Critical role of estrogen in the progression of chronic liver diseases
2020-10-23DevarajEzhilarasanMedical
Devaraj Ezhilarasan Medical
Keywords:
ABSTRACT
Introduction
The liver plays a pivotal role in the glucose homeostasis,metabolism, and detoxification of several drugs and xenobiotics in the body [1].When the liver is chronically affected by various etiological factors such as ethanol, aflatoxin B contaminated food, hepatitis B and C virus infections, and hepatotoxic drugs, the normal biochemical and physiological functions are affected, resulting in chronic liver diseases (CLD) [2].The acute liver injury is often reversible while chronic liver injury is responsible for various pathological manifestations like hepatic inflammation, steatosis, fibrosis,cirrhosis, portal hypertension, and hepatocellular carcinoma (HCC).Hepatic fibrosis is a highly orchestrated process that is characterized by the net accumulation of extracellular matrix (ECM) in perisinusoidal space resulting from the wound-healing response to chronic liver injury of any etiology [3].Hepatic fibrosis accounts for 45% of all deaths in industrialized nations, making this one of the largest unmet needs in clinical medicine, apart from cancer [4].
CLD and hepatic stellate cells (HSCs)
Chronic liver injury causes sustained scarring response which gradually disrupts the liver architecture and its functions through a net accumulation of ECM in perisinusoidal space or space of Disse that eventually causes liver cirrhosis and its associated portal hypertension and liver failure [ 5 , 6 ].Therefore, fibrogenesis is considered to play a central role in the onset of cirrhosis.Hepatic fibrosis is a common pathway that represents a convergent point from many etiologies, most prominently hepatitis B and C viral infections, nonalcoholic steatohepatitis (NASH) and alcohol [7].If left untreated, fibrosis progresses into cirrhosis and subsequently HCC [8].
HSCs are non-parenchymal and resident perisinusoidal cells in the liver commonly implicated in the progression of hepatic fibrosis [7].In normal liver, HSCs contribute to development and regeneration, regulation of ECM synthesis and degradation, retinoidmetabolism, endothelial cell-mediated vasoregulation, secretion of a variety of cytokines, immune regulation, lipid metabolism, and detoxification [9].Following chronic liver injury, HSCs undergo phenotypic transdifferentiation and acquire myofibroblast-like phenotypes which secrete various profibrogenic cytokines responsible for the synthesis of an enormous amount of ECM in the diseased liver [10].
Burden of CLD
Cirrhosis is one of the common sequels of persistent hepatic fibrosis and the major cause of significant morbidity and mortality of patients with CLD [11].Currently, 844 million people are affected by various forms of CLD, with a mortality of 2 million per year worldwide [ 12 , 13 ].Globally, 400 million people are chronically infected with HBV and up to 2 billion people are with the evidence of exposure [14].Approximately 3% of the world population is infected with HBV and 170 million people are infected with HCV [15].Obesity has become a global public health problem with increasing incidence in both adults and children in the 21st century and it produces NASH [16].Alcohol is one of the main causes of CLDs-associated mortality worldwide.Alcohol abuse results in steatosis, alcoholic steatohepatitis, fibrosis and cirrhosis [5].A recent report from the World Health Organization indicates that 3.3 million deaths (6% of all global deaths) are attributable to alcohol use alone [17].Thus, CLD represent a major, unneglectable global health burden [11].Therefore, we need to understand the influencing role of several regulators in the onset of CLD.Estrogens have an influencing role in the progression of CLD.Therefore, the purpose of the review is to discuss the role of estrogens on several forms of liver diseases.
Estrogen: synthesis, receptors, and function
Estrogens are regulators of sexual function and also play a significant role in various pathophysiological processes [18].Three types of estrogens are naturally produced in the female body.Firstly, 17β-estradiol (estradiol or E2) is endogenously synthesized by the ovary from cholesterol in large amounts in premenopausal women.Secondly, estrone (E1) is a metabolite of E2 considered less potent than its parent compound.It is normally secreted in women when ovary ceases producing E2 secretion in the postmenopausal stage.Thirdly, estriol (E3) is also considered a metabolite of E2 and it is synthesized in small quantities than E2 and E1 by the placenta during pregnancy [19].Among these three hormones, E2 is the most potent estrogenic hormone due to its high affinity for estrogen receptors (ER).Estrogens act on its receptors i.e.ER-α, and ER-βnuclear receptors and membrane-bound G protein-coupled estrogen receptors (GPER or GPR 30) [20].All these receptors are predominantly expressed in reproductive organs such as prostate, ovary, uterus, testis, and breast and also these subtypes are highly expressed in the liver of humans in both sexes and also in experimental animals [21].Estrogenic hormones have been considered for several decades only as sexual function regulators.The myriad biologic response produced by estrogenic hormones is not limited to effects on reproduction.Estrogenic hormones have a non-reproductive role on immunomodulatory, growth, neuronal function, and metabolism [22].ER-αis the major ER expressed in the liver and impaired ER-αfunction was implicated with obesity and liver associated metabolic dysfunctions [22 , 23].
Role of estrogen in liver diseases
To our knowledge, in clinical studies, the role of estrogen metabolism in the liver was first described by Long and Simmons in 1951.They reported that endocrine abnormalities can lead to liver dysfunction [24].Later, Kappas postulated that estrogen could aggravate liver injury in 1967 [25].After the identification of ER,the role of estrogen in liver diseases has been explored.Estrogens play a key role in maintaining homeostasis and make the liver less susceptible to several diseases in healthy premenopausal individuals [26].This review discusses the critical roles of estrogens in different forms of CLD.The modulatory role of estrogen in the liver is depicted in Fig.1.
Hepatic fibrosis
Estradiol is a major factor of sex-based disparity in the progression of hepatic fibrosis which is more common in men than in women, and this intrinsic sex difference is attributed due to presence of E2 in women [27].In experimental studies, E2 administration protected the liver from hepatic fibrosis by down-regulation of the hepatic collagen, hyaluronic acid andα-SMA expression in male and female rats [28 , 29].It has been observed that the fibrogenic response of the female liver to carbon tetrachloride (CCl 4 )administration was significantly weaker than that of the male liver due to the physiological level of estrogen present in females indicating the sex-associated disparities in the onset of fibrosis and this has been confirmed in ovariectomy (OVX) rats, in which CCl4administration induced prominent hepatic fibrosis than the female rats with intact ovary [29].In a similar study, increased ER expression was correlated with the protective effect of E2 on CCl 4 -induced fibrotic rats [30].In a dimethylnitrosamine (DMN) and pig serum-induced hepatic fibrosis models, administration of E2 at gestation related doses (0.033, 0.33, and 3.3 mg/kg/day) for 2 weeks resulted in significant decrease in the collagen content,α-SMA and desmin expressions indicating the probable antifibrotic effect of E2 and intrestingly, E2 treatments also inhibited the transformation of quiescent HSCs into myofibroblasts in the injured liver [31].It was demonstrated that the fibrogenic response in the male liver was stronger than that in the female liver after a single dose of DMN administration, and in contrast, female rats with OVXhave developed prominent fibrosis.In this model, E2 treatments reduced lipid peroxidation, tissue inhibitor of metalloproteinase-1,and deposition of type I and III fibril forming collagens [32].These data showed that estrogens could reverse the oxidative stressinduced activation of HSCs, the myofibroblast-like phenotype of activated HSCs and fibrosis markers against profibrogenic agents induced experimental fibrosis.A randomized clinical trial in postmenopausal patients reported the profibrogenic effect of tamoxifen, an anti-estrogen, was due to induction of profibrogenic cytokine i.e., transforming growth factor-β(TGF-β) [33].Thus, tamoxifen treatment during postmastectomy radiotherapy enhances the risk of fibrosis due to an antagonistic effect of estrogen.Therefore, Bissell [34]inan editorialon Yasudaetal.[32]suggested the importance of analyzing the TGF-βin antifibrotic studies with HSCs.However, in a study, tamoxifen did not interfere with TGF-βdownstream Smad signaling, in contrast, it blocks non-Smad signaling through ERK1/2 MAP-kinase and the AP-1 transcription factor FRA2 and thereby inhibits the TGF-β-mediated activation in myofibroblasts [35].Interestingly, treatment with diarylpropionitrile, a selective ER-βagonist inhibits activation and proliferation of HSCs thereby attenuates liver cirrhosis.In this study, the antifibrotic effect of estrogen was mainly mediated via ER-βagonists rather than ER-αand GPER agonists [36].In view of cirrhotic complication, in female rats, administration of E2 attenuates endothelin-1 induced portal hypertension [37].In another study,E2 enhances the production of nitric oxide by sinusoidal endothelial cells in the cirrhotic liver and reduces portal hypertension in rats [38].Moreover, E2 treatment upregulates the miR-19b expression thereby inhibits the proliferation of HSCs [27].Previous studies have shown that downregulation of miR-29a and miR-29b in livers of male mouse is associated with the early progression of hepatic fibrosis [5 , 39].E2 administration protects the liver via miR-29 induction in CCl 4 -induced hepatic fibrosis in mice [40].In a study, saikosaponin-d, a plant-derived triterpenoid saponins suppressed oxidative stress-mediated HSCs activation via modulation of ER-β[41].However, over several decades the role of estrogen in CLD is ambiguous and it has been reported that increased estrogen levels were found in the liver microenvironment and endocrine disturbance in patients with CLD [22 , 26 , 42].Clinically, increased serum E2 levels have been reported in patients with liver cirrhosis secondary to hepatitis B [43].Interestingly, Jiang et al.[44]correlated the excess estrogen level in CLD to NF-κB mediated inflammatory induction of steroid sulfatase, which facilitates the conversion of inactive estrogen sulfates to active estrogens and eventually, excess estrogen, in turn, attenuates the NF-κB-mediated inflammation.
Nonalcoholic fatty liver disease (NAFLD)
Estrogen receptors play a key role in the regulation of metabolic diseases such as NAFLD, obesity and insulin resistance [45].Estrogen deficiency exacerbates steatohepatitis.Interestingly, in highfat and high-cholesterol diet in mice, estrogen treatment improved inflammation, liver injury, and serum cholesterol level [46].Impaired estrogen signaling is associated with several metabolic diseases and the anti-lipogenic role of ER-αis well established especially against NAFLD.Loss of estrogen signaling in the liver caused oxidative stress induced by low levels of peroxisome proliferatoractivated receptor-γcoactivator 1α, which aggravates steatohepatitis induced by high fructose and high-fat diet in mice [47].ER-αseems to protect the liver from hypercholesterolemia [48]and it has been clinically correlated with a previous study in which a male patient without functional ER-αhas been reported with dyslipidemia indicating the fact that estrogens regulation on cholesterol homeostasis is important in the liver [49].Interestingly, it was found that OVX in rats did not alter the ER-αexpression in the liver rather it significantly increased after E2 treatment when compared to normal endogenous levels in rats with intact ovary [50].In the OVX model, ER agonists were studied previously against NAFLD induced by high-fat diet and such studies have also shown that increased levels of liver triglyceride and diacylglyceride in ER-αknockout male mice under high-fat diet feeding [46 , 51].E2, ER-α, and ER-βagonists treatments reduced triglyceride accumulation in high-fat diet induced liver of rats with OVX [52], suggesting that activation of ER could reduce lipid accumulation in the liver.These experimental studies were well correlated with clinical studies in which, the occurrence of NAFLD is highly common in postmenopausal women due to the lowest level of estrogen than in premenopausal women suggesting the protective role of estrogens in NAFLD [20 , 53].Furthermore, a previous study showed that serum E2 levels were associated with the presence of NASH in postmenopausal women [54].ER-αseems to have an antilipogenic effect while the ER-βrole in the liver is not consistent.For instance, a study from Foryst-Ludwig et al.[55]reported that ER-βdeficient mice have higher body weight but lower liver weight due to increased insulin sensitivity and decreased triglyceride accumulation in the liver, indicating that ER-βmight be lipogenic and diabetogenic in the liver.In contrast, Weigt et al.showed the anti-lipidemic property of ER-β[52].Therefore, it has to emphasize that ER-βfunction in the liver is ambiguous and its role in NAFLD is still subjected to debate and further studies are warranted on these lines.
HCC
The chronic HBV and HCV infections are major risk factors for the development of cirrhosis.It is estimated that chronic HBV is etiologically implicated in 50% to 80% of all HCC cases [56].The chronic infection rate after acute infection with HCV is 75% to 85%,with 60% to 70% developing CLD, which leads to cirrhosis in 5% to 20% and 1% to 5% dying from liver failure or cancer [56].Cirrhosis and liver failure occurs in 11% −20% in NASH patients [57].Clinical observations show that HBV-associated HCC tends to be higher in males and postmenopausal females than menopausal females [58].The HBV, chronic HCV infections seem to develop more quickly in men than in women.These observations suggest that estrogens may influence the development of chronic hepatitis.Clinically,Iyer et al.[59]confirmed an altered expression of ER subtypes in the liver that contribute to the progression of cirrhosis and cancer development during HCV-pathogenesis.In contrast, a recent study [60]reported a higher estrogen level in cirrhotic women with HCV infection.
Ample evidence reveals that the sex disparity seems to be mediated by the stimulatory effects of androgens and the protective effects of estrogen in the development and progression of HCC.Therefore, HCC is more frequently observed in men as compared to women [61].The protective role of estrogens in HCC was reported widely and clinical data also confirm the increased HCC morbidity and mortality among males [62], which could be due to the presence of estrogen hormones in females.Mechanistically,nuclear translocation of ER-αand ER-βproteins and subsequent activation of NF-κB were noticed in patients with HCV infections and its relation to HCC.Therefore, it is suggested that ER-α/ER-βmodulator could be a therapeutic drug candidate to use in HCC patients [59 , 62].A previous study showed an increase in the liver mRNA expression of ER-αand ER-βreceptors in patients with chronic HCV infection-related HCC [59].There was a positive correlation observed in the increased sub-cellular expression of ER subtypes with the expression of proinflammatory and cancer markers [59].Therefore, modulation of ER expression in the liver is responsible for the progression of HCC related cirrhosis and HCC.ER-αgene is reported as a tumor suppressor and is expressed inthe liver tissue.The ER-αpromoter hypermethylation has been reported in HCC [63].In view of the above study, modulation of ER-αexpression in males with HCC indicate that males are highly susceptible to the HCC development and this finding paves the way towards the use of ER-αmodulators as a potential anti-HCC therapy.Studies have also confirmed the oncogenic and anticancer potential of estrogens in HCC [59,62,64].It has been hypothesized that the liver expresses different ER isoforms in various stages of liver disease.ER also regulates various signal transduction process and one or more single nucleotide polymorphisms ofER-αandER-βgenes are related to the increased risk in the development of HCC [65].The positive rate of ER-αand ER-βexpressions in the liver of Budd-Chiari syndrome-related HCC was 71.1% and 68.4%respectively and it was said to be higher than that of HBV related HCC [64].The mechanism of estrogen-mediated anti-oncogenic signaling and its action on ER in HCC progression are unclear and controversial.Therefore, further studies are warranted on these lines.
Current scenario and future directions
In early 20 0 0, several experimental studies were started exposing the sex disparity and antifibrotic properties of estrogens.Since then several studies reported the protective role of estrogen in the progression of various CLDs.In contrast, a study by Serin et al.reported increased estrogen levels in CLDs [43].Therefore, several issues need to be addressed before coming into any conclusion.Hence, at this juncture, we do not know the exact role of ER-βon lipogenesis whether it has lipogenic and dyslipidemic properties.Are ER-αand ER-βexpressions disease-specific? For instance, in-creased ER-αand ER-βwere observed in the liver of patients with Budd Chiari-syndrome related HCC than those of HBV related HCC.Further, most of the studies reported only with membrane-bound ER and estrogens action on GPERs has not been studied widely.Several such questions remain unanswered and results were inconclusive.The critical role of estrogen in the onset of liver disease is presented in Fig.2.
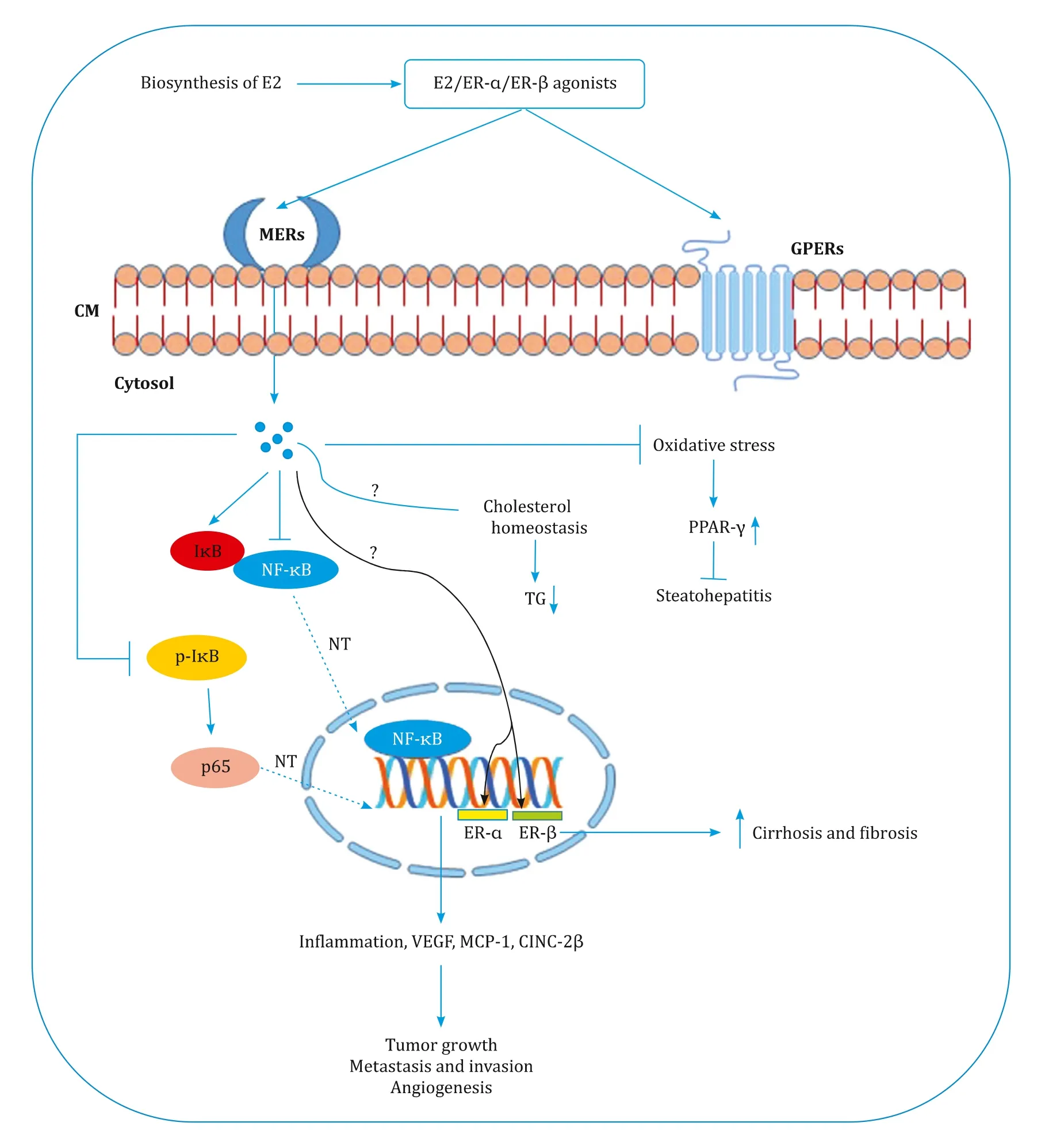
Fig.2.Modulatory effect of estrogen in the progression of chronic liver diseases.E2: estradiol; ER: estrogen receptor; MERs: membrane estrogen receptors; CM: cell membrane; GPERs: G protein-coupled receptors; TG: triglyceride; NF- κB: nuclear factor kappa-light-chain-enhancer of activated B cells; I κ B: inhibitory proteins of κ B family;p-I κB: phosphorylated I κB; NT: nuclear translocation; PPAR- γ: peroxisome proliferator-activated receptor gamma; VEGF: vascular endothelial growth factor; MCP-1: monocyte chemoattractant protein-1; CINC-2 β: cytokine induced neutrophil chemoattractant 2 β.
Conclusion
The present review confirms the imperative role of estrogen in various forms of CLDs.Several studies reported the protective role of estrogens in CLDs and this has been widely accepted and confirmed using OVX models in female rats.However, in a few clinical studies, increased estrogen levels are also implicated in CLDs.Therefore, further studies are warranted at molecular level to explore the role of estrogen in various forms of CLDs.
Acknowledgments
I thank Dr.K.Madhavi Latha (Hyderabad, India) for language editing.
CRediT authorship contribution statement
Devaraj Ezhilarasan:Conceptualization, Formal analysis,Methodology, Project administration, Resources, Software, Validation, Writing - original draft, Writing - review & editing.
Funding
None
Ethical approval
Not needed
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatobiliary&Pancreatic Diseases International
- No difference in mortality among ALPPS, two-staged hepatectomy, and portal vein embolization/ligation: A systematic review by updated traditional and network meta-analyses
- Telomerase reactivation is associated with hepatobiliary and pancreatic cancers
- Robotic isolated partial and complete hepatic caudate lobectomy: A single institution experience
- C -C motif chemokine ligand 16 inhibits the progression of liver cirrhosis via inactivating hepatic stellate cells
- Dynamic expression of hepatic GP73 mRNA and protein and circulating GP73 during hepatocytes malignant transformation
