Dynamic expression of hepatic GP73 mRNA and protein and circulating GP73 during hepatocytes malignant transformation
2020-10-23WenLiSiMinYoShuiJieShenWenJieZhengJinYingSunMengWuLiWngDengFuYo
Wen-Li Si , , # , Min Yo , # , Shui-Jie Shen , c , # , Wen-Jie Zheng , Jin-Ying Sun ,Meng-N Wu , Li Wng , Deng-Fu Yo , ∗
a Research Center of Clinical Medicine, Affiliated Hospital of Nantong University, Nantong 226001, China
b Departments of Medical Immunology & Medical Informatics, Medical College of Nantong University, Nantong 226001, China
c Department of Oncology, Nantong Hospital of Traditional Chinese Medicine, Nantong 226001, China
d Department of Oncology, Affiliated Hospital of Nantong University, Nantong 226001, China
Keywords:
ABSTRACT
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide [1], and still ranks the 1st tumor of incidence among all malignancies and the poorest survival rate in Qidong area, China [2].The molecular mechanisms of HCC development have been explored with a great progress in recent years [3-5], including some alterations of genes such as tumor growth/suppressor genes, apoptosis/anti-apoptosis genes, and specific biomarkers such as microRNAs [6], lncRNAs [7], exosomes [8],growth factors, molecules of signaling pathway, and so on [9-11].However, the prognosis of HCC patients is still poor with high recurrence.Early detection and effective treatment of HCC are of the utmost importance, and more sensitive and specific diagnostic tools should be explored [12 , 13].Golgi-specific membrane Golgi protein-73 (GP73, GOLPH2) is normally presented in the epithelial cells with GP73 mRNA transcript (about 3.0 kb), and consists of a short N-terminal cytoplasm-, a transmembrane- and a larger C-terminal-domain located in the luminal surface of the Golgi apparatus [14 , 15].Biliary epithelial cells express GP73 in normal livers.Overexpression of hepatic GP73 is closely related to HCC progression and worse outcome [16 , 17].
Recent studies discovered that GP73 mediates chronic liver diseases immunologically [18], regulates hepatic steatosis by enhancing the sterol regulatory element-binding proteins (SREBPs)-SREBP cleavage activating protein (SCAP) interaction [19], promotes the epithelial-mesenchymal transition and invasion by TGF-β1/Smad2 signaling activation [20], and causes chemotherapeu-tic resistance [21]in HCC.Accumulating data have demonstrated that HCC tissues overexpress GP73 which then secretes into blood.Serum GP73 levels are significantly higher in the HCC tissues than those in patients with liver cirrhosis or chronic hepatitis, and may be a new tumor diagnostic and prognostic biomarker for HCC.However, the evidences in previous studies are heterogeneous and inconsistent, especially at early HCC [22 , 23].In this study, therefore, we investigated the dynamic alterations of GP73 at HCC staging.We also confirmed the diagnostic values by the models of hepatocarcinogenesis in rats.
Methods
Liver specimens
Eighty-eight pair of fresh human HCC tissues and their surrounding tissues were intraoperatively collected from HCC patients(68 male, 20 female; age ranged from 30 to 68 years old) between March 2006 and August 2014 at the Affiliated Hospital of Nantong University, China, and snapped frozen immediately in liquid nitrogen.There were 71 cases with HCC sizes more than 2 cm and 17 less than 2 cm; 24 cases with AFP levels more than 400 ng/mL and 64 less than 400 ng/mL; 72 cases with single and 16 with multiple tumors; 30 cases with well-, 45 moderate- and 13 poordifferentiation according to the Edmondson grading system; 45 cases at stage I (51.1%), 25 at stage II (28.4%), 13 at stage III (14.8%),and 5 at stage IV (5.7%) based on the TNM classification of IUAC(6th edition); and 72 cases with positive HBsAg and 63 with cirrhosis.Criteria of HCC diagnosis were based on National Collaborative Cancer Research, China [24].HCC grading and staging were finally confirmed by the histopathological hematoxylin and eosin(HE) staining.This study was approved by the Ethics Committee of Affiliated Hospital of Nantong University, China (TDFY2013008).
Rat HCC models and histology
Rat hepatoma models were created with 2-fluorenyl acetamide(2-FAA) using diet plus 0.05% of 2-FAA (Sigma, St Louis, MO, United States).In brief, Sprague-Dawley (SD) rats (n= 42) at 4-6 weeks,100-120 g were divided randomly into the control (n= 6), and experimental groups (n= 36 for 6 groups) according to the previous method [25]and housed under bio-clean at 22 ± 2 °C with a 12-h light/dark and 55% humidity at the Animal Center, Nantong University, China, and were monitored daily for survival and weight loss,recorded signs, and sacrificed at every two weeks.According to the HE staining, the rats were divided into 4 groups: control (n= 6),hepatocyte degeneration (n= 18), precanceration (n= 9), and HCC(n= 9), which were approved by the Animal Care and Use Committee of Nantong University, China.
Preparation of liver total RNA and PCR amplification
Total RNAs from rat livers were purified using TRIzolkit(Fermentas, Vilnius, Republic of Lithuania), and then reversetranscribed to cDNA with the RevertAidTM first strand synthesis reagents.The cDNA was amplified by a nested PCR with pairs of primers according to the rat GP73 sequence (AF_057308): rGP73-P1 (sense): 5 ′ -GGT GCT AAC AGA TGA TGG TG-3 ′ (nt 329-348) and rGP73-P2 (anti-sense): 5 ′ -CTG GCT CAT AAC CCA TCA AC-3 ′ (nt 542-561).Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was an internal control:rGAPDH-P1 (sense): 5 ′ -CAG TGC CAG CCT CGT CTC AT-3 ′ (nt 50-69) and rGAPDH-P2 (anti-sense): 5 ′ -AGG GGC CAT CCA CAG TCT TC-3 ′ (nt 625-644).PCR amplification for GP73-cDNA was performed in a TC412 DNA thermal cycler (Techne, London,UK) using the Premix Taq DNA polymerase kit (TaKaRa, Tokyo,Japan).Total 25 μL contained 1 μL of GP73-cDNA (0.1 μg/μL),2 μL of sense primer (10 μmol/L), 2 μL of anti-sense primer(10 μmol/L), 12.5 μL of Premix solution, and ddH 2 O at 94 °C, 5 min, then 94 °C, 10 s, 55 °C, 30 s and 72 °C, 30 s for 35 cycles, and extended at 72 °C for 10 min.PCR production was electrophoresed on 1.5% agarose-TAE (Tris/Acetic acid/EDTA) buffer with ethidium bromide staining, and the fragment sizes were examined under a UV light with DNA ladder (Tamara, Aobaku,Japan).
Total protein preparation
Liver tissues (100 mg) were homogenized in 1.0 mL of an ice-cold homogenization buffer containing 50 mmol/L 3-(NMorpholino) propanesulfonic acid buffer (pH 7.4), 100 mmol/L KCl, 320 mmol/L sucrose, 50 mmol/L NaF, 0.5 mmol/L MgCl 2 ,0.2 mmol/L dithiothreitol, 1 mmol/L EDTA, 1 mmol/L EGTA,1 mmol/L Na 3 VO 4 , 20 mmol/L sodium pyrophosphate, 20 mmol/Lβ-phosphoglycerol, 1 mmol/L p-nitrophenyl phosphate, 1 mmol/L benzamidine, 1 mmol/L phenylmethylsulfonyl fluoride, and 5 μg/mL each of leupeptin, aprotinin, and pepstatin A.The homogenates were centrifuged at 800 ×gfor 10 min at 4 °C.The supernatants were collected, and total protein concentrations were determined by an enhanced bicinchoninic acid protein assay kit(Beyotime Institute of Biotechnology, Shanghai, China).
Western blotting
A total of 100 μg liver tissue protein that has been prepared was lysed in a radioimmunoprecipitation assay (RIPA) buffer containing phenylmethanesulfonyl fluoride, separated on 10% sodium dodecyl sulfate polyacrylamide gel (SDS-PAG), and electrotransferred to a PVDF membrane.The membrane was blocked with skim milk(5%) for 1 h under room temperature and probed with primary mouse anti-human anti-GP73 antibodies (1:300, SC-48010, Santa Cruz Biotech, CA, USA) for 1 h at room temperature.After washing for three times, the membrane was incubated with the horseradish peroxidase-conjugated anti-goat IgG antibodies at 37 °C for 30 min and then visualized with an enhanced chemiluminescence reagent.Mouse anti-human GAPDH antibodies (Santa Cruz Biotech) were used for internal control.The band images of rat hepatic GP73 protein were analyzed using Quantity One (ver.4.62) software (Bio-Rad, Hercules, CA, USA).The relative ratio from GP73 to GAPDH was calculated according to both staining strength in each correspondent liver.
Immunohistochemistry
Slides of rat livers were dewaxed, rehydrated, antigen retrieval,and incubated with primary rabbit anti-rat anti-GP73 antibodies(1:10 0 0, ab109628, Abcam, UK) at 4 °C overnight.After incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG, the slides were stained with diaminobenzidine, and counterstained with hematoxylin, cleared in xylene, and covered.The levels of GP73 expression were calculated with the Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA).Blinded evaluations of GP73 immunostaining and independent observation were carried out simultaneously, and then the expression level of GP73 in tissue was evaluated with the Image-Pro Plus 6.0 software with integral optic density (IOD) value [26].
Enzyme-linked immunosorbent assay
GP73 levels were quantitatively detected according to manufacturer’s instructions of the enzyme-linked immunosorbent assay kit(CLOUD-CLONE, Houston, USA) with positive/negative quality control by two independent researchers.Absorbances were read at 450 nm (Synergy HT, Florida, USA).Serum GP73 (nmol/mL) and supernatant GP73 (nmol/mg wet liver) of liver homogennants were calculated based on the standard curve.
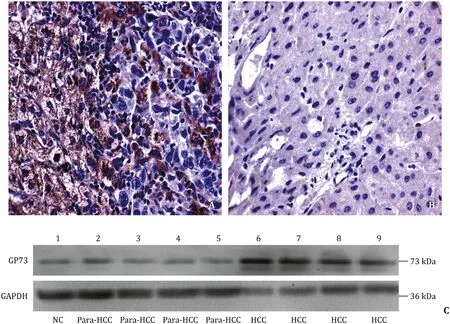
Fig.1.Abnormal expression of GP73 in human HCC tissues.A: the positive cells were localized in periportal areas in human HCC tissues (HE staining, original magnification×400); B: little or none expression in the paracancerous tissues (HE staining, original magnification ×400); C: the GP73 expressions of different tissues were confirmed by Western blotting.GP73: golgi protein 73; HCC: hepatocellular carcinoma tissues.
Statistical analysis
Data were presented as mean ±standard deviation (SD) and were analyzed with the SPSS software (version 20.0, IBM, New York, NY, USA) plus Image Pro Plus 6.0 image system (Media Cybernetics).Differences between the two groups were analyzed by the Student’st-test or Chi-square test, and among the groups using one-way analysis of variance.Overall survival curves of HCC patients were calculated with the Kaplan-Meier method or log-rank test.Significant difference was set at a value ofP<0.05.
Results
Overexpression of GP73 in human HCC tissues
The representative images of the GP73 immunohistochemical staining in a cohort of 88 HCC and their para-HCC tissues are shown in Fig.1.The GP73 expression was mostly located in the nucleus and cytoplasm of liver cancer cells, and the strongest GP73 expression or nuclear-positive cells was presented in the HCC tissues ( Fig.1 A) with advanced stages than that in the para-HCC tissues or at early HCC formation ( Fig.1 B).Increasing GP73 level was observed in advanced stage of HCC compared with that in the para-HCC tissues by the Western blotting ( Fig.1 C ).The gray values from GP73 to GAPDH were significantly increased in the HCC tissues than those in their para-HCC tissues (480.7 ±68.0 vs.209.0 ±243.2,P<0.001).
Relationship between GP73 and TNM staging of HCC
The relationship between GP73 and TNM staging of HCC and comparative analysis of HCC and their para-HCC tissues are shown in Fig.2.The staining feature of GP73 in HCC showed cytoplasm immunoreactivity for HCC tissues ( Fig.2 A), and the positive rate of GP73 in 88 HCC was 53.3% (24/45) at stage I, 84.0% (21/25) at stage II, 84.6% (11/13) at stage III, and 60.0% (3/5) at stage IV, respectively.Compared with para-HCC tissues, the GP73 expression was significantly higher in HCC tissues (P<0.001).Furthermore, the level of GP73 expression was parallel to the stage of HCC ( Fig.2 B).The cumulative survival curves of HCC patients were compared according to different HCC stages (P<0.001, Fig.2 C) and further confirmed that increased GP73 level was significantly associated with shorter overall survival of HCC patients.
GP73 up-regulation at protein level
The dynamic expression of hepatic GP73 during rat HCC development is summarized in Table 1.The positive rates of rat hepatic GP73 expression were 50.0% in low level ( + , 9/18) and 16.7%in high level ( ++ , 3/18) in degeneration; 11.1% in low level ( + , 1/9)and 77.8% in high level ( ++ / +++ , 7/9) in precanceration; and 11.1%in low level ( + , 1/9) and 88.9% in high level ( ++ / +++ , 8/9) in HCC,respectively.

Fig.2.Hepatic GP73 expression related to clinical staging of HCC.A: the representative images of the immunohistochemical staining with the increasing GP73 expression in 88 HCC tissues was associated with clinical staging from left to right (HE staining, original magnification ×200); B : the quantitative analysis of GP73 expression correspondence to above images; C: the cumulative survival curves were calculated by the Kaplan-Meier method in HCC patients with low or high GP73 expression.∗P < 0.001,compared with the paracancerous group.GP73: Golgi protein 73; HCC: hepatocellular carcinoma tissues; Para-HCC: para-cancerous tissues; IOD: integral optic density.

Table 1Dynamic alteration of hepatic GP73 expression in rat hepatocarcinogenesis.
Hepatic GP73 expression at mRNA level
Alterations of hepatic GP73 mRNA amplification during rat HCC development are shown in Fig.3.The fragments of rat GP73 mRNA amplification with GAPDH as a control ( Fig.3 A), no positive fragments were detected from the control group; the positive fragments were detected from degeneration, precanceration, and rat HCC tissues.The GP73 mRNA fragments were none in the control, 44.4% in degeneration, 77.8% in precanceration, and 100% in rat HCC tissues, respectively ( Fig.3 B).The GP73 alteration at mRNA level was progressively increased during rat hepatocarcinogenesis, especially in precancerous or HCC livers.
Quantitative analysis of hepatic and serum GP73
Quantitative analysis of GP73 expression in rat livers and blood is shown in Table 2.Cytoplasm GP73 levels in rat liver had a rising tendency along with the histomorphological alteration during the rat hepatocarcinogenesis.Cytoplasm GP73 specific level (nmol/mg wet liver) in the precancerous and rHCC group was significantly higher than that in the control group (P<0.001).Also, the serum GP73 levels had a clear rising gradient expression.The serum GP73 level in the precancerous group (P= 0.021) and in the rHCC group(P<0.001) was significantly higher than that in the control group.Moreover, there was a closely positive correlation between serum GP73 and hepatic GP73 (r= 0.91,P<0.01).
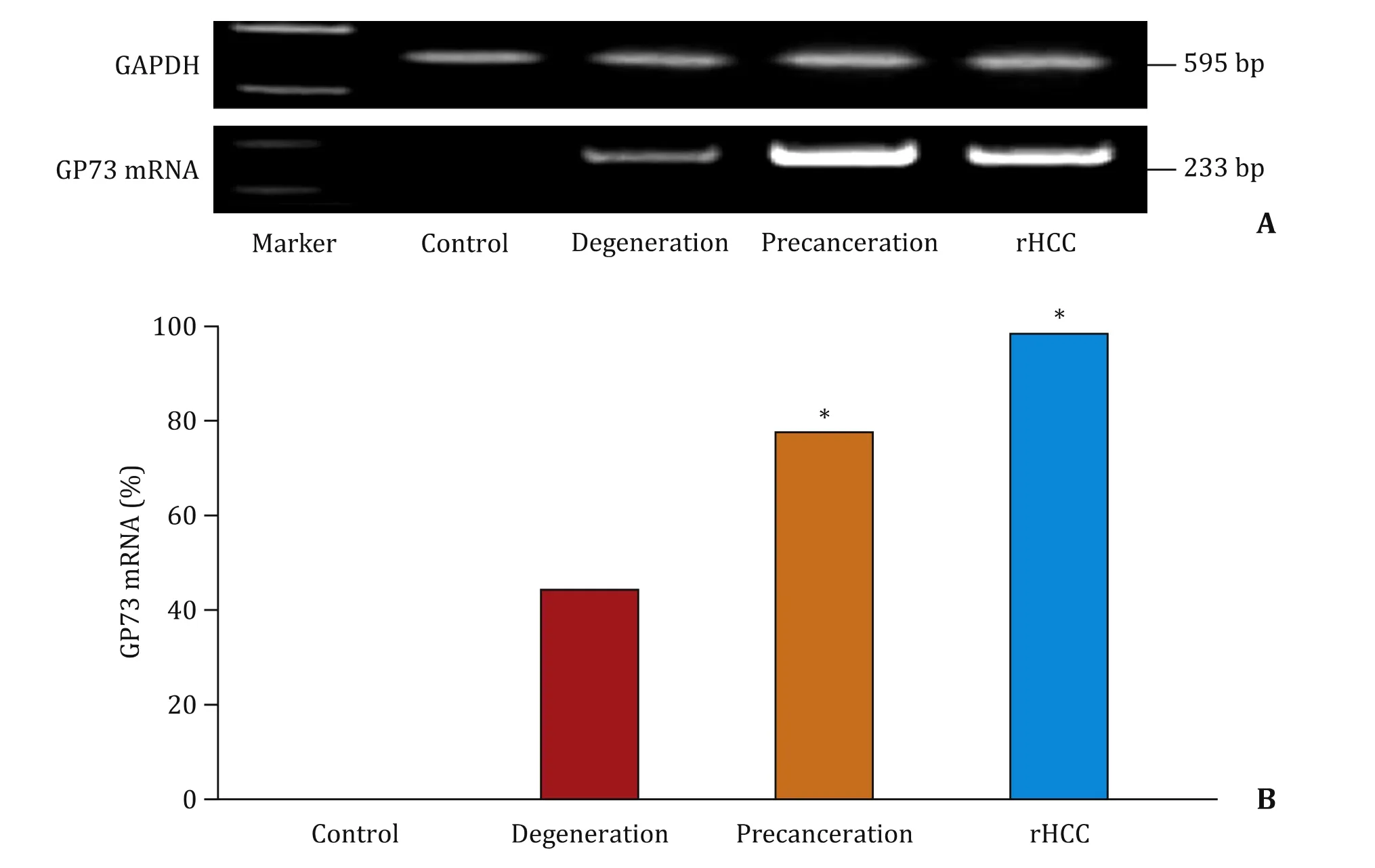
Fig.3.Alteration of GP73 mRNA amplification analysis during malignant transformation of rat hepatocytes.A: The amplified fragments of rat GP73 mRNA by the reverse transcription nested polymerase chain reaction (RT-PCR) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a control; B: The positive percentage of GP73 RNA fragments in rat livers of different groups.∗P < 0.001, compared with the control group.

Table 2Quantitative analysis of GP73 level in rat livers or blood during malignant transformation of hepatocyte.
Discussion
Patients with chronic liver diseases caused by viral infection or metabolic abnormalities are still at a high risk of HCC development.Early monitoring HCC or prevention of HCC is one of the most impactful strategies to improve the prognosis for these patients.Routine AFP test is a useful test for HCC detection.However,40% patients with early HCC show low AFP and this false-negative AFP value delays the diagnosis [27].Even in advanced HCC, circulating AFP levels still remain normal range from 15% to 30% of HCC patients [10 , 27 , 28].GP73 is a protein of Golgi apparatus located in human genome chromosome 9q21.33, a specific type II transmembrane protein.Some studies showed that GP73 is overexpressed in HCC which may be a useful biomarker for HCC diagnosis and prognosis [29 , 30].However, how to utilize circulating GP73 as a marker to screen HCC at early stage still remains controversial.The present study showed the status of GP73 expression in HCC at different stages and demonstrated that GP73 expression was gradually increased following hepatocarcinogenesis in rats.
Human HCC tissues can synthesize various tumor-related proteins, polypeptides, and isoenzymes and secrete them into blood.Accumulating evidences have suggested that serum GP73 is widely used as a sensitive marker of HCC, with significantly high GP73 similar to AFP in sera of HCC patients [22 , 30].Correlation was reported between GP73 expression and HBV infection, HCC metastasis and recurrence [23].Moreover, our study found that the higher GP73 level was detected in more advanced HCC and GP73 increase was parallel with HCC staging.
Although GP73 was inherent membrane protein in the Golgi apparatus, and significant difference was found between benign and malignant liver diseases [23], the dynamic features of GP73 alteration in HCC progression are still not clear.The present study found that the GP73 expression was longitudinally increased in hepatocarcinogenesis in rat HCC models.
There was a parallel positive correlation between hepatic GP73 and serum GP73 during hepatocytes malignant transformation,suggesting that the dynamics of GP73 expression in hepatocarcinogensis may be a valuable biomarker for the early diagnosis of HCC [29].
Recently, clinical and molecular HCC risk scores enable precise HCC risk prediction followed by tailored HCC of individual patients,maximizing cost-effectiveness and optimizing allocation of limitedmedical resources [6].GP73 expression in epithelial cells is closely associated with liver disease and is especially highly expressed in HCC.Therefore, monitoring GP73 change in high risk population of HCC, such as patients with chronic hepatitis B and C, and nonalcoholic fatty liver diseases (NAFLD) may lead to early diagnosis of HCC [19].
In conclusion, to the best of our knowledge, this is the first report to investigate hepatic GP73 dynamics at mRNA and protein levels in rat HCC and to indicate that GP73 may be a monitoring biomarker for hepatocytes malignant transformation.These novel findings are promising, and the initial evidence has shown that GP73 is one of the early alterations of HCC occurrence.Future studies should clarify the GP73 molecular mechanisms of promoting HCC and how to down-regulating its gene transcript in time to avoid hepatocytes malignant transformation in high risk population of HCC.
Acknowledgments
None.
CRediT authorship contribution statement
Wen-Li Sai:Conceptualization, Data curation, Investigation,Methodology.Min Yao:Funding acquisition, Project administration, Writing - original draft.Shui-Jie Shen:Data curation, Formal analysis, Investigation, Methodology.Wen-Jie Zheng:Funding acquisition, Project administration, Validation.Jian-Ying Sun:Formal analysis, Investigation, Validation.Meng-Na Wu:Formal analysis,Investigation, Validation.Li Wang:Funding acquisition, Supervision, Writing - review & editing.Deng-Fu Yao:Funding acquisition,Supervision, Writing - review & editing.
Funding
This study was supported by grants from the Ministry of S& T National Key Research and Development Program of China( 2018YFC0116902 ), the National Natural Science Foundation of China ( 81673241 , 81702419 , 31872738 , 81873915 ), and Project of Jiangsu Medical Science ( BE2016698 ).
Ethical approval
This study was approved by the Ethics Committee of the Affiliated Hospital of Nantong University, China (TDFY2013008).
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatobiliary&Pancreatic Diseases International
- No difference in mortality among ALPPS, two-staged hepatectomy, and portal vein embolization/ligation: A systematic review by updated traditional and network meta-analyses
- Telomerase reactivation is associated with hepatobiliary and pancreatic cancers
- Critical role of estrogen in the progression of chronic liver diseases
- Robotic isolated partial and complete hepatic caudate lobectomy: A single institution experience
- C -C motif chemokine ligand 16 inhibits the progression of liver cirrhosis via inactivating hepatic stellate cells
