Microbiology and risk factors for gram-positive Cocci bacteremia in biliary infections
2020-10-23IkHyunJoYeonJiKimWooChulChungJaeyoungKimSeonhooKimEunSunLimHonggeunAhnSeongYulRyu
Ik Hyun Jo, Yeon-Ji Kim , WooChul Chung, Jaeyoung Kim, Seonhoo Kim, EunSun Lim,Honggeun Ahn, SeongYul Ryu
Division of Gastroenterology, Department of Internal Medicine, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon 16247,Korea
Keywords:
ABSTRACT
Introduction
Acute cholangitis and acute cholecystitis are gallstone-related infectious diseases that are frequently encountered in the inpatient setting.A previous study reported that the hospitalization rate of these two diseases is approximately 257 per 100 000 individuals from the general population [1].In addition to their ubiquity, these disease entities are important because they pose medical and economic burden to both patients and society.For instance, a total of 1 million people are newly diagnosed with gallstone-related disease, which accounts for 5.8 billion dollars of medical expenditure annually in the United States [2].Moreover, the proportions of patients who meet the criteria for severe acute cholangitis and acute cholecystitis based on the Tokyo guidelines are 12.3% and 6.0%, respectively [3].
Clinical guidelines recommend early antibiotic therapy in most cases [ 4 , 5 ].The common pathogens in biliary tract infections are gram-negative bacteria, and other types including gram-positive bacteria and anaerobes account for a relatively minor proportion of the etiologies [6].Thereby, antibiotic agents are mainly chosen to target the causative species, based on the well-known microbial prevalence.However, in severe cases in which poor prognosis is expected, such as septic conditions,immediately using more than one type of broad-spectrum antibiotics might be helpful to improve the outcome [7].From this point of view, if biliary sepsis is caused by gram-positive pathogens, it could be assumed that rapid antibiotic administration against gram-positive bacteria will be beneficial to a patient’s clinical course.Unfortunately, current guidelines on empirical therapy do not offer enough coverage with respect to these pathogens [ 4 , 5 ].In addition, there is no proper clinical or diagnostic tool for detecting the disease in the early-stage, regardless of whether biliary sepsis is caused by gram-negative or gram-positive pathogens.
The aims of this study were to understand the spectrum of bacteremia resulting from biliary tract infectious diseases and to determine how to distinguish between gram-positive and gramnegative pathogens in the early-stage.
Patients and methods
Study population
We performed a retrospective study in patients with biliary tract infections at St.Vincent’s Hospital.Biliary tract infection was defined as the presence of all of the following three criteria: (i) fever (>37.5 °C) or abdominal pain, (ii) findings suggestive of ascending cholangitis or cholecystitis on computed tomography(CT) or magnetic resonance imaging, and (iii) hyperbilirubinemia(bilirubin level>1.2 mg/dL).
All patients who were admitted to our hospital and underwent two consecutive blood culture examinations before beginning the antibiotic treatments, and those who were identified to have bacteremia were enrolled in this study.Cases which were suspicious as a contamination were excluded according to following definitions: first, pathogens detected only once in two consecutive blood culture; second, the possibile contamination was mentioned by the laboratory report.Over 48 months (from January 2013 to December 2015), 151 patients were admitted and followed up using their electronic medical records through December 2018.We excluded patients according to the following criteria: (i) age<18 years, (ii)suspicion of contamination in the culture report, (iii) concomitant infectious diseases other than biliary system diseases, and (iv) inappropriate medical records for review.This study was approved by the institutional review board of the Catholic University of Korea (VC19RESI0191).All investigations adhered to the tenets of theDeclarationofHelsinki.
Data collection and interpretations
We inspected all patients’ demographic characteristics, including age, sex, body mass index, medical histories, laboratory findings, and results of CT within 12 h of hospital visit.The presences of gallbladder, intrahepatic duct, or common bile duct stones; the size of the stones; and common bile duct dilatation more than 7 mm on CT images were designated as variables.The number of each emergent intervention [defined as one of the following procedures conducted within the first 24 h of admission: percutaneous biliary drainage, endoscopic retrograde cholangiopancreatography(ERCP), or surgery]was collected.Further, the length of hospital stay, types of confirmed pathogens, re-admission for biliary infection up to December 2018, and time interval from discharge to readmission were also investigated.
The basic characteristics of the entire study population were analyzed.Thereafter, all patients were divided into three groups according to the type of bacteremia: gram-negative, gram-positive,or both gram-negative and gram-positive bacteremia.We attempted to determine whether there is any difference in the numerous variables among the three groups.Additionally, the same analysis was also conducted between two groups: gram-negative alone or gram-positive alone.Finally, we attempted to create a prediction model using information that was available at the time of the first hospital visit, such as medical histories or laboratory and imaging findings.
Statistical analysis
We used R Studio for data analysis (ver.1.0.153, R Foundation for Statistical computing, Vienna, Austria).Variables with a normal distribution were presented as mean and standard deviation (SD),and non-normally distributed variables were presented as median and interquartile range (IQR).The differences between two groups were analyzed using an independentt-test or the Mann-WhitneyUtest as appropriate.Categorical variables were presented as frequency and percentage, and analyzed using the Chi-square test.To predict gram-positive bacterial infection, we fit a logistic regression model, with the presence of gram-positive bacteremia as a binomial variable.A stepwise method was adopted for the optimal selection of the prediction model.APvalue less than 0.05 was considered statistically significant.
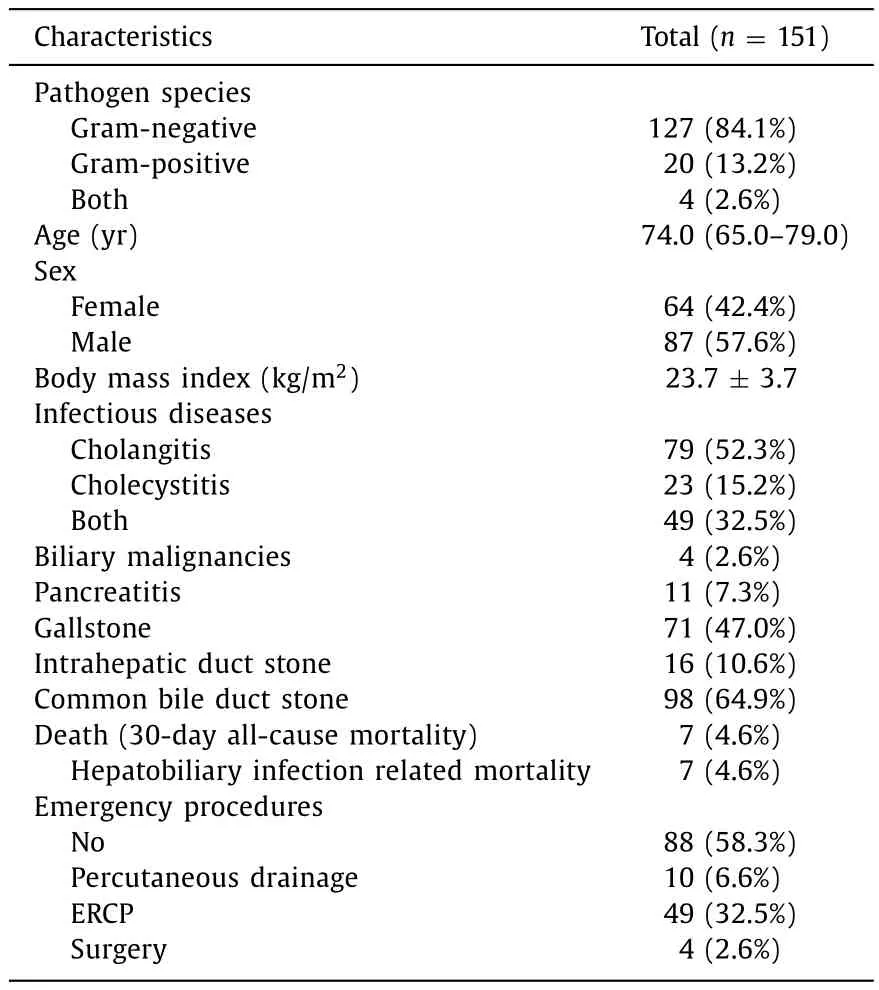
Table 1Baseline characteristics of the study population.
Results
Study population and baseline characteristics
Except for 57 contaminated patients, a total of 151 patients were eventually analyzed.The characteristics of the entire study population were summarized in Table 1.Gram-positive pathogens were isolated in 20 patients (20/151, 13.2%), whereas gramnegative pathogens were isolated in 127 patients (127/151, 84.1%).In 4 patients (4/151, 2.6%), both gram-positive and gram-negative bacteria were isolated at the same time.Candidaalbicansfungemia was detected in 2 patients, as a form of mixed infection.Coexisting biliary tract malignancies were found in 4 patients.Mortality rate for enrolled patients was 4.6%.The median age was 74.0 (IQR 65.0-79.0) years, and bacteremia was more common in male patients than in female patients (57.6% vs.42.4%).Emergency interventions were needed in 41.7% of the patients.There were no significant differences between the gram-positive isolate group and the gramnegative isolate group in clinical findings and demographic factors except previous gastrectomy (P= 0.018) ( Table 2 ).
Spectrum of bacteremia according to gram staining
The most frequently detected bacterium in gram-negative group(n= 132) of bacteremia originating from the biliary tract wasEscherichiacoli(n= 91, 68.9%), followed byKlebsiellapneumoniae(n= 31, 23.5%).In the gram-positive group (n= 24),EnterococcuscasseliflavusandEnterococcusfaecaliswere the most common cause of bacteremia (n= 3, 12.5%, respectively), followed byBacillusspecies,Enterococcusfaecium,Staphylococcusaureus, andStaphylococcushaemolyticus(n= 2, 8.3%, respectively).The distribution of all gram-positive and gram-negative bacteria was demonstrated in Figs.1 and 2.
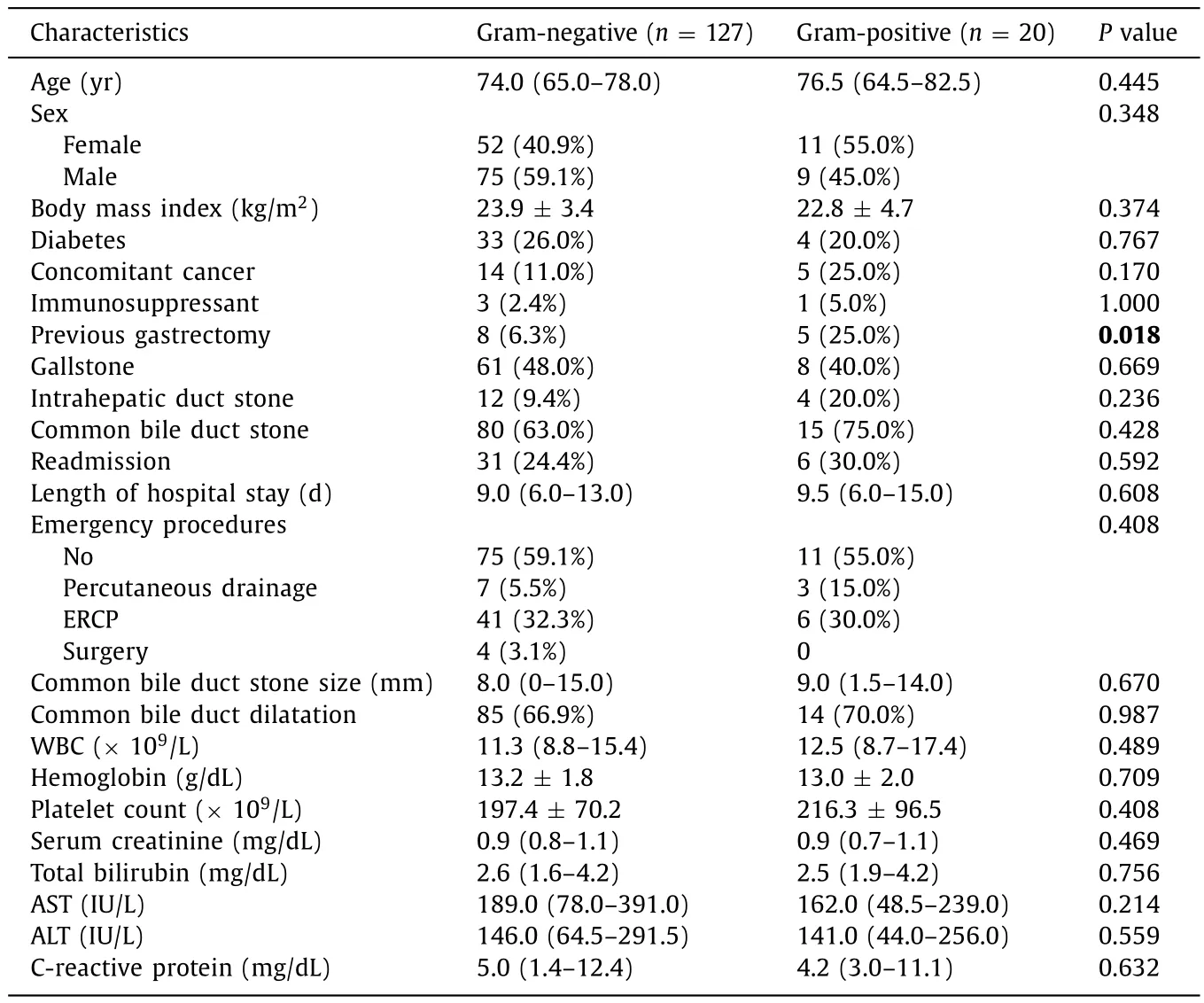
Table 2Clinical characteristics in gram-negative and gram-positive detected groups.
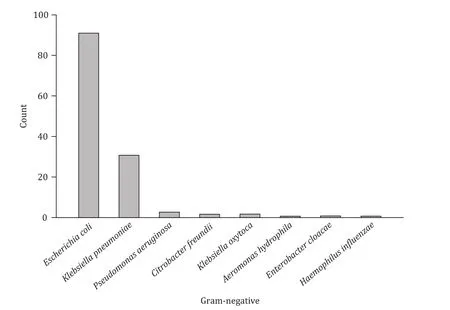
Fig.1.Spectrums of gram-negative pathogens isolated in blood cultures.Two bacteria ( Escherichia coli and Klebsiella pneumonia) were cultivated in one patient.
Univariate analysis of gram-positive bacteremia
In univariate analyses, only history of a previous gastrectomy was positively associated with gram-positive bacteremia [odds ratio (OR) = 3.91, 95% CI: 1.16-13.23;P= 0.028]( Table 3 ).Information on CT images, demographic factors, and initial laboratoryfindings did not show any significant correlation with grampositive bacteremia.
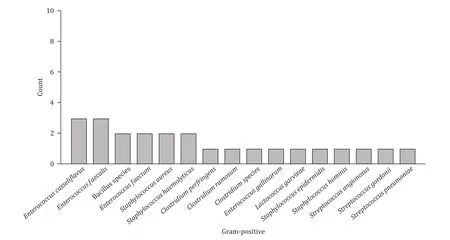
Fig.2.Spectrums of gram-positive pathogens isolated in blood cultures.

Table 3Univariate logistic regression analysis of gram-positive bacteremia in biliary infections.
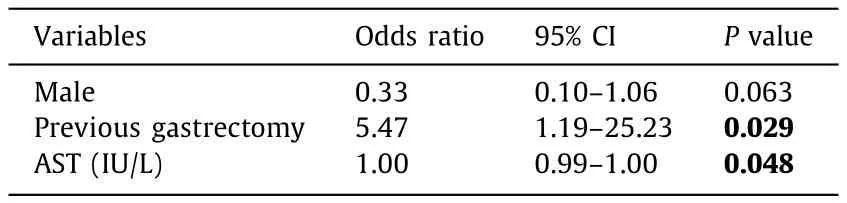
Table 4The final prediction model for gram-positive bacteremia using stepwise method.
Multivariate analysis and prediction model for gram-positive bacteremia
We conducted a multivariate analysis with all factors used in the univariate analysis ( Table 4 ).Among these variables, history of gastrectomy showed the strongest correlations (OR = 5.47, 95% CI:1.19-25.23;P= 0.029).The ORs of variables in the final predictive model were presented in Fig.3.
Discussion
Generally, biliary tract infection is a common cause of bacteremia and is associated with high mortality rates.In this study,we evaluated the clinical characteristics of bacteremia induced by biliary tract infection and tried to find a method for predicting the causative pathogens of bacteremia.Previous studies reported that gram-negative organisms cause most episodes of biliary tract infection-induced bacteremia [ 6 , 8 , 9 ].In our study, of approximately 16 percents of cases (13.2% as monomicrobial, and 2.6% as polymicrobial), detected pathogens were gram-positive organisms.Most of the positive cultures were documented before biliary tract decompression procedures (e.g., ERCP and percutaneous drainage),and all enrolled patients had systemic inflammatory responses with definite evidence of biliary tract infection.To date, several studies have investigated the spectrum of pathogens in biliary infectious diseases, and the range of the reported prevalence of gram-positive pathogens in other papers varied from 19.3% to 40.0% [ 6 , 10-13 ].Compared with past studies, the proportion of gram-positive infection has shown an increasing trend in recent research [ 8 , 9 ].
We focused on gram-positive organisms in biliary tract infection-induced bacteremia, and our model for predicting grampositive bacteremia combined with biliary infections consisted of three parameters: history of previous gastrectomy, sex, and serum aspartate aminotransferase (AST) level.It was unlikely that sex and serum AST level had meaningful correlations with gram-positive bacteremia.For this reason, care must be taken when analyzing each variable as a single predictor of gram-positive bacteremia in biliary infections, even though sex and serum AST level were included in the final prediction model.
Patients with biliary infectious diseases who underwent any kind of gastrectomy had an OR of 5.47 for gram-positive bacteremia, compared with those who did not.We believe that this relationship could be explained by the alteration of gut microbiota after surgery.Bacterial invasion through the sphincter of Oddi isa well-known pathogenic mechanism of ascending biliary infections [14-16].A series of events created a favorable condition for the overgrowth of certain microorganisms, and finally caused dysbiosis.Previous studies investigated the change in gastrointestinal bacterial flora in post-gastrectomy patients [ 17 , 18 ].In a study of bacterial flora in the gastric chambers of patients who underwent open-banded Roux-en-Y gastric bypass, gram-positive bacteria were the predominant organisms among all detected microbes [17].Similarly, it was observed that all patients had significant small-intestinal bacterial overgrowth in the mucosa under the esophagojejunostomy site after total gastrectomy, andstreptococciwere the most common species in the bowel [18].Therefore, infections induced by atypical pathogens are likely to develop in postgastrectomy patients and in this context, we may explain the correlation between gastrectomy states and the occurrence of biliary sepsis by gram-positive strains.
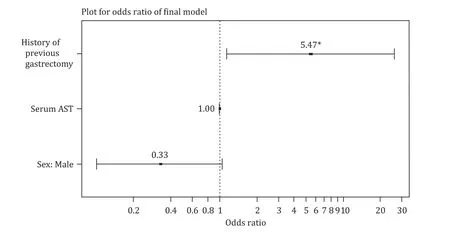
Fig.3.Plot for odds ratio of final prediction model of gram-positive biliary bacteremia ( ∗P < 0.05).AST: aspartate aminotransferase.
This study has several limitations.First, this was a retrospective and single-center analysis.Therefore, due to the limitation of the small sample size, we could not perform the detailed subgroup analysis according to the type of bacteria.Second, the original definition of biliary tract infection-induced bacteremia involves the same organisms identified from bile juice and blood samples.Although we did not analyze bile samples, our study had definite criteria for the presence of “biliary tract infection”(i.e., fever, abdominal pain, positive imaging results, abnormal biochemical findings,and simultaneous positive blood bacterial cultures).Third, our results show only 24 patients with gram-positive bacteremia, and the proportion is somewhat less than those of other literatures.Therefore, the spectra of the causative gram-positive strains we demonstrated may be insufficient to reflect the phenomenon in the real world.Nevertheless, our data follows some general tendency: the most frequent gram-positive pathogens areEnterococcusspecies (9 of 24), as many literatures have reported.In the future, multicenter, prospective studies with a larger cohort are anticipated, and it should be investigated whether early antibiotic therapy against the predicted pathogens of biliary sepsis could really improve the clinical outcomes as well as reduce the mortality and morbidity of biliary tract infection-induced bacteremia.
In conclusion, in a considerable proportion of patients, biliary sepsis could be induced by gram-positive bacteria.History of a previous gastrectomy is a helpful marker for predicting gram-positive bacteremia.Prompt detection of gram-positive pathogens would enable offering early antibiotic therapy with a more appropriate coverage to the patients.
Acknowledgments
None.
CRediT authorship contribution statement
Ik Hyun Jo:Data curation, Formal analysis, Methodology, Writing - original draft.Yeon-Ji Kim:Conceptualization, Methodology,Supervision, Writing - original draft, Writing - review & editing.Woo Chul Chung:Conceptualization, Supervision, Writing - review& editing.Jaeyoung Kim:Data curation, Formal analysis, Writing- original draft.Seonhoo Kim:Formal analysis.Eun Sun Lim:Formal analysis.Honggeun Ahn:Formal analysis.Seong Yul Ryu:Formal analysis.
Funding
None.
Ethical approval
This study was approved by the Institutional Research Ethics Board of the Catholic University of Korea (VC19RESI0191).All investigations adhered to the tenets of theDeclarationofHelsinki.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatobiliary&Pancreatic Diseases International
- No difference in mortality among ALPPS, two-staged hepatectomy, and portal vein embolization/ligation: A systematic review by updated traditional and network meta-analyses
- Telomerase reactivation is associated with hepatobiliary and pancreatic cancers
- Critical role of estrogen in the progression of chronic liver diseases
- Robotic isolated partial and complete hepatic caudate lobectomy: A single institution experience
- C -C motif chemokine ligand 16 inhibits the progression of liver cirrhosis via inactivating hepatic stellate cells
