Telomerase reactivation is associated with hepatobiliary and pancreatic cancers
2020-10-23VitoSnsoneMrcoLeGrzieJennyRoselliSimonePolvniAndreGlliFrncescoTovoliMirkoTrocchi
Vito Snsone Mrco Le Grzie Jenny Roselli Simone Polvni Andre Glli Frncesco Tovoli Mirko Trocchi
a Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy
b Department of Experimental and Clinical Biomedical Sciences "Mario Serio", University of Florence, 50139 Firenze, Italy
Keywords:
ABSTRACT
Introduction
Telomeres are the terminal DNA sequences on chromosome ends, composed of repeated hexameric sequences of the TTAGGG bases, protecting chromosome ends from the attack of exonuclease and ligase.These sequences are continuously shortened by a defective activity of the DNA-dependent DNA-polymerase in replicating the 5 ′ end of the DNA sequence; telomeres shorten at every cellular division, and repeated divisions reduced telomeres until to a critical size which signals the cell to stop dividing, thus becoming senescent.Telomerase is an enzyme, a ribonucleoprotein that partially corrects this error, synthesizing the telomeric DNA onto chromosomal ends, using a segment of its RNA component as a template.When its activity is impaired, DNA sequences are shortened.Telomerase is composed of an RNA component(TERC), which serves as a template for the synthesis of telomere sequences, and the reverse transcriptase (TERT), the catalytic component [1].Telomeres are the mitotic clock of human somatic cells, as the shortening in length is associated with the decrease of cellular division, thus functioning as a “counter”of their division cycles [2 , 3].
While in normal cells the loss of telomeres occurs gradually,in neoplastic ones this process is faster, and causes genetic instability, leading to the immortalization of those cells.Telomere dysfunction could lead to mitotic disturbances, giving rise to chromatin anaphase bridges and multipolar mitosis, causing genomic imbalances and chromosomal rearrangement [4].Telomeres are stabilized at a length that depends on a balance between the loss of nucleotides at each cycle and the telomeric elongation by the enhancement of the telomerase activity -there is speculation on the fact that telomerase is repressed in normal, somatic cells to reduce the probability of cancer.TERC, for instance, is ubiquitously expressed in humans, while TERT expression is suppressed,and is the limiting factor in telomerase activity in most somatic cells.Overexpression of TERT immortalizes a variety of primary human cells [1].However, the maintenance of telomeres and the activity of telomerase is of critical importance in the development of tumors, as cancer cells have short but stable telomerelength, maintained in such state by the reactivation of the telomerase [2]( Fig.1 ).
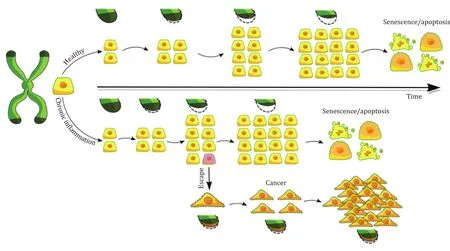
Fig.1.Review healthy and inflammation telomerase pathways.
In the last decade, a limited amount of data has been published on the role of telomere regulation in hepatic, pancreatic or gut pathophysiology.This review aims to recapitulate the present understanding of telomere maintenance and telomerase activity during hepatobiliary and pancreatic diseases and their relationship with cancer progression.
Liver
Many studies have been conducted on hepatic diseases,especially in hepatocellular carcinoma (HCC) and in cirrhosis.
HCC
HCC has recently become the second cause of cancer death:therapeutic options right now are expanding [5], however, the prognosis is dismal in advanced stages and prompt surveillance is essential in reducing the disease mortality.Currently, surveillance in the population at risk is based on ultrasonography: no serum test is recommended in current guidelines, thus having less diagnostic tools useful in primary prevention [6].
Gathering studies on this subject presents a fundamental bias,consisting mainly of the different etiologies of liver damage:hepatitis C virus (HCV), hepatitis B virus (HBV) and alcohol are the most frequent agents damaging the liver in the articles on the subject, but there are many doubts on the representation of the correspondent quota of these etiologies in real-life situations, due to the small sample size of the studies.
Progressive telomere shortening in advancing liver lesions from cirrhosis through dysplasia to HCC has been found.Telomerase activity is not detected in normal liver tissues, and only rarely in liver cirrhotic and in chronic hepatitis tissue (HCV, HBV as well as virusnegative cases), but almost universally detected in HCCs.Telomere length shortens with the progression of the disease towards HCC.Oh et al.found that telomere length was ranging between 7.3 and 9.2 kb in normal liver, 6.7 and 9.4 kb in chronic hepatitis, 4.6 and 10.5 kb in cirrhosis, 4.2 and 9.2 kb in regenerative nodules,3.6 and 5.1 kb in low-grade dysplastic nodules, 2.9 and 5.1 kbs in high-grade dysplastic nodules, and 3.4 and 9.3 kbs in HCCs [7].
Telomere shortening could be not only a representation of cellular aging but a prerequisite for the development of malignancy, as some of the telomeres critically shortened may suffer changes from genetic instability: most of those cells could become senescent, some of them, instead, upregulate telomerase,progressively immortalizing in this process.Telomeres reach a critically short length and lose their “capping”function at the ends of chromosomes, causing DNA-damage like responses involving the p53 pathway [1].Viral proteins could have a role in this process, especially HBV ones, that inactivate p53 oncosuppressor [2].Therefore, steady, stable and short telomeres, with an increased telomerase activity characterizing dysplastic nodules, are in fact the signs of a precancerous lesion, considering the high telomerase activity and short telomeres that are almost always present in HCC nodules.Those two are considered early hallmarks of hepatocarcinogenesis [7].
It was experimentally demonstrated that p21 and p16, markers of cell cycle regulators (respectively of p53 and Rb) are elevated in cirrhosis and large cell changes dysplasia, while decrease dramatically in small cell changes dysplasia and HCC.Their absence causes cell cycle blocks, and the loss of DNA damage checkpoint,thus leading to major chromosome instability [8].
Moreover, an increased rate of telomerase mutation in patients with liver cirrhosis compared to non-cirrhotic patients was reported, as it could imply a reduced telomerase activity and a deficit in telomere maintenance, predisposing to cirrhosis development in consequence of chronic liver damage [9].In an experimental model of liver fibrosis, conducted by Rudolph et al.,TERC knockout gene developed more pronounced fibrosis [10].Regarding carcinogenesis, telomere shortening causes chromosomal instability that leads to the development of HCC in patients with various underlying etiologies.Instability of chromosome 8 causes overexpression of theMYCgene that transactivates the TERT promoter and the telomerase activity itself.p53 has a proven interaction, as in cases of p53 deficiency hepatocarcinogenesis is attenuated when telomerase activity is reduced, while deletion of p53 accelerates carcinogenesis in TERC-deficient mice [11].
In non-tumor cells of the microenvironment surrounding HCC,significant attrition of telomeres and increased telomerase activity in cancer-associated fibroblasts (CAF) was observed, in relation to larger tumor size and the presence of vascular invasion.Shortened telomeres in tumor cells or CAFs were associated with reduced survival and increased recurrence and were identified as independent predictors of HCC patients [12].Similarly Park et al.reported that the telomerase activity in cirrhotic tissue surrounding HCC was significantly lower compared with the one reported in HCC tissues.In non-tumor liver tissue it was reported an enhancement of the telomerase activity, more evident in cirrhosis than in chronic hepatitis.It is inferred that the tumor cells with no activation of DNA polymerase are due to tissue factors inhibiting the activity of the Taq DNA polymerase used in the PCR [3].In dysplastic, precancerous nodules a similar degree of telomerase activity was reported as to HCC nodules, probably occurring before microscopic features of carcinosis are recognizable [13].
Human telomerase reverse transcriptase (hTERT) mRNA levels were significantly higher in HCCs than in surrounding nontumorous livers and associated with longer telomeres, and in HCCs with high telomerase activity showed minimal shortening of telomeres, being associated with advanced tumor stage but not with prognosis.Higher telomerase activity (TA) and hTERT levels were related to an increased rate of multipolar mitoses and anaphase bridges, as unstable tumors require telomerase to prevent complete genomic deterioration [4].
TERT mRNA expression was higher in every HCCs compared to non-tumorous liver, but the mutation did not affect the mRNA expression level in HCCs [14].In the literature, many microRNA have a role in regulating telomerase activity: in particular, miR-155 [15],a silencer of pro-apoptotic molecule TP53INP1, and miR-103 [16],a silencer of tumor suppressor molecule AKAP12 were associated with higher telomerase expression and activity in tissue and blood samples from HCC patients.TERT is a target of miR-138, a tumor suppressor, which lowers its activity, inducing cell senescence.
Nault and colleagues established that TERT promoter mutation is the first and most frequent genetic event identified in the generation of HCCs, independent of the most frequently mutated genes in liver cancer similar mutations in HCCs.Mutations were found in 59% of the total HCCs, while in 44% of hepatocellular carcinoma (HCA) with malignant transformation a somatic mutation was present, associated with aCTNNB1(a gene encodingβcatenin) activating mutation, therefore indicating a role in malignant transformation [17].In a cohort of Korean patients with HBV-related HCC, the prevalence of TERT mutations were found in 39.0%of the sample, CTNNB1 in 14.6%, while 83.3% of the HCV-related HCC had TERT promoter mutations [18].
TERT promoter mutations are identified in dysplastic nodules and associated with the transformation to early HCC, while classical cancer gene transformations are associated mainly with larger nodules.Therefore, TERT promoter mutations are early events in the progressive path that leads from cirrhosis to HCC.TERT promoter mutations were associated with histological and immunohistochemical malignant features, with a fundamental role in premalignant lesions on a cirrhotic background [19].Many promoter mutations have been identified, most recently C15orf55 and C7orf43 which have been associated with neoplastic progression and poorer prognosis [20].
The rate of shortening of telomere restriction could be accelerated by oxidative stress, as well as an enhanced sensitivity of the telomeric sequence to the oxidative damage [21].Liu et al.explored the relationship between reactivation of telomerase in HCC and oxidative stress and found that telomerase activity was detected in all HCCs and correlated significantly with malonildialdehyde (MDA).Telomere shortening is accelerated by oxidative stress when telomere became too short, causing the transcription of TERT [22].
Tumor-associated macrophages (TAMs) are an important component of the immune cell populations within the tumoral microenvironment.They are considered the main actor of the cancerrelated inflammation, promoting tumor growth by suppression of effective anticancer immunity, inducing angiogenesis, and tissue remodeling.Macrophages have been assigned a classically activated (proinflammatory) M1 or an alternatively activated (antiinflammatory) M2 phenotype.During tumor progression in HCC,macrophages shift from M1 to M2 phenotype.This polarization seems not only to depend on the disease stage but also within different areas of the same tumor [23].A recent study linked the density of TAMs with the survival of patients with HCC: in particular high density of CD68 + TAMs is associated with a more advanced tumoral stage and worse overall survival.A high density of M1 predicts better survival in these patients, while a high density of M2 or CD169 + TAMs predicts worse survival [24].
To date there are not many studies linking TAMs with telomerase activity or telomerase length: different TAMs may be related to different telomere maintenance mechanisms in glioblastoma.An increased content of TAMs was associated with a poorer outcome from high-grade glioma [25].Another group seemed to correlate a reduced macrophage infiltration in bladder cancer with amplified TERT, and a poor prognosis [26].
Telomerase activity in viral hepatitis
In HBV-related HCCs, TERT could be activated by the HBx protein, by direct integration of the genome in the TERT gene promoter, or by upregulating TERT expression [14].The frequency of TERT promoter mutations was determined by sequencing the gene in a region containing already known mutations.Mutations were described in the 29.2% of HCCs and observed more frequently in groups with HCV positivity and in older patients.However, these findings were not independently associated with this mutation:this was not the case of HBsAg positive patients.Survival was similar in TERT positive and negative HCCs [14].
Correlation between telomeres length, telomerase activity and viral infections in HCC was also evaluated by Piciocchi et al.: HCV infection was associated with earlier oxidative damage and shorter telomeres (P= 0.03), while telomerase activity resulted higher in HCC patients with both HCV and HBV infections but its activity increased earlier in HCV infections than that in HBV ones.Methylation of TERT promoter was higher in tumor and peritumor tissues in both groups [27].
In HCC, HBV DNA integration into TERT locus, mostly in the HBx region, resulted in mutually exclusive to TERT promoter mutations that, on the other hand, were slightly more frequent but also associated with shorter disease-free survival and overall survival [28].A significant TERT activator was identified in PROX1, although in HBV-related HCC it does not have a pivotal role, given that the HBx expression inhibits PROX1 binding to the promoter region [29 , 30].
Therefore, TERT promoter mutation seems to be a mandatory passage in HCC development regardless of etiology, even if at different stages of neoplastic evolution.This hypothesis seems confirmed even by Kim et al., who found this alteration in a high percentage of NAFLD related HCC (82%), in association with a high frequency of chromosome 8p loss [31].
Integration of HBV and human papilloma virus (HPV) in hTERT gene has been studied in liver and cervical cancers.Both viruses’actions are associated with small deletions at the site of integration that do not interfere with its coding sequence.HBV, more specifically its enhancer sequence, integrates into the promoter region of the gene and transactivates hTERT.This gene is more prone to viral insertion without the production of large deletions.Tumor phenotype may be determined by the site of viral integration [32].
Chromosome 10 has been identified as a preferential site of integration of HBV genes and hTERT —correlating with its overexpression and de-stabilizing cellular homeostasis.The region in which the HBV genome transfects into the promoter results in a c-terminated truncated HBx protein that blocks apoptosis and enhances the cellular proliferation [33].HBc, as well, enhances HCC cell proliferation and upregulates hTERT expression linking in c-Ets2 binding site, thus enhancing the promoter activity and resulting in higher expressions of hTERT in HBc-positive than those in controls [34].
Some have speculated [35]on the role of tenofovir in preventing HCC in HBV patients by inhibiting TERT activation [36], on the basis of an activity observed in HIV patients.
Diagnosis
hTERT is a known primer in the progression of HCC and its inhibition has been demonstrated as a blocking progression bothin vitroandinvivoof HCC [37].Activating pathways are multiple: cmyc expression via its binding with c-Myc/Max/Mad network [38]and binding with hTERT promoter [39].The absence of phosphatase and tensin homolog gene (PTEN) mRNA has been reportedly associated with positive staining of hTERT in HCC cells,with no coexpression of both genes [40].Moreover, genes such asRBFOX3[41]orBPTF[42]that have been linked with a worse prognosis in HCC patients: in the latter case its silencing has been linked with enhanced tumor apoptosis, therefore identifying a possible therapeutic target [42].
Telomeric Repeat Amplification Protocol (TRAP) has a high sensitivity in detecting telomerase activity in tissue extract.However,given the different cell types and population involved, attempts in better assessing the telomerase expression indirectly have been performed withinsituhybridization of telomerase reverse transcriptase (hTERT) mRNA and telomerase RNA (hTR), two major components of telomerase, but none of those correlated strictly with telomerase activity: the first one is widely expressed, while the second, even if closely related, presents more variability due to alternative splicing and post-translational modifications [43].
InsituTRAP assay (ISTRAP), allowing direct visualization of telomerase activity in tissue sections has been compared to the traditional TRAP assay.85% of tested HCC were positive for ISTRAP,mostly with zonal distribution.A direct comparison between the two techniques showed substantial correlation [43].
Presence of hTR and hTERT mRNA in serum has been ascertained in breast cancer, suggesting a possible role in its diagnosis.As telomerase are reactivated in HCC, Miura’s group has tried to use hTERT as a marker for this disease.
hTERT positivity rate increased gradually with disease progression, being highest in HCC with a sensitivity of 89.7% and a specificity of 63.6%.Alpha-fetoprotein (AFP), tumor size and differentiation degree of HCC were significantly and independently associated with hTERT expression [44].Later, a new PCR assay, more sensitive and specific in the detection of hTERT mRNA in HCC diagnosis, proved to be superior to AFP especially in early HCC diagnosis.Moreover, mRNA expression was associated with HCC differentiation.Most importantly mRNA in sera correlated with the one in HCC tissue [45].Serum TERT mRNA has been searched in HCC patients for early diagnostic purposes.PCR methodic was reported to be sensitive in the detection of tumor-derived hTERT mRNA, with high sensitivity and specificity.A correlation between the size of the tumor, AFP and hTERT has been reported, as well as one between the AFP and a tumoral burden>2 cm [46].
Observed data, together with technological progress, allowed to implement the evaluation of TERT in patients affected by HCC in a clinical diagnostic and prognostic context: the assessment of circulating tumor DNA (ctDNA) allows to identify TERT mutations in these patients, with potential therapeutic and prognostic consequences [47].In particular, despite the quantitative level of ctDNA based on hTERT as analyzed seems to be unrelated to the probability of a relapse of HCC after treatment, the evaluation of ctDNA looking for specific alterations of genes involved in carcinogenesis as TERT, showed a significant relation between the presence of tumor-associated mutations and a higher probability of relapse of HCC after surgical treatment [48].On the basis of these data, it is possible to affirm that the more in-depth understanding of the role of TERT and its mutations in the context of ctDNA in patients affected by HCC may lead to its more appropriate use as diagnostic tool in patients under follow-up for hepatic cirrhosis and for the evaluation of the therapeutic outcome of HCC.
Treatment perspectives
Townsley et al.observed the therapeutic effect of Danazol(400 mg twice daily), a male hormone, in lowering the attrition rate of telomeres in a sample of 11 of 12 men (92%) with known telomere-associated disease and obtained a hematological response in 79% of patients [49].
Acyclovir, a nucleotide analogue highly selective for virusinfected cells is often paired with adenovirus carrying herpesvirus thymidine-kinases, which impedes the DNA replication.Acycloguanosyl 5 ′ -thymidyltriphosphate (ACV-TP-T), its prodrug, is a telomerase substrate that inhibits tumor growth.Interestingly the hTERT modulation influences the tumor cell susceptibility to ACV-TP-T: transfection of a dominant-negative hTERT gene increased the half maximal inhibitory concentration (IC 50 ) of the prodrug, thus showing a diminished use of the molecule.ACV-TP-T blocks the targeted cell cycle, inducing apoptosis, and has a selective action, inducing the regression in transgenic models of HCC.Telomerase, a prominent feature of many HCCs, can be used as a therapeutic perk to counter the proliferation of the same tumoral cell.An association with sorafenib is pictured, even if statistically significant results cannot be obtained [50].
Furthermore, it is interesting to note that some drugs, such as Azidothymidin [51]and PI3Kδinhibitors [52], despite not appearing to have a direct effect on TERT, have shown promising results in murine models, also presenting a reduction of TERT activity itself.It is therefore important to understand the involvement of TERT in the carcinogenesis pathway of HCC, given the pivotal role that it seems to have, with a subsequent therapeutic potential.
Intrahepatic cholangiocarcinoma
In a small series of surgically resected intrahepatic cholangiocarcinomas (ICCs) plus biliary epithelial dysplasia, mRNA of hTR and telomerase-associated protein 1 (TP1) were present in 85% of ICC cells, in biliary epithelial dysplastic cells while it was absent in normal biliary epithelial cells.This finding leads to the hypothesis that telomerase mRNA expressed by neoplastic and preneoplastic lesions of the hepatobiliary tree detected in endoscopic retrograde cholangiopancreatography (ERCP) brushing samples could be an important diagnostic tool in the early diagnosis of ICC [53].
Transfecting the HBx gene in HCCs and ICCs cell lines resulted in upregulation of hTERT expression and subsequent telomerase activity.HBV genome integration upstream of the hTERT promoterand the upregulation of the telomerase caused an increased hTERT expression.Both cell lines, when transfected, expressed increased hTERT mRNA.It could be inferred that HBV contributes to carcinogenesis in two ways, first activating proto-oncogenes by integrating in the promoter (in the case of HBV genome), a newly found model of HBV carcinogenesis, and secondly when virus protein HBx directly contributes to cell transformation and carcinogenesis, suppressing p53 and Rb pathways, and upregulating hTERT promoter activity [54].
The role of common TERT promoter mutations is arguably less important: the most common mutations (C124T and C146T) were not detected in a sample of 55 patients, probably indicating a different activation method for the telomerase [55].In another large study comprising 799 samples from 13 different tumors, only 9 ICC samples were obtained, however, with no TERT promoter mutation [56].
In combined hepatocellular-cholangiocarcinomas (CHC) and ICC in liver cirrhosis: while CHC presented alterations of TERT in 80%of samples, TERT mutations were identified only in a single case of ICC, demonstrating, once again, that genomic profiles of HCC and ICC present some relevant differences, even regarding telomerase activity [57].
An interesting development was retrieved while fabricating an ICC cell line, ETK-1, a stem-cell-like that can be transdifferentiated on a hepatocyte lineage: the telomerase activity measured by TRAP assay was reduced to nearly 60% from the starting cell line.Other cell lines, while having a different maturation stage, had lower hTERT activity while advancing with their maturation stage [58].
What about therapeutic perspectives? As discussed above, to date it is difficult to propose therapeutic options that could interfere with the telomerase pathway.Jo et al.evaluated the role of sodium meta-arsenite (KML001), an anticancer agent that acts on the telomerase pathway, on biliary tract cancers refractory to gemcitabine.Only 3 of the 44 enrolled patients had a progression-free disease after 1.5 months of therapy and, of these,only 1 patient completed 6 treatment cycles without disease progression [59].
Pancreas
Alterations in telomerase expression and activity have been shown to be involved in the physiopathology of pancreatic diseases.
The growing interest in this enzyme and related pathways led to deepening our knowledge about the development and function of both endocrine and exocrine pancreatic cells.As telomerase is universally involved in mechanisms of cellular proliferation and tissue regeneration, its activity appears to be involved even in the physiologic mechanism of damage repair of exocrine cells: von Figura et al.have studied pancreatic regeneration after cerulein-induced pancreatitis in telomerase knockout rats, reporting alterations in the regeneration of exocrine tissue compared to wild type rats with subsequent metaplasic acinar cells persistence,and absence of proliferation were noticed [60].
Benign pancreatic diseases
Unfortunately, little evidence regarding the physiopathological role and possible therapeutic use of telomerase in benign pancreatic diseases are available.An interesting field of research could be diabetes mellitus.Only one study on animal model deficient for TERC subunit of telomerase has suggested its involvement in impaired glucose-stimulated insulin secretion (GSIS)associated to reduced pancreatic islet size, probably due to an impaired replicative capacity of insulin-producing beta-cells in these mice [61].Thus, it is possible that hopefully, future studies may lead to the development of new drugs for the treatment of this pathologic condition that precedes diabetes.
Malignant pancreatic diseases
Regarding pancreatic neoplasms, significant evidence of telomerase involvement in tumor progression and its possible use in diagnosis and therapy were evaluated.It is likely that the same proliferative boost caused by telomerase in pancreas development and regeneration could be pathologically altered in neoplastic progression of pancreatic cells.Weng et al.[62]evaluated TERT expression in human pancreatic duct cell adenocarcinoma cell line AsPC-1in vitroandinvivoas a xenograft implanted in severe combined immunodeficiency (SCID) mouse.These cells were characterized by the overexpression of CD133, a transmembrane protein which is used also as a marker for normal stem cells and that is overexpressed in some tumor histotypes.CD133 cells showed properties of induced stem cells, added to a significantly increased proliferative rate, tumorigenic power, angiogenetic activity, migration and resistance to chemotherapics.Thus, Weng suggested that the stem cell phenotype of this cell line could be related the role of CD133 not only in activation of EGFR, but also in enhanced telomere transcriptase expression, and as a transcriptional modulator of the Wnt-beta catenin signaling pathway.
Further studies showed the impact of genetic predisposition based on TERT mutations for pancreatic cancer development in the human population.TERT variant rs401681 has been shown to be an SNP related to a higher risk of pancreatic cancer [63].Another SNP coding for another TERT variant (rs2853677) was found to be associated with higher risk of pancreatic cancer independently from rs401681 in patients affected by pancreatic adenocarcinoma in comparison to controls taken from PANDoRA and Pan-Scan consortia, suggesting a potential usefulness of TERT as a genetic marker and as a prognostic tool for this class of neoplasia.
Pancreatic endocrine tumors
It is important to highlight that research studies paid more focus on pancreatic adenocarcinoma, the most frequent type of pancreatic neoplasia, while little information is available on other kinds of pancreatic tumors, as results indicated less telomerase involvement and potentials clinical usefulness.Conflicting evidence about pancreatic endocrine tumor (PETs) is available.In a first study [64]done on 10 histological samples of PET, Lam et al.found an increased telomerase activity in more aggressive pathologies characterized by vascular and perineural infiltration, suggesting that telomerase activity might be useful for distinguishing between benign and malignant PETs.On the other hand, a second study [65], based on the evaluation by TRAP of 30 samples obtained from frozen surgical archived neoplastic tissue, showed only three samples with high telomerase activity initially defined as “non-functional endocrine tumors”, two of which associated to extra-pancreatic disease.Subsequently, all three telomerasepositive cases were reclassified as either acinar cell carcinoma(two cases) or mixed acinar-endocrine cell carcinoma.The authors stated that telomerase activity could help in identifying acinar tumors simulating PET; in the second place, they suggested that absence of telomerase activity in confirmed PETs could be responsible for their clinical indolence.In conclusion, it is not clear whether telomerase activity is involved in PETs themselves or if its increase is a step to evolving into a mixed form of pancreatic neoplasia.
Pancreatic adenocarcinoma
In regard to pancreatic adenocarcinoma, far more evidence is available about telomerase relevance even in the related preneoplastic lesion.The importance of this evidence is related to the difficulty in distinguishing intraductal papillary mucinous tumor of the pancreas (IPMN) from adenocarcinoma; of course, these two diseases have a very different therapeutic approach and prognosis, thus it would be important to develop systems capable of defining nature of suspected malignant lesions before surgery.As first, a study tried to define the clinical role of telomerase activity evaluation by TRAP on pancreatic juice obtained via ERCP in case of suspected malignant lesions.Compared to cytology [66]:pancreatic juice was collected from 28 patients (13 with intraductal carcinoma and 15 with adenoma), and two samples of pancreatic juice were collected from each patient to compare examination by cytology for Papanicolaou staining and by TRAP assay; this study revealed a low accuracy (31%) of cytology alone for diagnosis of intraductal papillary mucinous tumor (IPMT) and showed subsequently how TRAP could raise accuracy up to 81%.Once confirmed the importance of telomerase in development of pancreatic lesions, the subsequent step regarded trying to figure out at which stage of IPMN progression mutation in telomerase occurs.Hashimoto et al.[67]analyzed telomerase expression and telomere length in 68 IPMN samples in comparison to 15 samples of adenocarcinoma and 10 samples of chronic pancreatitis, finding out a significant difference (P<0.001) in telomere length between borderline IPMN andinsituIPMN with an increasing telomerase expression beginning from borderline form (P= 0.02 for carcinomainsituvs.borderline IPMNs,P= 0.002 for invasivevs.carcinomain situIPMNs).The relevance of TERT mutation in IPMN progression was suggested also by Nissim et al.[68]who conducted a metaanalysis to define genetic mutations involved in malignant transformation of IPMT: this meta-analysis evidenced a strong association of malignant IPMN with hTERT upregulation, while none of the other examined mutations appeared to be similarly associated to disease progression.
Telomerase and diagnosis of pancreatic lesions
Considered the characteristic telomerase activity in pancreatic lesions, this enzyme was evaluated as a possible diagnostic tool for pancreatic adenocarcinoma.Two studies [69 , 70]investigated telomerase activity by TRAP in this neoplasia, comparing neoplastic tissue with samples of the normal pancreas, pancreatitis and benign pancreatic neoplasia: as expected, telomerase activity was significantly increased in malignant neoplasia, in particular, Pearson et al.[69]found five telomerase-positive "normal"specimens, suggesting that telomerase expression could be an initial step of microinvasion of surrounding normal tissue by adenocarcinoma.Then, Büchler et al.[71]introduced telomerase mRNA expression by real-time PCR as a more accurate neoplastic marker: they detected a significant correlation between telomerase mRNA expression and pancreatic adenocarcinoma while no relationship was found between telomerase activity and the fold increase of telomerase mRNA, indicating that detection of telomerase activity using the TRAP assay has limitations in defining malignant nature of pancreatic disorders.In particular,Büchler found a significant increase of hTERT mRNA expression in pancreatic cancer (relative increase 23.9-fold above normal) and in chronic pancreatitis (relative increase 5.5-fold above normal)in comparison with normal controls.No association was found between the fold increase of hTERT mRNA above normal and the age of the cancer patients, tumor stage and tumor grade or histology.Telomerase activity is always associated to hTERT mRNA expression, even if no association was found between the level of mRNA expression and the level of telomerase activity nor between the histologic tumor phenotype and telomerase activity or hTERT mRNA expression.Unexpectedly, further data seem to have countered this statement.Grochola et al.[72]showed a significant correlation between low mRNA expression and worse prognosis of pancreatic adenocarcinoma (P= 0.013)whereas undetectable expression showed an intermediate mortality rate.
Most recent studies suggest both telomerase activity detection by TRAP and hTERT mRNA expression evaluation by real-time PCR in pancreatic juice collected during the performance of ERCP are more accurate than cytology made on brushing samples obtained through ERCP or from surgical specimens.In particular, Uehara et al.[73]found that TRAP essay has a higher sensitivity, specificity, predictive value for positive results and predictive value for negative results in defining pancreatic cancer compared with cytology.In particularinsitutelomerase activity was described as high,low, or none according to the intensity of the fluorescence: in this evaluation, high telomerase activity was observed only in pancreatic cancer, while IPMNs showed low telomerase activity and normal pancreas was negative.On the other hand, interesting results came from Nakashima et al.[74], who found a higher sensitivity,specificity and overall accuracy of hTERT expression (85.1%, 82.1%and 84.3%, respectively) compared with cytology (47.1%, 89.3% and 57.4%, respectively) for the differentiation between carcinoma and benign diseases; he pointed out also an increase of accuracy and sensitivity combining the results of cytology and hTERT expression(87.8% and 92.0%, respectively) even if a lower specificity was noticed (75.0%).Even if no quantitative evaluation of hTERT expression was done, hTERT mRNA was found to be expressed in neoplastic disease since the stage of borderline IPMN (56%), with an increasing tendency toward to pancreatic adenocarcinoma (84%).
These data were confirmed by Hata et al.who evaluated telomerase activity by PCR in 219 surgically-aspirated cystic lesions.In their study, high-grade dysplasia + / −associated invasive cancer was associated with higher telomerase activity [median(interquartile range), 1158 (295.9-13033) copies/μL]of cyst fluid than those without [19.74 (2.58-233.6) copies/μL](P<0.001).In particular, telomerase activity was found to be higher in cystic lesion under preoperative evaluation that presented worrisome features and high-risk stigmata, with subsequent detection of high-grade dysplasia or invasive cancer after surgical resection.Telomerase activity, with a cutoffof ≥ 730 copies/μL, was defined as an independent predictor of invasive cancer/high-grade dysplasia in pancreatic cysts even in multivariate analysis (23 vs.6; odds ratio = 20.698; 95% CI: 4.195-140.94;P<0.0 0 01) [75].
To date, new molecular tests regarding telomeres are available or still under development.For example, Hata et al.developed nested real-time quantitative PCR method for telomere fusion detection in pancreatic ductal adenocarcinomas, IPMNs, and IPMN cyst fluids and tested it on pancreatic cysts fluid.The presence of telomeres fusions was significantly associated with high telomerase activity and with the presence of high-grade displasia/invasive cancer (P= 0.0414) and resulted in an independent predictor of high-grade displasia/invasive cancer by multivariate analysis (odds ratio = 6.23; 95% CI: 1.61-28.00) [76].
As all studies cited above evaluated tissue specimens obtained through invasive methods, Yamaguchi et al.[77]tried to understand the possible clinical role of telomerase activity in peripheral blood samples of patients affected by benign and malignant diseases of the pancreas, biliary ducts and liver, focusing on the possible use of this biomarker as a screening tool.However, the results were not satisfying, because in this contest telomerase activity seemed to have a possible role only in detecting aggressive and metastatic tumors, making it useless to screen clinically sane patients.The role of leukocyte telomere length is presently con-sidered but still unclear.A prospective study by Hamada’s group has identified a role of a shorter prediagnostic leukocyte telomere length in predicting a shorter overall survival of patients diagnosed with pancreatic adenocarcinoma [78].A much larger Chinese study [79]demonstrated an association between longer telomere length and a shorter survival: this discrepancy could be explained by the presence of different risk factors (such as age, body mass index, smoking) in the two populations.More studies are required on this particular topic.
New promising diagnostic methods related to telomerase activity are under development, such as C-circle essay [80], which identifies C-circle (partially single-stranded closed circular DNA molecules containing telomeric repeat tracts) levels in peripheral blood, that showed to be higher in case of alternative lengthening of telomere, and circulating tumor cells evaluation using the telomerase specific adenovirus OBP401 [81], but further studies are needed to define their clinical relevance.
In conclusion, it is possible to say that available data and subsequent systematic reviews with meta-analysis [82 , 83]recently performed suggest a possible central role of telomeres and telomerase activity in diagnostic workup and staging of pancreatic lesions.
Telomerase as a possible treatment target
Vaccination [84]with dendritic cell transfected with hTERT mRNA has been tested, showing a complete response in a case report of a patient with a post-surgical recurrence treated for three years with radiologic and biological negativity for relapse of the disease without significant side effects.Other authors thought to take advantage of elevated telomerase activity of the pancreatic adenocarcinoma absent in normal pancreatic tissue to directly target the tumor with chemotherapy [85]: in particular they developed a thymidine analogue prodrug, acycloguanosyl 5 ′ -thymidyltriphosphate (ACV-TP-T), that is metabolized by telomerase, releasing the active form of acyclovir, and evaluated its efficacyinvitroand in mice; there was a reduced proliferation and an increased apoptosis of pancreatic adenocarcinoma compared to normal pancreatic tissue bothinvitroandinvivo.
Further studies on human will probably define possible clinical usefulness of these therapeutic approaches.
Conclusion
Many factors are known to regulate telomerase activity and its subunits.Our analysis of the present publications disclosed that telomerase reactivation, overexpression of hTERT or an alteration in its physiological activity can be found in several hepatobiliary and pancreatic diseases.Interestingly this condition might be used as biomarkers for progression from precancerous lesions to cancer;furthermore, hTERT might be a predictor of poor prognosis in some class of neoplasia.On the other side, disrupting the transcriptional activation of hTERT represents a promising therapeutic target for the treatment of a subset of hepatobiliary and pancreatic diseases with a particular interest in solid cancers.
Acknowledgments
None.
CRediT authorship contribution statement
Vito Sansone:Writing - original draft, Writing - review & editing.Marco Le Grazie:Writing - original draft, Writing - review &editing.Jenny Roselli:Writing - original draft, Writing - review &editing.Simone Polvani:Formal analysis.Andrea Galli:Resources.Francesco Tovoli:Supervision.Mirko Tarocchi:Supervision.
Funding
None.
Ethical approval
Not needed.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatobiliary&Pancreatic Diseases International
- No difference in mortality among ALPPS, two-staged hepatectomy, and portal vein embolization/ligation: A systematic review by updated traditional and network meta-analyses
- Critical role of estrogen in the progression of chronic liver diseases
- Robotic isolated partial and complete hepatic caudate lobectomy: A single institution experience
- C -C motif chemokine ligand 16 inhibits the progression of liver cirrhosis via inactivating hepatic stellate cells
- Dynamic expression of hepatic GP73 mRNA and protein and circulating GP73 during hepatocytes malignant transformation
