No difference in mortality among ALPPS, two-staged hepatectomy, and portal vein embolization/ligation: A systematic review by updated traditional and network meta-analyses
2020-10-23PschlisGvriilidisRoertSutcliffeKeithRoertsMdhvPiDuncnSpldingNgyHiLongJioMikelSodergren
Pschlis Gvriilidis , Roert P Sutcliffe , Keith J Roerts , Mdhv Pi Duncn Splding Ngy Hi Long R Jio Mikel H Sodergren
a Department of Hepatopanceaticobiliary Surgery, Imperial College Healthcare NHS Trust, Hammersmith Hospital, London W12 0HS, UK
b Department of Hepato-Pancreato-Biliary and Liver Transplant Surgery, Queen Elizabeth University Hospitals Birmingham NHS Foundation Trust,Birmingham B15 2TH, UK
Keywords:
ABSTRACT
Introduction
The principal cause of death after major hepatectomy is posthepatectomy failure due to insufficient functional liver remnant(FLR) [1].Currently, to manage insufficient FLR, seven hypertrophy strategies have been developed: portal vein embolization (PVE),portal vein ligation (PVL), two-staged hepatectomy (TSH), associating liver partition and portal vein ligation for staged hepatectomy (ALPPS), radiofrequency ALPPS (RALPPS), tourniquet ALPPS(TALPPS), and liver venous deprivation (LVD).
Makuuchi et al.[2]introduced the era of hypertrophy management by developing PVE to increase FLR in patients with large tumors in the right lobe extending up to segment IV.It is reported that 20% of patients dropped out owing to either insufficient hypertrophy of the FLR or disease progression [3].In 20 0 0,Adam et al.introduced TSH for bilobar unresectable colorectal liver metastases [4].PVE or PVL performed during the first stage of TSH can induce 27% −39% hypertrophy of the FLR in 4 to 8 weeks [5].A major caveat of TSH was a failure to proceed to the second stage in about one-third of patients, due to either insufficient hypertrophy or disease progression [6].Individual studies reported the impact of PVE on the growth promotion of colorectal liver metastases [ 7 , 8 ].
In 2012, the ALPPS technique was introduced with the hope of increasing the rate of hepatectomy in this group of patients by achieving hypertrophy of the FLR in a shorter time compared with TSH.However, in 2012 the first study by Schnitzbauer et al.on this technique reported major morbidity and mortality rates of 68% and 12%, respectively [9].In 2015, the first meta-analysis reported that ALPPS can induce hypertrophy of up to 84% compared to PVE or PVL.However, major morbidity and perioperative mortality occurred in 44% and 11% of patients, respectively.As oncological outcomes were not well documented in the included studies it was not possible to report results on oncological safety [10].The most recent meta-analysis demonstrated that the ALPPS technique was associated with a greater increase of the FLR and kinetic growth rate.However, major morbidity and perioperative mortality remained significantly higher compared to TSH [11].Since this publication there have been a number of studies published on this topic including randomized controlled trials (RCTs) [ 12 , 13 ].Therefore, updated and network meta-analyses (NMA) were conducted to track the accumulation of evidence over time.Mortality and major morbidity were selected as primary outcomes.Based on the fact that Bayesian NMA does not usePvalues in order to facilitate the readership to make easier comparisons, we decided to use this and the frequentist meta-analysis simultaneously which usesPvalues.Consequently, the results of both were compared to detect any discrepancies.We believe this approach increased the intrinsic value of the sensitivity analysis of the present study.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist were followed in this study [14].
Literature search
With the use of search terms in the free text and Medical Subject Headings (“associating liver partition and portal vein ligation”, “ALPPS”, “portal vein embolization”, “portal vein ligation”,“two-staged hepatectomy”, “liver venous deprivation”“TSH”, “PVE”,“PVL”, “randomised or randomized controlled trial”, “retrospective studies”, or “prospective studies”), a systematic search of literature published over the last 18 years up to December 2019 was performed using the EMBASE, MEDLINE (PubMed), Cochrane Library,and Google Scholar databases.A grey literature search was also performed in the clinicaltrials.gov website.References of the retrieved articles were checked manually for additional studies.Disagreements between authors were resolved by consensus-based discussions.
Study selection and inclusion and exclusion criteria
Both RCTs and retrospective studies that compared ALPPS or its modifications with TSH and PVE or PVL were included in this study.In addition, studies that compared PVE with PVL or LVD were also included.In cases of multiple publications by the same institution, only the most recent publication was included.Abstracts and non-English publications were excluded from the analysis.
Data extraction and outcomes
Two reviewers (Gavriilidis P and Sodergren MH) independently extracted the following summary data from the included studies:name of authors; year of publication; number of patients included in the ALPPS and TSH cohorts; age; body mass index (BMI); sex;tumor size; neoadjuvant treatment; pure volume increase of FLR;regeneration rate; kinetic growth rate; time to intervention; definitive hepatectomy; cleaning of FLR; R0 resection; Clavien-Dindo IIIIV; recurrence rate; 90-day mortality; 1-year overall survival; and 1-year disease-free survival.
Statistical analysis
The methodological quality of all included studies was evaluated using the validated Newcastle-Ottawa scale (NOS) [15].Studies scoring ≥7 were considered high quality (Table S1).
Cochrane’s criteria were used to assess the methodological quality of included RCTs.Two authors (Gavriilidis P and Sutcliffe RP) independently, assessed the risk of selection bias, attrition bias,detection bias, performance bias and reporting bias.Consequently,included RCTs were categorised as unclear, high, or low [16].
First an updated meta-analysis was conducted for studies comparing two of the hepatic hypertrophy approaches.Traditional meta-analysis of the portal vein occlusion (PVO) group included hypertrophy approaches of PVE, PVL, and TSH and compared with ALPPS.Consequently, subgroup analysis of each approach was done, when possible.NMA was conducted to compare ALPPS vs.PVE vs.PVL vs.RALPPS vs.TALPPS vs.LVD.Statistical analysis was performed using the Review Manager 5.3 software (Cochrane Collaboration, Oxford, UK) [16], Stata software (version 16, Stata Corp.LP, College station, TX, USA) [17], and General mixed treatments comparisons (GeMTC) software [18].Heterogeneity was assessed using theI2test, and cut-offvalues of 25%, 50%, and 75% were considered low, moderate, and high heterogeneity, respectively [19].
For studies that did not report the mean and variances of the two groups, these values were estimated from the median, range,and sample size, using the technique described by Hozo et al.,where possible [20].Analysis of long-term survival was performed by combining the hazard ratios (HRs) and 95% confidence intervals(95% CIs) from the included studies.These were rarely reported and, thus, were estimated using the method described by Parmar et al., where possible [21].
NMA was conducted using hierarchical random-effects models [22].A fixed-effects model was also used to estimate whether any discrepancy could be detected between the results of the two models.Quantitative data synthesis of the connected network of the studies was performed using the software package WinBUGS(version 1.4.3, MRC Biostatistics Unit, Cambridge, UK) [23].For each model, 20 0 0 0 0 simulations were generated for the two sets of initial values, and the first 50 0 0 were discarded as the burn-in period.The Brooks-Gelman-Rubin statistic was used for the assessment of convergence [22].The point-estimate was defined as the median of the posterior distribution based on 200 000 simulations;the corresponding 95% credible intervals (95% CrIs) were obtained using the 2.5th and 97.5th percentiles of the posterior distribution,which can be interpreted in a similar way as 95% CI [ 22 , 23 ].Inconsistency and heterogeneity of the direct and indirect evidence for the six hepatic hypertrophy approaches were estimated.
Frequentist NMA was conducted with Stata and the extent of uncertainty in the estimated treatment effects was estimated not only with the CIs but with the predictive intervals that incorporated the extent of heterogeneity.The predictive interval was defined as the interval within which the relative treatment effect of future studies is expected to lie [17].
In all analyses, the point estimate was considered significant atP<0.05.
Sensitivity analysis
Analyses of both primary and secondary outcomes were calculated using the random- and fixed-effects models to assess the impact of heterogeneity on the robustness of the conclusions.Afterthe traditional meta-analysis, NMA was conducted and the results of both were compared to detect any discrepancies between them.Subgroup analysis of the RCTs and the PVE and TSH studies was conducted and the results were compared between them and with the whole sample.
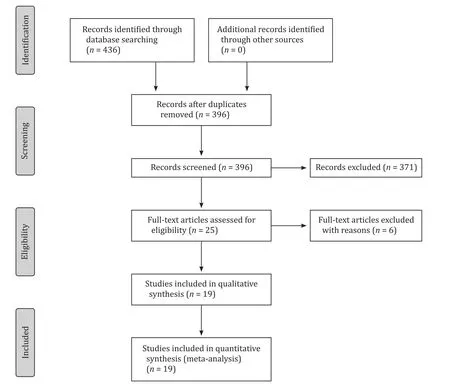
Fig.1.Flow diagram of the search strategy.
Results
Search strategy and included study characteristics
Nineteen studies (1200 patients), were selected from a pool of 436 studies ( Table 1 , Fig.1 ).Of these patients, 315 (31%) and 702 (69%) underwent ALPPS and PVO, respectively [ 12 , 13 , 24-35 ].The studies compared: ALPPS vs.TSH (n= 4) [ 12 , 28 , 29 , 31 ]; RALPPS vs.PVE (n= 1) [13]; ALPPS vs.PVE (n= 6) [ 24-27 , 30 , 33 ]; TALPPS vs.TSH (n= 1) [34], and ALPPS vs.PVE/PVL/TSH (n= 1) [35].Four studies (155 patients) compared PVE (84, 54%) vs.PVL (71,46%) [36-39].One study compared PVE (n= 15 patients) vs.LVD(n= 13 patients) [40].Of these studies, 2 were RCTs and 17 retrospective studies ( Table 1 ).No significant differences were found in the demographic characteristics between cohorts.However, there was evidence that the ALPPS cohort included significantly smaller tumors (by about 4.84 mm) compared to the PVO cohort ( Table 2 ).The methodological quality of the RCTs was high.Fifteen of the nineteen studies scored ≥7 and were characterised as high quality( Table 1 ).
The assessment of RCTs according to Cochrane criteria detected that the Achilles’ heel of both of them was lack of blinding of participants, personnel, and assessors of the outcomes (Table S2).
Primary outcomes
Perioperativemortality
All studies evaluated perioperative overall mortality.There was evidence of significantly higher overall mortality in the ALPPS cohort (8%, 22/284 patients) compared to the PVO cohort (4%, 29/669 patients; OR = 1.93; 95% CI: 1.10-3.39;P= 0.002;I2= 0%).Eleven of fourteen studies reported 90-day mortality [ 12 , 13 , 25-28 , 31-35 ].There was marginal significantly higher mortality in the ALPPS cohort (8%, 20/266) compared to the PVO cohort (5%, 28/600;OR = 1.78; 95% CI: 0.99-3.20;P= 0.06;I2= 0%).In addition, subgroup analysis of the RCTs demonstrated no significant differences in 90-day mortality between the ALPPS (7%, 5/74) and PVO cohorts(4%, 3/73; OR = 1.62; 95% CI: 0.41-6.49;P= 0.49;I2= 0%) ( Tables 2 and 3 ).Moreover, Bayesian NMA demonstrated no significant differences in 90-day mortality between ALPPS and the other six hypertrophy approaches ( Fig.2 , Table S3).Furthermore, frequentist NMA demonstrated no significant differences in 90-day mortality as well between all types of hepatic hypertrophy approaches except for ALPPS vs.PVE, and additionally, the predictive intervals demonstrated low heterogeneity and no significant differences of the relative effect of future studies ( Fig.3 ).
Majormorbidity
There was evidence that the ALPPS cohort (47%, 113/242)had significant higher major morbidity compared to the PVO cohort (22%, 122/545; OR = 3.19; 95% CI: 2.26-4.52;P<0.001;I2= 59%).Subgroup analysis of the RCTs demonstrated no significant differences in major morbidity between the ALPPS (35%,26/74) and PVO cohorts (18%, 13/73; OR = 2.90; 95% CI: 0.85-9.93;P= 0.09;I2= 31%) ( Tables 2 and 3 ).Furthermore, in Bayesian NMA, the PVO cohort demonstrated significantly lower major morbidity rates compared to ALPPS cohort ( Fig.4 , Table S2).Frequentist NMA demonstrated that the PVE and PVL cohorts had significantly lower major morbidity compared to ALPPS cohort.However, the predictive intervals demonstrated that the relative effect of future studies were expected to be nonsignificant except for PVE.Of note, indirect evidence demonstrated that LVD had significantly lower major morbidity rates compared to ALPPS( Fig.5 ).
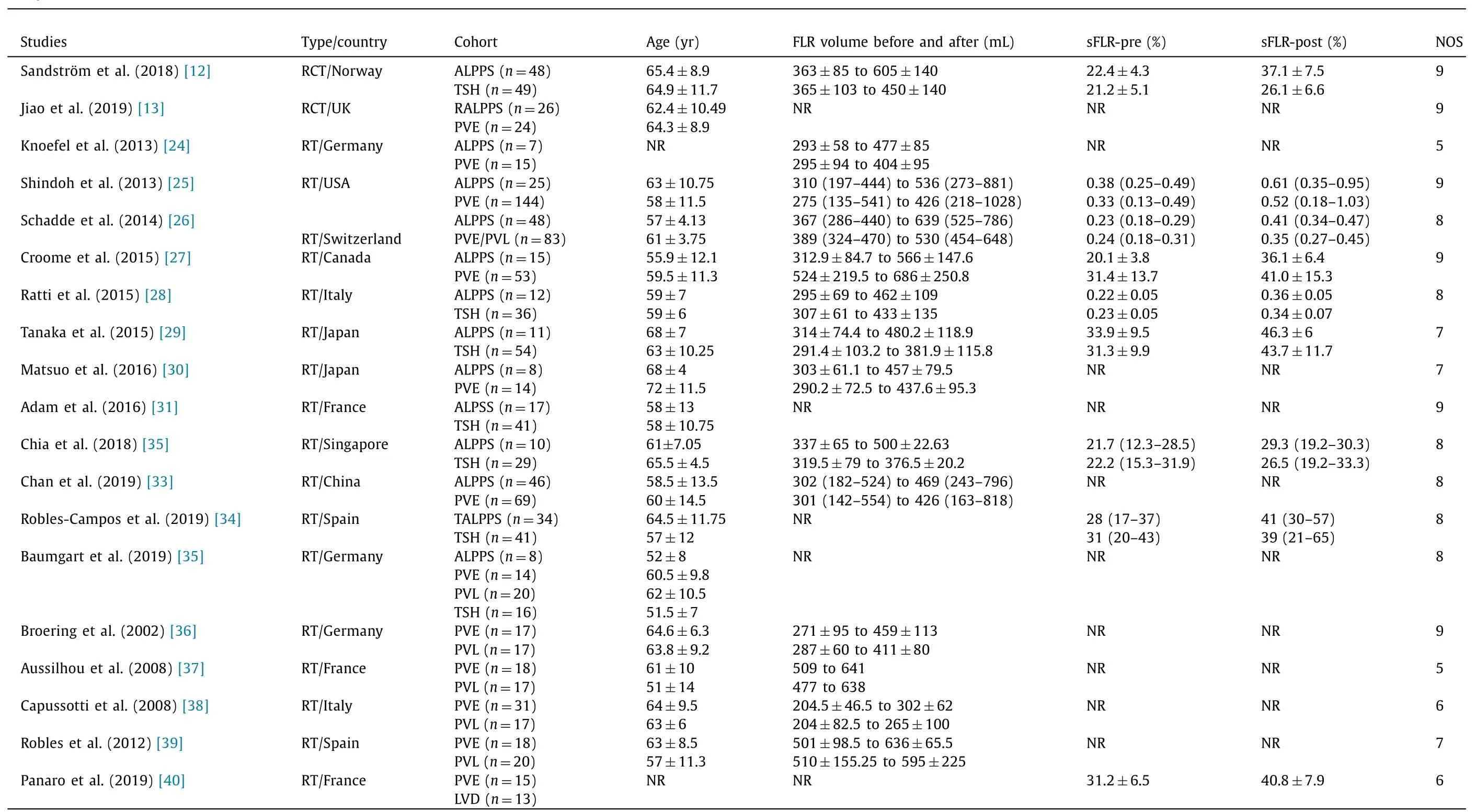
Table 1Study characteristics.
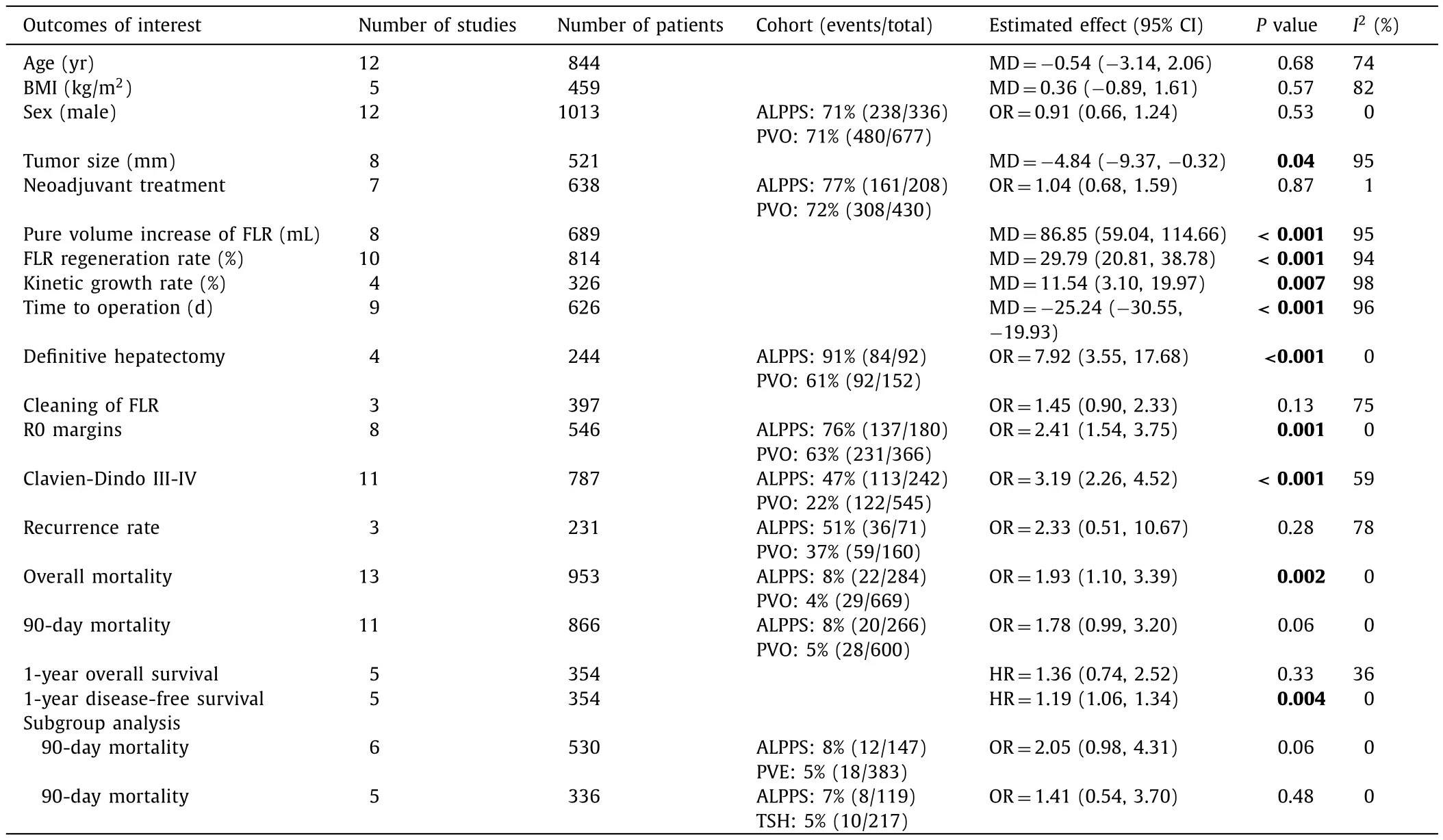
Table 2Outcomes of interest of ALPPS vs.PVO cohorts.
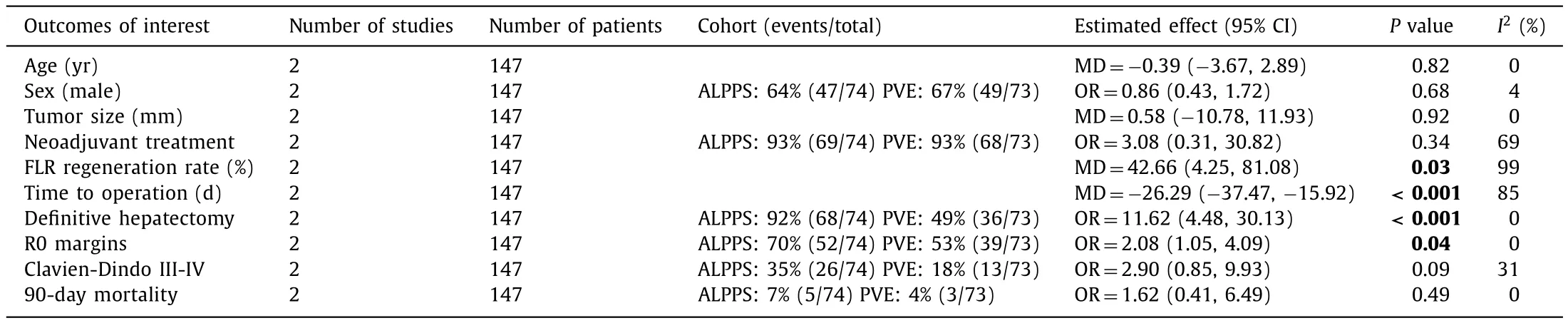
Table 3Outcome of interests of ALPPS vs.PVO in RCTs.
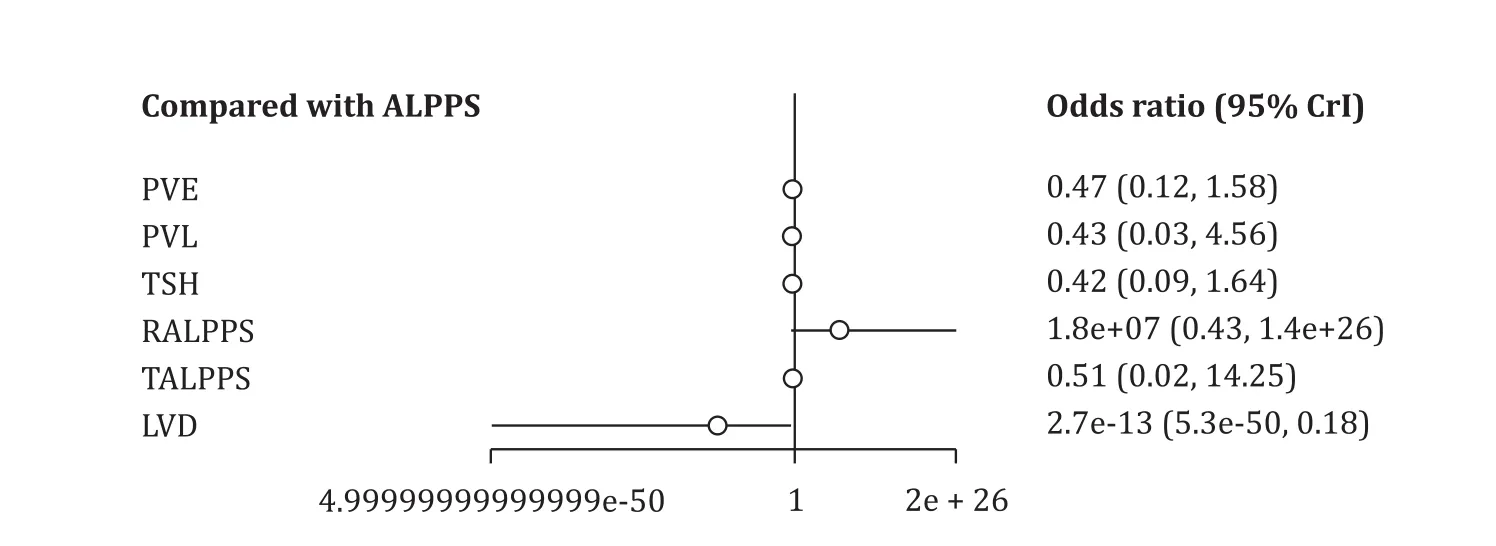
Fig.2.Comparison of 90-day mortality of hepatic hypertrophy approaches with ALPPS, using Bayesian NMA.ALPPS: associating liver partition and portal vein ligation for staged hepatectomy; PVE: portal vein embolization; PVL: portal vein ligation; TSH: two-staged hepatectomy; RALPPS: radiofrequency ALPPS; TALPPS: tourniquet ALPPS; LVD:liver venous deprivation; 95% CrI: 95% credible interval.
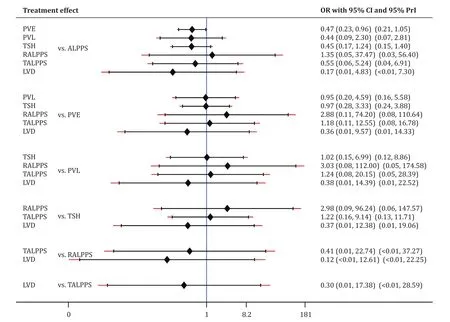
Fig.3.Comparison of 90-day mortality of hepatic hypertrophy approaches with ALPPS, using frequentist NMA.ALPPS: associating liver partition and portal vein ligation for staged hepatectomy; PVE: portal vein embolization; PVL: portal vein ligation; TSH: two-staged hepatectomy; RALPPS: radiofrequency ALPPS; TALPPS: tourniquet ALPPS; LVD:liver venous deprivation; OR: odds ratio; 95% CI: 95% confidence interval; 95% PrI: 95% predictive interval.
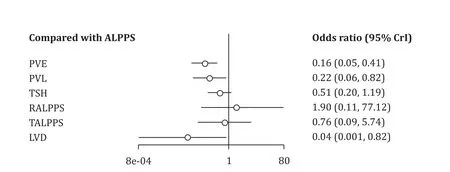
Fig.4.Comparison of major morbidity of hepatic hypertrophy approaches with ALPPS, using Bayesian NMA.ALPPS: associating liver partition and portal vein ligation for staged hepatectomy; PVE: portal vein embolization; PVL: portal vein ligation; TSH: two-staged hepatectomy; RALPPS: radiofrequency ALPPS; TALPPS: tourniquet ALPPS; LVD:liver venous deprivation; 95% CrI: 95% credible interval.
Secondary outcomes
PurevolumeincreaseoftheFLR
There was evidence of a significantly larger increase of the final FLR of the ALPPS cohort compared to the PVO cohort(MD = 86.85 mL; 95% CI: 59.04-114.66;P<0.001;I2= 95%).
FLRregenerationrateandkineticgrowthrate
There was evidence that the FLR regeneration rate(MD = 29.79%; 95% CI: 20.81% −38.78%),P<0.001;I2= 94%)and kinetic growth rate (MD = 11.54%; 95% CI: 3.10% −19.97%;P<0.001;I2= 98%) were faster in the ALPPS cohort compared to the PVO cohort.
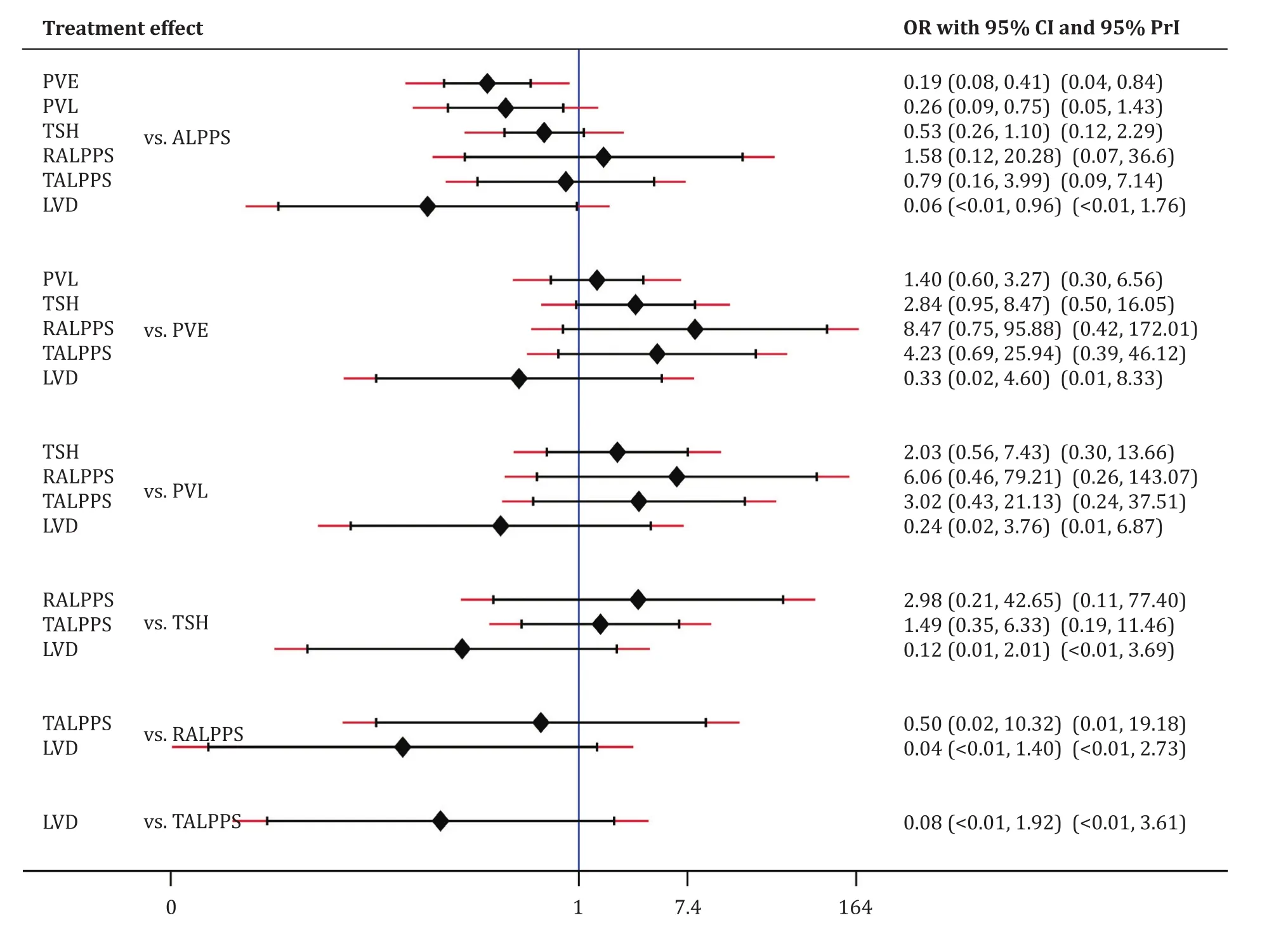
Fig.5.Comparison of major morbidity of hepatic hypertrophy approaches with ALPPS, using frequentist NMA.ALPPS: associating liver partition and portal vein ligation for staged hepatectomy; PVE: portal vein embolization; PVL: portal vein ligation; TSH: two-staged hepatectomy; RALPPS: radiofrequency ALPPS; TALPPS: tourniquet ALPPS; LVD:liver venous deprivation; OR: odds ratio; 95% CI: 95% confidence interval; 95% CrI: 95% credible interval.
Timetooperation
The time to operation was significantly shorter (by 25 days) in the ALPPS cohort compared to the PVO cohort ( Table 2 ).
Definitivehepatectomy
There was evidence that significantly more patients in the ALPPS cohort (91%, 84/92 patients) underwent definitive hepatectomy compared to the PVO cohort (61%, 92/152 patients; OR = 7.92;95% CI: 3.55-17.68;P<0.001;I2= 0%).
R0margins
There was evidence that significantly more operations with R0 margins were performed in the ALPPS cohort (76%, 137/180) compared to the TSH cohort (63%, 231/366; OR = 2.41; 95% CI: 1.54-3.75;P= 0.001;I2= 0%).
One-yeardisease-freesurvival
There was evidence that the ALPPS cohort demonstrated significantly worse 1-year disease-free survival compared to the PVO cohort (HR = 1.19; 95% CI: 1.06-1.34;P= 0.004;I2= 0%) ( Table 2 ).
Statisticallynonsignificantresults
The parameters of age, BMI, sex, neoadjuvant therapy, FLR cleaning, recurrence rate, and 1-year overall survival demonstrated no significant differences between the ALPPS and PVO cohorts( Table 2 ).
Sensitivity analysis
There was no significant differences in findings between the fixed- and random-effects models.Subgroup analysis of the two RCTs demonstrated nonsignificant results on the parameters of tumor size and major morbidity and 90-day mortality, whereas the whole sample of the study demonstrated significantly better results in the PVO cohort compared with the ALPPS cohort.In the remaining parameters, there were no discrepancies between RCT results and those of the whole study sample ( Tables 2 and 3 ).
Both Bayesian and frequentist NMA demonstrated no significant differences in 90-day mortality rate, without any discrepancies between them.Regarding secondary outcomes, result comparisons of the three methods of meta-analysis did not detect any discrepancies.
It was demonstrated that the ALPPS cohort included significantly smaller tumors compared to the PVO cohort.However, further analysis detected that the study by Tanaka et al.[29]was an outlier and when removed, it rendered the findings nonsignificant.
Discussion
This study demonstrated a change of paradigm regarding the 90-day mortality of the ALPPS technique compared with other types of hepatic hypertrophy approaches.Namely, updated traditional meta-analysis of the whole sample and subgroup analysis of two high quality RCTs demonstrated no significant differences in 90-day mortality of the ALPPS technique compared to the PVOtechnique; of note, both of the above results were produced with 0% heterogeneity.In addition, Bayesian NMA comparing each of the techniques (PVE, PVL, TSH, RALPPS, TALPPS, and LVD) with ALPPS demonstrated no significant differences in 90-day mortality without observed discrepancies between the direct and indirect evidence.Furthermore, frequentist NMA demonstrated similar results with the above two methods and predicted that the relative effect of future studies was expected to demonstrate no significant differences.A marginal discrepancy was detected between Bayesian and frequentist NMA regarding the comparisons of ALPPS vs.PVE,where the first demonstrated no significant differences while the second was marginally significant.These slight differences may result from the way each method evaluated the heterogeneity, and the contributed percentage weight of each study in the whole sample.
It has been reported that age (>60 years) is a risk factor for increased perioperative mortality [41].However, in the present study, the two RCTs included patients with age>60 years demonstrated low mortality and nonsignificant differences in two approaches [ 12 , 13 ].
So far, the role of preoperative chemotherapy has not been clearly defined as a cause of increased morbidity.However, it has been reported that prolonged chemotherapy may cause steatotic changes and sinusoidal injury of the liver parenchyma which may increase major morbidity [ 42 , 43 ].
All FLR hypertrophy parameters favored ALPPS and the time to operation was significantly shortered by 25 days.Consequently,these advantages translated to significantly better rates of definitive hepatectomies in the ALPPS cohort (91%) compared to the PVO cohort (61%), with 0% heterogeneity.
Regarding oncological safety parameters, R0 margins rates were significantly higher in the ALPPS cohort (76%) compared to the PVO cohort (63%), with 0% heterogeneity.The recurrence rate was 51%in the ALPPS cohort and 37% in the PVO cohort, and the difference was not significant and with high heterogeneity (78%); consequently, this led to significantly better 1-year disease-free survival in the PVO cohort compared to the ALPPS cohort, whereas 1-year overall survival demonstrated no significant differences between cohorts.As data regarding tumor stage, volume load and distribution was not available, it is not possible to assess for these differences in the study populations which may contribute to oncological outcomes.Since the included studies did not report longterm survival benefits, we were not able to assess them.
The results of the present study should be interpreted in the context of its limitations.The ALPPS cohort sample consisted of 31% of the total sample.Apart from the two high quality RCTs, the remaining studies were retrospective and from different centers.Therefore, an underpowered sample, and national and institutional bias may have influenced the results.In non-randomized studies selection bias is likely to be significant.In addition, another limitation was the inability to assess long-term oncologic outcomes and survival benefits as they have been underreported.Of note,from the five studies that reported survival only two those of Adam et al.[31]and Robles-Campos et al.[34]were performed survival analysis by intention to treat.They defined overall survival as the time interval between the diagnosis of liver metastasis or the date of the first hepatectomy until death or date of the last follow-up.Disease-free survival was defined as the period from the date of last hepatectomy until the first recurrence.Therefore, survival results should be interpreted cautiously because selection bias may have influenced the results.Risk of bias assessment detected that the Achilles’ heel of the RCTs was high risk of bias in the domains of blinding participants, personnel and assessors of outcomes.Furthermore, there was significant heterogeneity in the definition of the individual surgical techniques which would limit applicability of these data.
In conclusion, the present study, using updated traditional meta-analysis and Bayesian and frequentist NMA, demonstrated that perioperative mortality of ALPPS is statistically comparable to any other hepatic hypertrophy approach.Furthermore, two RCTs demonstrated no significant differences in 90-day mortality and major morbidity between the ALPPS and PVO cohorts.Therefore,these data suggest that ALPPS and its modifications can be used as an alternative hepatic hypertrophy approach in specialized centers and in adequately selected patients.
Acknowledgments
None.
CRediT authorship contribution statement
Paschalis Gavriilidis:Conceptualization, Formal analysis, Investigation, Methodology, Software, Validation, Writing - original draft, Writing - review & editing.Robert P Sutcliffe:Formal analysis, Investigation, Methodology, Validation, Writing - original draft,Writing - review & editing.Keith J Roberts:Formal analysis, Investigation, Methodology, Validation, Writing - original draft, Writing - review & editing.Madhava Pai:Formal analysis, Investigation, Methodology, Validation, Writing - original draft, Writing -review & editing.Duncan Spalding:Formal analysis, Investigation,Methodology, Validation, Writing - original draft, Writing - review& editing.Nagy Habib:Formal analysis, Investigation, Methodology, Validation, Writing - original draft, Writing - review & editing.Long R Jiao:Formal analysis, Investigation, Methodology, Supervision, Validation, Writing - original draft, Writing - review & editing.Mikael H Sodergren:Formal analysis, Investigation, Methodology, Supervision, Validation, Writing - original draft, Writing - review & editing.
Funding
None.
Ethical approval
Not needed.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.hbpd.2020.07.005.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatobiliary&Pancreatic Diseases International
- Telomerase reactivation is associated with hepatobiliary and pancreatic cancers
- Critical role of estrogen in the progression of chronic liver diseases
- Robotic isolated partial and complete hepatic caudate lobectomy: A single institution experience
- C -C motif chemokine ligand 16 inhibits the progression of liver cirrhosis via inactivating hepatic stellate cells
- Dynamic expression of hepatic GP73 mRNA and protein and circulating GP73 during hepatocytes malignant transformation
