Antitumor effect of VEGFR2-targeted microbubble destruction with gemcitabine using an endoscopic ultrasound probe: In vivo mouse pancreatic ductal adenocarcinoma model ✩
2020-10-23NnShimmotoMskiItoMsfumiChiSdmuHonmHirooImzuKzukiSumiym
Nn Shimmoto , Mski Ito , Msfumi Chi , Sdmu Honm , Hiroo Imzu ,Kzuki Sumiym
a Department of Endoscopy, The Jikei University School of Medicine, 3-25-8 Nishi Shinbashi, Minato-ku, Tokyo 105-8461, Japan
b Division of Oncology, Research Center for Medical Science, 3-25-8 Nishi Shinbashi, Minato-ku, Tokyo 105-8461, Japan
c Division of Gastroenterology and Hepatology, Department of Medicine, Nihon University School of Medicine, 30-1 Oyaguchikami-cho, Itabashi-ku, Tokyo 173-8610, Japan
Keywords:
ABSTRACT
Introduction
Ultrasound-targeted microbubble destruction (UTMD) is an effective method for delivering antitumor drugs to various cancers.UTMD is initiated by intravenous administration of a microbubble(MB) and antitumor drug.Subsequent topical application of an ultrasound beam induces sonoporation, the temporary and reversibleintroduction of holes in the surface of vessels to enable passage of the antitumor drugs.The acoustic response of gas bubbles after the oscillation also causes cavitation, allowing MB destruction [ 1 , 2 ].Invitroexperiments using UTMD combined with gemcitabine showed a 60% reduction in the viability of pancreatic ductal adenocarcinoma (PDAC) cells as well as significant tumor growth delay [3].In addition, Dimcevski and colleagues [4]conducted a successful phase I trial of UTMD with systemic gemcitabine(GEM) administration for inoperable PDAC patients.Using commercially available non-targeted MB, they demonstrated significant survival prolongation without increasing the occurrence of side effects and adverse events compared with patients treated with GEM alone.
Various molecules are relevant to the abnormal angiogenesis of PDAC.In particular, the activation of vascular endothelial growth factor (VEGF) and its receptor 2 (VEGFR2) triggers various signaling networks to activate multiple PDAC growth at levels [5].Consequently, MBs coated with antibodies to VEGFR2 were developed to facilitate cancer diagnosis [6]and to monitor angiogenesis during chemotherapy [7-11].We previously evaluated the chronological changes in tissue enhancement of VEGFR2-targeted MB in PDAC tissues by time intensity curve (TIC) analysis and revealed more selective and longer lasting localization in those samples than in tissues treated with non-targeted MB [12].The sonoporation effect is influenced by the duration of ultrasound application [13]and the tumor cell line [14], therefore TIC analysis of VEGFR2-targeted MB could optimize UTMD, and such protocols should increase the effi-cacy of antitumor drugs for PDAC treatment.
In this study, we evaluated whether GEM-mediated UTMD using VEGFR2-targeted MB could enhance locoregional antitumor effects with a novel protocol of ultrasound application, in which low and high ultrasound intensity was combined to maximize the MB properties during a short period of ultrasound application.For future clinical applications, we also tested a novel protocol using endoscopic ultrasound (EUS) which allows the most sensitive imaging technology to visualize pancreatic masses in a minimally invasive fashion.
Methods
Animals
Female C57BL/6 J mice, 8-10 weeks of age (25-30 g body weight), were purchased from CLEA Japan, Inc.(Tokyo, Japan).All animals were housed in controlled conditions (12-h light-dark cycle, 24-25 °C, relative humidity: 40% −60%) withadlibitumfood(CE-2, CLEA Japan, Inc.) and water.This study protocol was approved by the Institutional Animal Care and Use Committee of the Jikei University School of Medicine (protocol number: 26016).
Cell line and tumor model
The Pan-02 murine pancreatic carcinoma cell line was obtained from the National Cancer Institute-Frederick cancer DCT tumor repository (Frederick, MD, USA).Pan-02 cells (1 ×106/mouse)were inoculated subcutaneously into the back of the mice, and the UTMD experiment was commenced once the tumors reached approximately 7-10 mm.
Ultrasound contrast agents and antitumor drug preparation
We used a VEGFR2-targeted MB (VISISTAR R ○, Targeson Inc., San Diego, CA, USA), which comprises a decafluorobutane-containing phospholipid shell binding to the single chain.According to information provided by the manufacturer, the VEGFR2-targeted MB was mixed with 2 mL of normal saline to a final concentration of 2 ×108MB/mL.The non-targeted MB (SONAZOID, Daiichi Sankyo Co., Ltd., Tokyo, Japan), comprising perflubutane, was shaken with 2 mL of saline to give a final concentration of 4.2 ×108MB/mL.The half-lives of VEGFR2-targeted MB and nontargeted MB were 15 and 5 min, respectively, according to our previous study [12]which also revealed the chronological change of MB concentration in tumor as that of the luminance in the tumor based on a TIC.After shaking preparation, MB size distribution was conducted by a Zetasizer nano series (Malvern Instruments, Worcestershire, UK).GEM (Eli Lilly and Company Indianapolis, IN, USA) was dissolved in normal saline to a concentration of 40 mg/mL.
Experimental protocols
To investigate the effects of single UTMD treatments, experiment 1 was conducted with 35 mice allocated to one of the following 5 groups based on injected drugs (7 mice per group):VEGFR2-targeted MB + GEM, VEGFR2-targeted MB, non-targeted MB + GEM, GEM alone, and a control group that received neither drug injection nor ultrasound application.The treatment protocol was as follows: After anesthesia with pentobarbital natrium (50 mg/kg) administered via intraperitoneal injection, tumor volume was calculated using the formula for a prolate ellipsoid: volume =π/6 × length × width × depth.In the VEGFR2-targeted MB + GEM and non-targeted MB + GEM groups, GEM(30 μL/mouse) was slowly injected over 10 s, followed by the VEGFR2-targeted or non-targeted MB (30 μL/mouse) injected similarly.All injections were via the mouse-tail vein using a 27-gage needle.The VEGFR2-targeted MB and GEM groups received only VEGFR2-targeted MB or GEM, respectively, injected as described for the other groups.After the injections, mice were stabilized on their back over the water and a 4-MHz convex type transducer of an EUS scope (GF-UCT 260, Olympus, Tokyo, Japan)was positioned on the tumor in the back skin.Ultrasound pulses with a mechanical index (MI) of 0.16, peak negative pressure of 0.65 MPa, pulse duration of 0.46 ms, pulse repetition frequency of 10 Hz, and a 1-cm imaging depth were applied to the tumor.Because our protocol aimed to maximize the MB properties using a time course of MB concentration, we decided the duration of ultrasound application based on the results of our previous study [12].Since TIC showing the time course of the MB luminance reflected the time course of the MB concentration, we ascertained that VEGFR2-targeted MB took 6 min to reach the maximum MB concentration and non-targeted MB took 3 min.Therefore, a 6-min application was used in the VEGFR2-targeted MB + GEM and VEGFR2-targeted MB groups, and a 3-min application was used in the non-targeted MB group.For the GEM group, ultrasound was applied for 6 min to compare the outcomes with the VEGFR2-targeted MB group.Finally, high-intensity ultrasound (MI of 2.0) was applied to the tumor for 1 s for MB destruction (manual flash) in all groups except for the control group.Two mice in each group were sacrificed by cervical dislocation at 3 h after the treatment to evaluate the immediate effect (sudden death group).The remaining mice (survival group) underwent tumor diameter measurement every other day, and when the diameter had increased 2-fold compared with the size before treatment (tumor diameter doubling time, TDT), the mouse was euthanized.
To verify the suppression of tumor growth with weekly GEM-mediated UTMD, experiment 2 used 15 mice randomly divided into 5 groups (3 mice per group).Grouping, anesthesia, tumor volume measurement, and the treatment process were conducted as for experiment 1.This treatment process was repeated once a week for 4 weeks, and the tumor diameter was measured once every twodays.Mice were sacrificed when the tumor diameter doubled regardless of the completion of the treatment.All experimental protocols are represented in Fig.1.
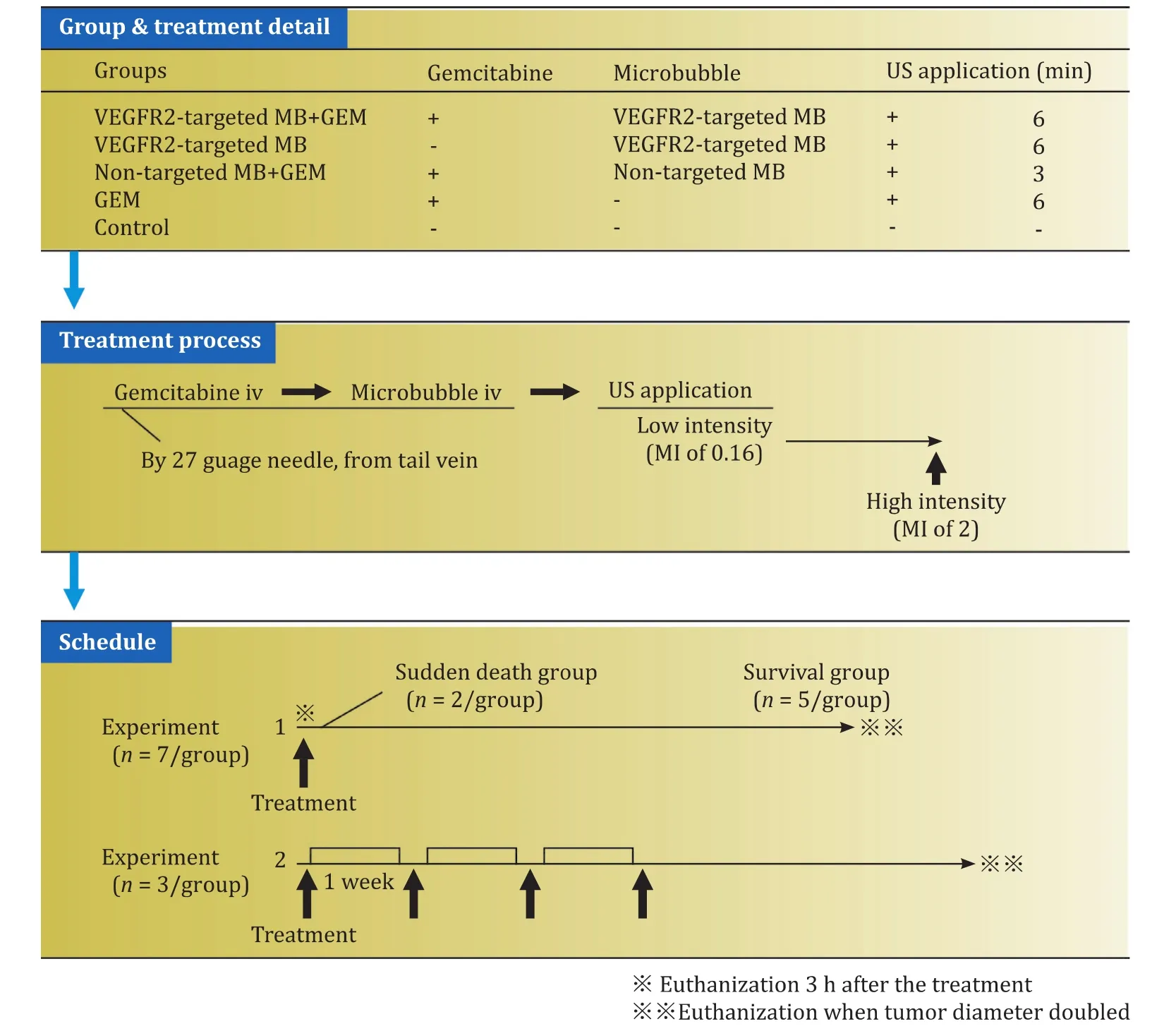
Fig.1.Experimental protocol.Each arrow in the schedule part showed the treatment.GEM: gemcitabine.
Histological analysis
All tumors were sampled and histologically evaluated at necroscopy.Eight-micrometer paraffin sections were prepared for hematoxylin eosin (H&E) staining to assess tumor morphology and tumor vasculature.Four- and six-micrometer sections were used for the immunohistochemical (IHC) staining to assess tumor microvascular density (MVD) and VEGFR2 expression, respectively.For the immunohistochemistry, we deparaffinized and rehydrated 4-micrometer sections for microwaving in 1 mmol/L sodium citrate buffer, and then incubated them with 0.3% H 2 O 2 in methanol to block endogenous peroxidase activity.The sections were washed in phosphate-buffered saline (PBS) before embedding in superblock and incubated overnight with an anti-mouse CD31 monoclonal antibody [anti CD31, Mouse (Rat), Dianova GmbH, Hamburg, Germany].Negative controls were prepared by substituting PBS for primary antibody staining.Sections were then rinsed in buffer and incubated with secondary antibody (ImmPRESS REAGENT Anti Rat IgG, Vector Laboratories, Burlingame, CA, USA) for 1 h, followed by incubation in peroxidase substrate solution for 5 min and rinsing in tap water.Finally, sections were counterstained and mounted.
The 6-micrometer sections were deparaffinized and rehydrated for antigen retrieval in microwave in 1 mmol/L sodium citrate buffer, and then incubated with 0.3% H 2 O 2 in methanol to block endogenous peroxidase activity.The sections were washed in PBS before embedding in superblock for overnight incubation with an anti-mouse VEGFR2 monoclonal antibody [VEGF Receptor2 (55B11)Rabbit mAb, Cell Signaling Technology, MA, USA].Negative controls were prepared by substituting anti-mouse IgG (DAKO Rabbit IgG# 0936, Agilent Technologies, Ltd., CA, USA) for primary antibody staining.Sections were then rinsed in buffer and incubated with secondary antibody (Anti-Rabbit Biotin #E0432, Agilent Technologies, Ltd.) for 1 h.The avidin-biotin-peroxidase complex method was completed by incubation in peroxidase streptavidin (Nichirei#426062, Tokyo, Japan) followed by the DAB substrate (ab64238,Abcam, Cambridge, United Kingdom).
The CD31 expression was assessed microscopically at magnifications ranging from × 40 to × 400.For the evaluation of MVD,two observers independently counted the CD31-stained vessels according to an established method.After identifying hot spots under ×40 magnification, three fields were randomly chosen,and the numbers of individual brown-stained luminal structures were counted at ×400 magnification for MVD measurements,performed 3 h after the experiment and at the time when tumor diameter was doubled in all groups.VEGFR2 density along the vessels (VEGFR2v) and in intra/peripheral cells (VEGFR2c) was counted microscopically in the same manner.VEGFR2v was recognized as a brownish linear stricture and VEGFR2c was recognized as cells stained brownish or surrounded by brownish areas ( Fig.2 ).
Statistical analysis
In experiment 1, differences in tumor diameter, tumor volume and TDT were statistically evaluated by analysis of variance(ANOVA).MVD, VEGFR2v, and VEGFR2c were evaluated by the Kruskal-Wallis equality of populations rank test in the sudden death group and by ANOVA in the survival group.Bonferroni correction was then conducted for each pair showing a significant difference by ANOVA.In experiment 2, differences in tumor diameter,tumor volume, TDT, MVD, VEGFR2v, and VEGFR2c were statistically evaluated by the Kruskal-Wallis equality of populations rank test.All analyses were performed using STATA 14 (Stata Corp LP, Texas,USA).Pvalues<0.05 were considered statistically significant.
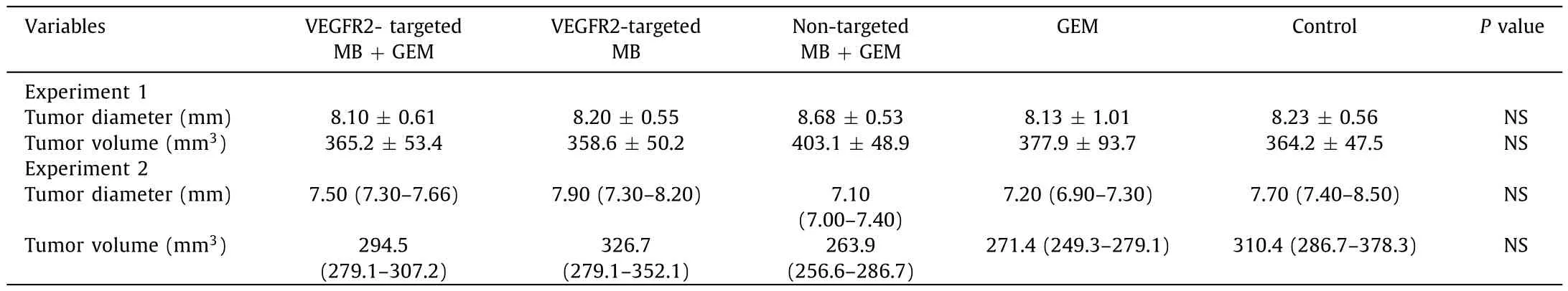
Table 1Tumor size before experiment.
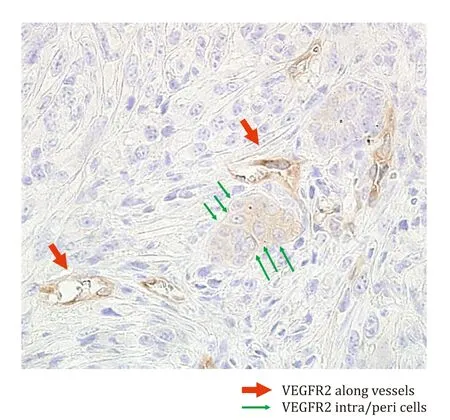
Fig.2.Examples of VEGFR2v and VEGFR2c.Brownish-stained round and luminal structures were counted as VEGFR2-positive vessels, while brownish-stained cellular and surrounding areas were counted as VEGFR2-positive intra/peripheral cells(original magnification ×400).
Results
Tumor diameters and volumes were not significantly different among the groups in both experiment 1 and 2 before treatments( Table 1 ).MB size analysis demonstrated that the average size of VEGFR2-targeted MB was 1.61 μm and that of the non-targeted MB was 1.99 μm.Each MB showed uniform internal size distribution( Fig.3 )
Experiment 1: single treatment
In experiment 1, a temporal decrease in mean tumor diameter was observed after treatment in the VEGFR2-targeted MB + GEM group and VEGFR2-targeted MB group, while an increase was observed in the other groups ( Fig.4 A).TDT was significantly longer in the VEGFR2-targeted MB + GEM group than that in the non-targeted MB + GEM, GEM, or control groups (28.0 ±2.5 vs.18.4 ± 1.5, 15.4 ± 2, and 11.6 ± 1.9 days, respectively; Table 2 ).Although the VEGFR2-targeted MB group showed longer TDT than the non-targeted MB + GEM, GEM, or control groups, there was no statistically significant difference.
H&E staining of tumor tissue from the sudden death group revealed an irregular arrangement of round or fusiform tumor cells associated with sporadic necrotic and desmoplastic areas.In the survival group, the tumors showed a palisade arrangement of cancer cells with sporadic necrotic and desmoplastic areas.The degrees of necrosis were macroscopically equivalent among the sudden death and survival groups.MVD in the VEGFR2-targeted MB + GEM group [3 (2-6) HPF/count]was significantly smaller than that in the non-targeted MB + GEM group [17.5 (14-26) HPF/count],GEM group [26 (19-34) HPF/count], and the control group [21.5(12-33) HPF/count]in the sudden death group ( Table 3 , Fig.5 );however, MVD showed no significant difference among the survival group (VEGFR2-targeted MB + GEM group: 31.40 ±2.14 HPF/count,VEGFR2-targeted MB group: 31.70 ±2.10 HPF/count, non-targeted MB + GEM group: 29.10 ± 1.90 HPF/count, GEM group: 32.50 ± 2.16 HPF/count, and control group: 30.30 ±2.48 HPF/count) ( Table 3 ).
VEGFR2v was significantly lower in the sudden death group,as compared the VEGFR2-targeted MB + GEM group [4.5 (3-6)HPF/count]and VEGFR2-targeted MB group [6.5 (4-9) HPF/count]with the non-targeted MB + GEM [10.5 (8-12) HPF/count], GEM alone [9.5 (8-11) HPF/count]and the control group [11 (9-12)HPF/count], respectively ( Table 3 , Fig.5 ).In contrast, VEGFR2v was not significantly different among the survival group, as was the case for VEGFR2c levels with different treatment in the sudden death and survival groups ( Table 3 ).
Experiment 2: weekly treatments over 4 weeks
In the experiment 2, 2 mice in the VEGFR2-targeted MB + GEM group, 3 mice in the VEGFR2-targeted MB group and non-targeted MB group, and none in the GEM group and the control group completed 4 courses of the treatment.The median tumor diameter temporally decreased after every treatment time point, except for the fourth treatment with VEGFR2-targeted MB + GEM and after the first treatment with VEGFR2-targeted MB, the median tumor diameter temporarily decreased.Meanwhile the median tumor diameter steadily increased in the other groups until the tumor size was doubled ( Fig.4 B).Median TDT was 35 (18-40) days in the VEGFR2-targeted MB + GEM group, 22 (22-26) days in the VEGFR2-targeted MB group, 24 (22-24) days in the non-targeted MB + GEM group, 14 (11-14) days in the GEM group, and 9 (9-11)in the control group; TDT was significantly longer in the VEGFR2-targeted MB + GEM than in the GEM and control groups ( Table 2 ).
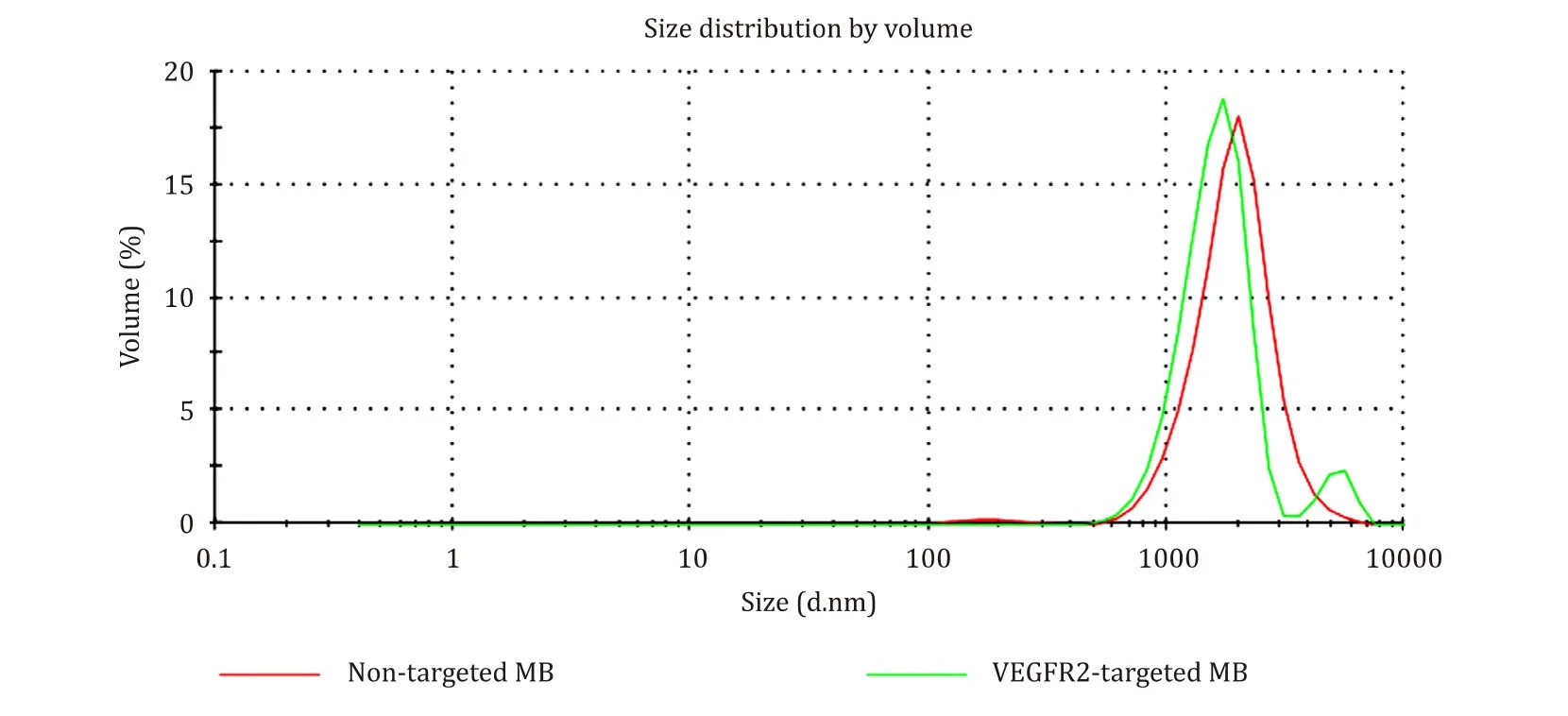
Fig.3.Size distribution result.The MB size analysis revealed the average size of VEGFR2-targeted MB described as green line to be 1.61 μm and that of the non-targeted MB described as red line to be 1.99 μm.Each MB showed uniform internal size distribution.
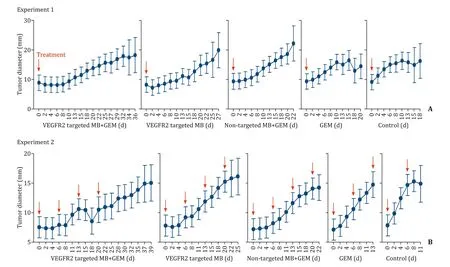
Fig.4.Transition of the tumor diameter.The red arrow showed the treatment day.A: Experiment 1; B: Experiment 2.GEM: gemcitabine.

Table 2Tumor diameter doubling time.
Among all groups in experiment 2, H&E staining of the tumor tissue revealed an irregular arrangement of round or fusiform tumor cells with some necrotic and desmoplastic areas covering the same extent among groups ( Fig.6 ).MVD at TDT was not significantly different among the groups [VEGFR2-targeted MB + GEM group: 30 (19-40) HPF/count; VEGFR2-targeted MB group: 28(24-45) HPF/count; non-targeted MB + GEM group: 28 (22-42)HPF/count; and GEM group: 33 (27-40) HPF/count; and control group: 36 (19-40) HPF/count]( Table 3 ).VEGFR2v density was significantly smaller in the VEGFR2-targeted MB + GEM group [13(10-16) HPF/count]and VEGFR2-targeted MB group [12 (9-15)HPF/count]than in the GEM group [17 (14-20) HPF/count]and control group [19 (16-22) HPF/count]( Table 3 ).Also, VEGFR2v density was significantly lower in the non-target MB + GEM group[14 (10-20) HPF/count]than in the control group [19 (16-22)HPF/count].VEGFR2c was significantly smaller at TDT in the VEGFR2-targeted MB + GEM group [13 (10-14) HPF/count]and the non-target MB + GEM group [14 (11-16) HPF/count]than in the control group [18 (13-23) HPF/count]( Table 3 ).
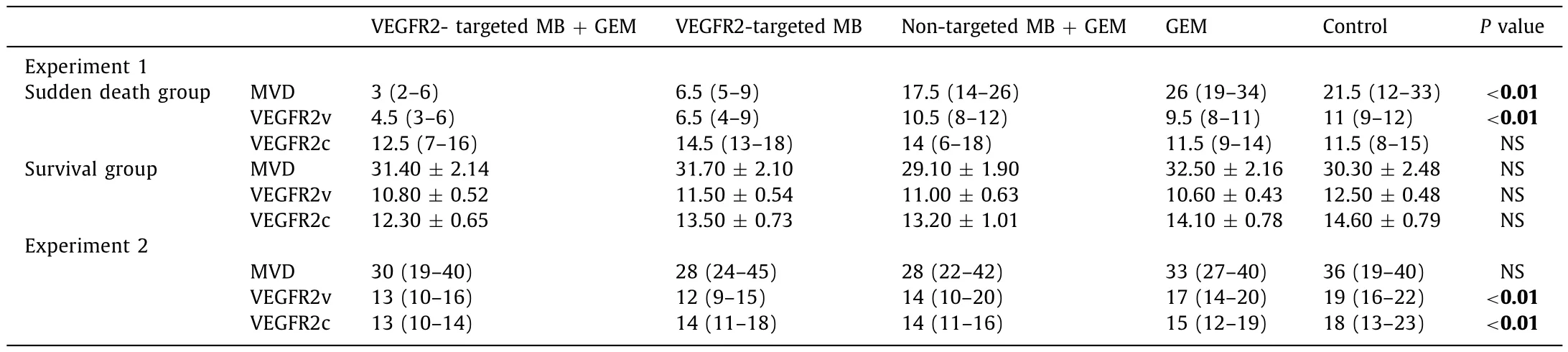
Table 3Histological results (HPF/count).

Fig.5.Histological results of experiment 1 (CD 31 staining and VEGFR2 staining) in the sudden death group (original magnification ×400).
Discussion
PDAC carries a dismal prognosis, with an overall 5-year survival rate of approximately 5% regardless of treatment [15].The ruinous tumor microenvironment causes acidification and deterioration of tissue osmotic pressure and disrupts the uniform dissemination of antitumor drugs into PDAC tissues.While GEM is the most effective chemotherapeutic agent for PDCA [ 16 , 17 ], the treatment effect is still limited because of poor drug distribution together with unavoidable damage to surrounding normal tissues.
To this end, various UTMD-mediated therapies were developed to treat PDAC.To enhance the selective extravasation of antitumor drugs to therapeutic sites, most UTMD-mediated therapies use antitumor drug placed inside nanoparticles, with or without targeted molecules [18-20].Another way to enhance the selective extravasation is to regulate the pulse intensity of UTMD.Cheng and colleagues [21]showed that low and high peak negative pressure(PNP) proportional to the pulse intensity induced sonoporation and cavitation, respectively.Moreover, sonoporation also influences the penetration efficiency of molecules into the nucleus [22]while cavitation induces eradication of the locoregional tumor vasculature [23].
In the present study, we regulated the ultrasound pulse intensity to maximize the pulse efficacy of VEGFR2-targeted or nontargeted MB, by initially applying low acoustic-intensity ultrasound(MI of 0.16) to induce sonoporation, followed by a flash of highintensity ultrasound (MI of 2.0) at the moment of maximal MB concentration in the tumor site to induce cavitation.The duration of the low-intensity application was decided according to our previous study wherein the concentration of VEGFR2-targeted MB and non-targeted MB in the tumor reached maximum at 6 min and 3 min, respectively, after the injection of MB.The single treatment of experiment 1 showed a substantial tumor reduction with a significant prolongation of TDT in the VEGFR2-targeted MB + GEM group.Histological analysis in the sudden death group revealed a significant reduction in MVD and VEGFR2v expression in the VEGFR2-targeted MB + GEM group and VEGFR2-targeted MB group than in other groups; however, MVD was not significantly dif-ferent between the survival groups.Since such a reduction reflects the cavitation effect [23], we surmised that a single treatment of GEM-mediated VEGFR2-targeted MB destruction realized the antitumor effect mainly via the cavitation effect.The weekly treatments of experiment 2 induced tumor suppression effects after the first, second, and third treatments with VEGFR2-targeted MB + GEM and after the first treatment with VEGFR2-targeted MB group, with significant TDT elongation also shown by the VEGFR2-targeted MB + GEM group.Histological analysis confirmed that in the VEGFR2-targeted MG + GEM group, VEGFR2v was significantly lower than in the GEM or control groups and also the VEGFR2c was significantly lower than in the control group, while only VEGFR2v showed significant suppression in the VEGFR2-targeted MB group compared to the GEM or control groups.Since a reduction of VEGFR2c reflects the suppression of tumor cell proliferation caused by the sonoporation of GEM [22]and that of VEGFR2v reflects the suppressed neogenesis of tumor vessels caused by cavitation, we speculated that a weekly treatment of GEM-mediated VEGFR2-targeted MB destruction induced the antitumor effect via VEGFR2 reduction caused by the sonoporation and cavitation effect.Therefore, VEGFR2-targeted MB destruction with GEM could mediate tumor suppression with a one-offtreatment whereby destruction of tumor vasculature occurs via cavitation.In addition,although the MVD reduction attenuated with repeated treatment,the cumulative treatment effect enhanced VEGFR2 suppression.
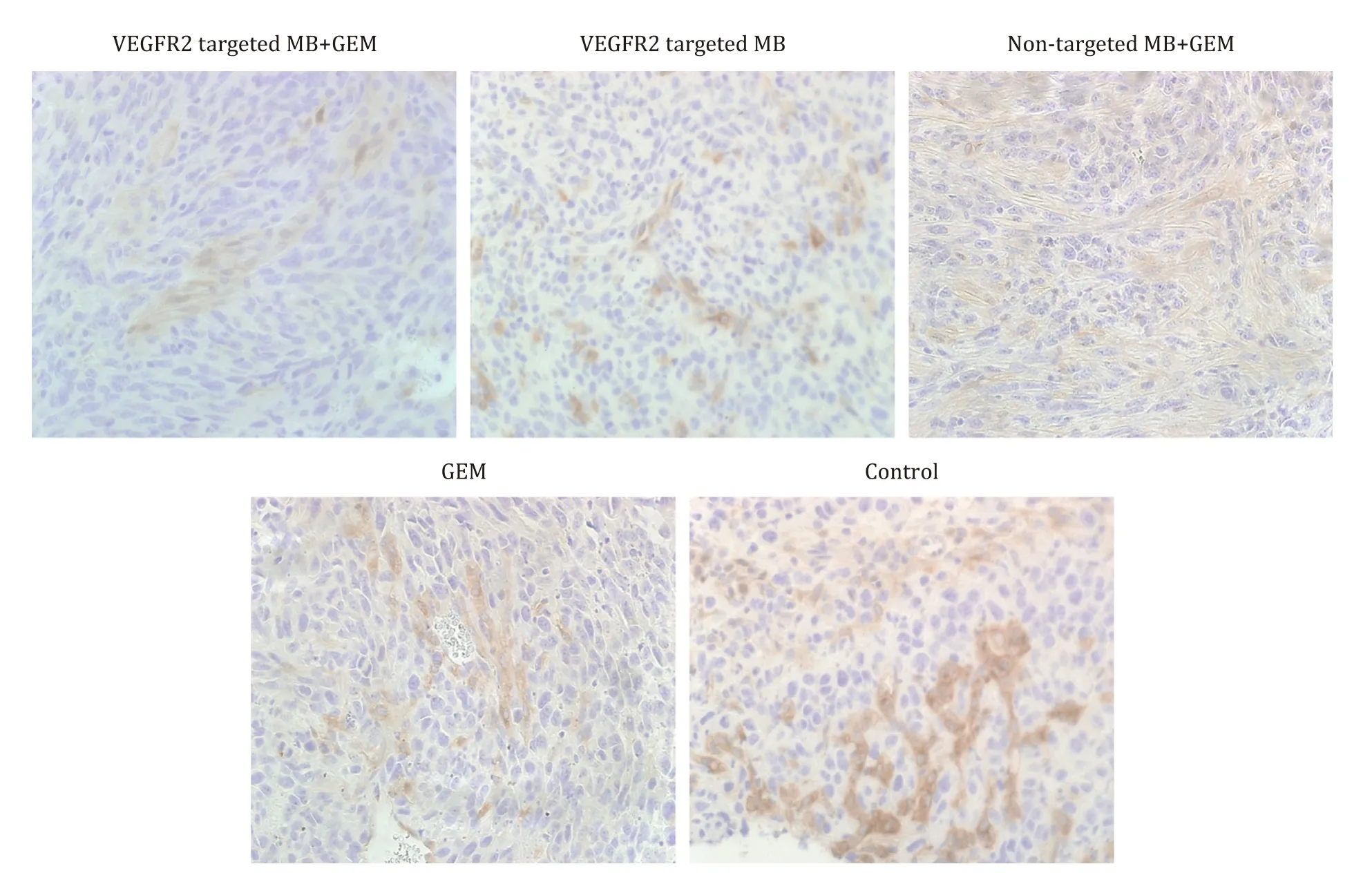
Fig.6.VEGFR2 staining result of experiment 2 (original magnification ×400).GEM: gemcitabine.
UTMD-mediated therapy requires ultrasound application at close range from the targeted site to increase the UTMD effect and reduce bystander tissue damage.Since the pancreas is located behind the stomach, US beam of transabdominal ultrasound (TUS) is often attenuated.Thus, the effect of UTMD using TUS is reduced and bystander tissue damage is inevitable.In this study, we tested a novel protocol using EUS probe.By applying the ultrasound beam from inside the stomach or duodenum, EUS has realized clearer visualization of pancreas without air interference compared to TUS,contributing to the improvement of the detection of a pancreatic mass [24].Although this heterotopic model study did not make use of this EUS advantage, future UTMD-mediated therapy should be continued based on EUS, particularly in cases of PDAC.
Our study has some limitations.First, the heterograft model might not adequately represent the tumor microenvironment in human PDAC.Considering that the heterotopic PDAC model showed lower chemosensitivity to the PDAC with more desmoplastic and hypovascular change than the orthotopic models [25],our novel protocol of gemcitabine-mediated UTMD with VEGFR2-targeted MB might showed higher efficacy in the orthotopic model.However, because the 4-MHz convex transducer of the EUS is not dedicated for small animals, the spatial resolution is insufficient to identify PDAC in the orthotopic or spontaneous model, making precise exposure of ultrasound beam to the PDAC impossible, as shown by Pysz and colleagues [6]using a 40-MHz nonlinear probe dedicated for small animals to identify PDAC in a spontaneous model.To establish UTMD-mediated chemotherapy using the EUS probe, another PDAC orthotopic model that could enable the identification and targeted exposure of PDAC through transluminal scanning is therefore needed to confirm the reproducibility.Second, the dose of GEM was decided according to a tolerant liquid volume for single slow injection (about 25 mL/kg).Since the dose of MB in this study was 30 μL/mouse, GEM was also injected at 30 μL/mouse (1.2 mg/mouse).Thus, the injected dose of GEM might not have been sufficient to achieve a significant difference between the GEM group and control group in experiments 1 and 2.Considering that the complex microenvironment in aninvivostudy might diminish the antitumor effect of GEM compared toinvitroresults [26], factors other than the GEM dose might impact the present results.Third, the number of mice (n= 3/group)used in experiment 2 might not be sufficient to draw a statistically strong conclusion or provide detailed explanations of the long-term therapeutic effects of VEGFR2-targeted UTMD.
In conclusion, this study demonstrated that VEGFR2-targeted MB destruction with GEM using EUS probe significantly inhibits PDAC growth compared with conventionally delivered systemic gemcitabine and gemcitabine-mediated non-targeted UTMD.In addition, such a treatment repeated weekly has a cumulative therapeutic effect, and reduces tumor size.
Acknowledgments
We are grateful to Mr.Kazutoshi Sakurai from the Department of Pathology at the Jikei University School of Medicine for his important contributions to the study.
CRediT authorship contribution statement
Nana Shimamoto:Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing - original draft.Masaki Ito:Data curation, Investigation, Methodology.Masafumi Chiba:Data curation, Investigation.Sadamu Honma:Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing - review & editing.Hiroo Imazu:Conceptualization, Methodology, Project administration, Supervision.Kazuki Sumiyama:Conceptualization, Formal analysis, Funding acquisition, Methodology, Supervision, Writing - review & editing.
Funding
This work was supported (in part) by a grant from Kudo Academic Foundation (Support for the academic researcher, 2017).
Ethical approval
This study protocol was approved by the Institutional Animal Care and Use Committee of the Jikei University School of Medicine(protocol number: 26-016).
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatobiliary&Pancreatic Diseases International
- No difference in mortality among ALPPS, two-staged hepatectomy, and portal vein embolization/ligation: A systematic review by updated traditional and network meta-analyses
- Telomerase reactivation is associated with hepatobiliary and pancreatic cancers
- Critical role of estrogen in the progression of chronic liver diseases
- Robotic isolated partial and complete hepatic caudate lobectomy: A single institution experience
- C -C motif chemokine ligand 16 inhibits the progression of liver cirrhosis via inactivating hepatic stellate cells
