 40 days based on the organic matter content (191.8 g · kg-1), pH (7.14±0.01), humus content (HA, 84.9±0.9, FA, 52.1±0.3),GI (91.8±0.3); and C/N ratio (18.9).During the composting process, the total heavy metal contents increased slightly.The contents of Cu, Pb, Zn and Cd in oxidizable and residual forms increased, whereas those in exchangeable forms decreased.The contents of carbonate Cu, Zn and Cd, and reducible Cu, Zn and Pb declined during the composting; however, those of carbonate Pb and reducible Cd increased.Generally,composting treatment reduced the contents of the mobile fractions for these metals.
Based on the observations, the distribution of heavy metal speciation during composting was dependent not only on the total contents of heavy metals, i.e.C(T), but also on the composting parameters, such as temperature, pH and OM contents.The contents of the mobile Pb could be used to predict the content of the total Pb.
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Effects of Drought Stress and Re-watering on Osmotic Adjustment Ability and Yield of Soybean
- Winter Hardiness Physiological Response with Dehydration in Winter Wheat
- Effects of Constant High Temperature on Survival, Development and Reproduction of Aphis glycines Matsumura
- Fine Genetic Mapping of Dwarf Trait in Cucumber (Cucumis sativus L.)Using a RIL Population
- Generation of a Canine-origin Neutralizing scFv Against Canine Parvovirus
- Study on Relationship Between Differential Proteins of Bacillus cereus LBR-4 and Its Salt Tolerance Mechanism
