代谢组学在肉及肉制品品质监测中的应用
2020-10-22梁荣蓉朱立贤杨啸吟韩明山成海建张一敏
陈 雪,罗 欣,2,梁荣蓉,朱立贤,杨啸吟,韩明山,成海建,张一敏
代谢组学在肉及肉制品品质监测中的应用
陈 雪1,罗 欣1,2,梁荣蓉1,朱立贤1,杨啸吟1,韩明山3,成海建4,张一敏1※
(1. 山东农业大学食品科学与工程学院,泰安 271018;2. 江苏省肉类生产与加工质量安全控制协同创新中心,南京 210095;3. 国家肉牛牦牛产业技术体系通辽站,通辽 028100;4. 国家肉牛牦牛产业技术体系济南站,济南 250000)
代谢组学是通过研究机体受外界干扰前后小分子代谢物(分子量<1 500 Da)的变化,进而探究其代谢机制的新兴科学。近年来,代谢组学在肉品科学研究领域受到广泛关注。但目前基于该技术监测宰前因素(遗传因素、肌肉部位及饲喂方式)及宰后成熟(时间、方式)对肉及肉制品品质影响的相关研究仍缺乏系统总结。同时,代谢组学技术的引入,也为肉品货架期预测、肉制品加工工艺优选、产地溯源及真伪鉴别提供了新的思路。因此,该研究概述了近年来代谢组学常用的分析检测技术(核磁共振技术、气相色谱质谱联用技术、液相色谱质谱联用技术)及数理统计方法(主成分分析、偏最小二乘判别分析等),重点对代谢组学在肉品生产诸多环节(动物饲喂、屠宰、加工等)中的最新研究进展进行综述,最后总结了目前肉品代谢组学研究中存在的代谢产物检测有限、试验重复性差等问题并认为多组学联合分析是监测肉品品质的未来发展方向,以期为其在肉品科学领域的应用提供参考。
肉;品质控制;代谢组学;货架期;溯源;生物标记物
0 引 言
肉品是消费者膳食营养的重要来源。近年来随着生活水平的改善,消费者对肉品品质的关注度也逐渐提高。在肉类生产过程中,由于受到各环节诸多因素的影响,肉品的生化代谢过程也随之发生改变,导致其品质产生差异。因此,改善和提高肉品质量对整个行业的发展具有重要意义。为此,现代肉品科学领域引入了代谢组学技术。代谢组学是继蛋白质组学、基因组学和转录组学之后系统生物学的重要分支,以高通量检测技术和多元数据处理为手段,通过研究机体受干扰前后小分子代谢物(分子量<1 500 Da)的变化,进而探究其代谢机制的新兴科学[1-2]。近年来,代谢组学技术在肉品科学领域的研究与应用不断拓展,涉及动物饲养-屠宰、加工-销售多个方面[3]。而基于代谢图谱分析和代谢标志物的筛选有助于揭示上述生产环节诸多因素对肉品品质的影响,完善从农场至餐桌的“全链条”质量监控,已经成为当前的研究热点。因此,本文从宰前因素(如遗传因素、肌肉部位及饲喂方式等)、宰后成熟(时间、方式)、肉制品加工、货架期预测、产地鉴别和掺假检验等多个方面对代谢组学技术在肉品科学领域的最新研究成果进行综述,旨在为进一步推动代谢组学技术在肉品科学中的应用提供理论支持。
1 代谢组学的主要分析技术及数理统计方法
1.1 主要分析技术
1.1.1 核磁共振技术
核磁共振技术(Nuclear Magnetic Resonance spectroscopy,NMR)是目前肉品代谢组学研究中应用最广泛的检测技术,该技术的优势在于样品前处理简单,可对所分析样品实现无损检测和无偏向分析,同时能够进行实时和动态检测[4-5]。目前,常用的有氢谱(1H-NMR)、碳谱(13C-NMR)和磷谱(31P-NMR)技术,其中1H-NMR对含氢化合物均有响应,能实现样品中绝大多数物质的检测[6],在肉品科学领域应用最为广泛,涉及品质判别、真伪检验及加工肉制品品质控制等多个方面[7-8]。但NMR技术也存在检测灵敏度相对较低,对痕量物质检测存在误差等缺点。近年来新发展的多维核磁共振技术(Multidimensional NMR,MNMR)、高效液相色谱-核磁共振联用(High Performance Liquid Chromatography- Nuclear Magnetic Resonance Spectroscopy,LC-NMR)及高分辨魔角旋转磁共振波谱(High-Resolution Magic Angle Spinning MR Spectroscopy,HRMAS MRS)等技术弥补了这一缺陷,提高了检测分辨率,使基于NMR的肉品代谢组学研究日趋完善。
1.1.2 气相色谱-质谱联用技术
气相色谱-质谱联用技术(Gas Chromatography-Mass Spectroscopy,GC-MS)具有较高的分辨率、重现性和检测灵敏度,可实现多组分混合物中未知组分的定性分析[9],是目前代谢组学研究中最成熟的分析技术。其中顶空固相微萃取-气相色谱-质谱联用技术(Headspace Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry,HS-SPME-GC-MS)在肉品科学中应用较为广泛,特别适用于分析挥发性的化合物,主要用于揭示不同肉品之间风味差异的潜在机制或预测贮藏期间肉品的货架期[10-15]。但GC-MS也存在一定的局限性,分析难挥发的代谢组分需经过衍生化处理(硅烷化试剂反应、烷基化反应和酰基化反应),若衍生方法应用不当,则会影响其检测灵敏度[16]。
1.1.3 液相色谱-质谱联用技术
液相色谱-质谱联用技术(High Performance Liquid Chromatography - Mass Spectroscopy,LC-MS)不需要对代谢物进行衍生化处理,即可进行定性和定量分析,具有较高的分辨率、检测灵敏度和分析速度,适合沸点高、极性强的化合物分析,应用范围更广,尤其适合代谢产物的代谢轮廓分析[17]。目前该技术已经成为肉品代谢组学研究中强有力的手段,主要用于分析宰后肌肉能量代谢变化,旨在从代谢层面完善肌肉到食用肉转化的内在生物机制[18-19]。但该技术也尚存在系统稳定性和轮廓谱重现性差,分析时间长,进行单一分析时得到的分析物有限等问题[20]。针对这些不足,超高效液相色谱-质谱联用、毛细管柱液相色谱-质谱联用、多维液相色谱-质谱联用技术(High Performance Liquid Chromatography - Mass Spectroscopy,HPLC-MS)等在其基础上应运而生,有效提高了对复杂样品的检测效率。
肉品代谢产物的复杂多样性使得对分析技术的灵敏度、分辨率、通量等提出更高的要求。但目前基于某一检测技术尚不能全面覆盖肉品中的代谢产物信息[21]。有学者提出将LC-MS、NMR和GC-MS联合应用可为探究肉品品质的调控机制提供更加全面的视角[22]。如,Warner等[23]就基于NMR和HPLC技术,发现肌苷酸(Inosine Monophosphate,IMP)对肌动球蛋白的弱化作用可能是超快速冷却对嫩化牛肉的一大原因。由此可见,为提高代谢组学技术在肉品科学中的应用潜力,多平台集成联合将会是未来的发展方向。
1.2 数理统计方法
肉品代谢组学研究中通过高通量分析仪器生成的海量、高维、高噪声、高变异性的数据,需要采用化学计量学(主要为模式识别技术)和生物信息学技术对其进行降维归类处理后才能有效地筛选出生物标志物[24]。常用的模式识别方法主要包括两种,一种为非监督学习方法,如主成分分析(Principal Component Analysis,PCA)、聚类分析(Cluster Analysis,CA)、非线性映射(Nonlinear Mapping,NLM);另一种为有监督学习方法,如K最邻近法(K-Nearest Neighbor Classification method,K-NN),辨别分析(Discriminate Analysis,DA)、偏最小二乘判别分析(Partial Least Squares Discrimination Analysis,PLS-DA)、基于正交信号校正的偏最小二乘判别分(Orthogonal-PLS-DA,OPLS-DA)、人工神经网络(Artificial Neural Network,ANN)、支持向量机(Support Vector Machine, SVM)等[17]。其中PCA和PLS-DA是肉品代谢组学研究中最常用的模式识别方法[25-26]。这两种方法通常以得分图获得对样品分类的信息,载荷图获得对分类有贡献的变量及其贡献大小,从而用于发现可作为生物标志物的变量。
2 代谢组学在肉品科学中的应用
本节分别从宰前因素(如遗传因素、肌肉部位及饲喂方式等)、宰后成熟(时间、方式)、肉制品加工、货架期预测、产地鉴别和掺假检验等多个方面对代谢组学技术在肉品科学领域的最新研究成果进行综述,如图1所示。

图1 代谢组学在肉及肉制品品质监测中的应用
2.1 代谢组学在研究宰前因素对肉品质影响的应用
2.1.1 遗传因素(品种、年龄等)
动物的品种、年龄及杂交种的亲本等均会影响肉的品质,明确这些因素与某些特征代谢产物之间的联系有助于揭示其品质差异机制,并为优质肉类资源的开发提供理论支持。近年来,相关学者就基于代谢组学技术对牛肉、羊、猪、鸡、鸭的种内品质差异进行了研究(表1)。Gomez等[27]筛选出内洛尔牛和内洛尔牛×安格斯杂交牛之间乙酰肉碱、丙氨酸等15种关键差异代谢产物,主要涉及谷氨酰胺和谷氨酸代谢、缬氨酸、亮氨酸和异亮氨酸生物合成、谷胱甘肽代谢等通路。Straadt等[28]分析了5种杂交猪肉的代谢图谱,发现了丙氨酸、肌肽及含胆碱化合物等10余种标志性代谢物,信息学分析发现:这主要归因于不同种间宰前能量代谢、肌纤维膜性质、糖酵解潜力以及蛋白质和脂肪水解程度的差异。此外,代谢产物的种类及丰度也是影响禽肉食用品质的重要因素。Wang等[29]和Kim[30]分别对不同品种的鸡肉和鸭肉进行了代谢组学分析,并鉴定出多种特征代谢产物为地方品种的保护提供了有力支持。
总体来看,目前的研究已经证实能量代谢、氨基酸代谢、蛋白质或脂肪水解程度等的不同是导致品种间肉质差异的重要原因。但关于上述代谢通路与表型之间的相关性分析相对较少,因此,未来可基于靶向代谢组学技术进一步明确差异代谢物对不同品种肉质的潜在影响。
另外,动物在生长过程中其代谢水平的改变也贯穿始终。因此,借助小分子代谢产物(如鹅肌肽和肌肽等)有助于对肉品质量进行合理评估,进而优选出最佳的养殖时间[31]。Liu等[32]发现随日龄(27~500 d)的增加樱桃谷鸭肉中乳酸和鹅肌肽的含量增加,而延胡索酸、甜菜碱、牛磺酸、肌苷等却呈现降低趋势。综合考虑(嫩度、风味、持水力等),50 d日龄的樱桃谷鸭品质最优。此外,还有学者对比了不同日龄(110~230 d)武定鸡的代谢图谱,发现140 d日龄的武定鸡中牛磺酸和肌肽及其相关化合物(Carnosine Related Compounds,CRCs)含量最高且与其他组间总代谢产物无显著差异;主要通过丙氨酸、天门冬氨酸和谷氨酸代谢、嘌呤代谢、甘氨酸、丝氨酸和苏氨酸代谢等生化通路来调控不同日龄鸡肉的风味[33]。除上述代谢产物外,后续研究中也需重点关注脂肪酸等风味前体物质的变化。总体来看,基于代谢图谱可以辅助对由年龄导致的禽肉肉质差异进行综合分析。

表1 代谢组学在研究不同品种肉品质中的应用
2.1.2 肌肉部位
不同部位肌肉间在代谢水平上(糖酵解、TCA循环、核苷酸代谢等)的差异,也会进一步影响肌肉到食用肉的转化及后续贮藏期间的品质[34]。基于代谢组学技术,England等[18]发现与糖酵解相关的内在生物因素(糖酵解相关内源酶的丰度、三磷酸腺苷(Adenosine Triphosphate,ATP)等是调控猪肉宰后24 h酵解型肌肉和氧化型肌肉pH值下降速率的关键。亦有学者发现亲水氨基酸和-丙氨酸及相关化合物与牛肉部位(背最长肌(,LL)和骨中间肌(,VI))存在一定的相关性;代谢通路分析显示糖酵解代谢、嘌呤代谢、氨基酸及多肽等生化通路对两部位肌肉品质起调控作用[34]。此外,LL和半膜肌(,SM)之间的宰后早期代谢模式也存在不同,其中内源酶(如乳酸脱氢酶、苹果酸脱氢酶等)的活性、丙酮酸的含量以及涉及三羧酸循环的代谢产物等均存在一定的差异[19]。与此同时,该学者在研究中也证实了牛肉不同部位肌肉宰后早期有氧代谢时间及强度、糖酵解潜力等的差异是影响其贮藏及零售期间品质的重要因素[19]。
目前,关于不同部位肌肉的生化代谢机制研究仍然十分有限,虽然已经初步表明宰后早期能量代谢是导致肉质差异的关键,但基于特征代谢产物对于不同部位肌肉肉质特性的调控机制尚不明确。因此,后期需进一步完善研究策略,可采用多组学集成联合将有助于更加系统地阐明不同部位肌肉到食用肉转化的生化机制及其对后续品质的影响。
2.1.3 饲喂方式
饲喂方式(日粮等)不仅影响动物的生产性能,也会对其宰后肉的品质及代谢产物产生影响。Antonelo等[35]研究发现与饲喂基础日粮相比,添加3.5%大豆油会影响牛肉中甜菜碱、甘油、延胡索酸和肌肽等代谢物质的含量,可能通过调控甘油酯类物质代谢、甘氨酸、丝氨酸和苏氨酸代谢,谷氨酰胺和谷氨酸代谢等通路,最终导致其较差的感官品质。若日粮添加抗氧化成分可通过影响肌肉代谢,提高宰后肉的抗氧化能力。Zawadzki等[36]对比了日粮中添加不同浓度的巴拉圭茶提取物对牛肉品质及代谢产物的影响。结果发现:在不影响动物生产性能的情况下饲喂添加该物质可提高肉中磷酸肌酸、肌酸、肌肽以及共轭亚油酸的含量,降低自由基的形成趋势,提高牛肉的氧化稳定性。类似的,Baira等[37]发现在肉鸡日粮中添加橙皮苷(1.5 g/kg)可增加宰后肉品中的酰基肉碱和脂肪酸的水平,降低脂质氧化程度。由此可见,基于宰后代谢组学的研究结果不仅能够有效区分饲喂方式的不同,同时也能为肉品品质的改善提供新的解决方案。
2.2 代谢组学在研究宰后成熟(成熟时间、方式)过程对肉品质影响的应用
宰后成熟是改善肉品适口性的重要过程,期间随脂肪酸、氨基酸的氧化以及蛋白质的降解等,会产生与肉风味相关的多种代谢产物。如氨基酸类(谷氨酸、蛋氨酸、亮氨酸等)可促进肉的滋味;核苷酸类(肌苷酸(Inosine Monophosphate,IMP),鸟苷酸等)可促进肉的鲜味[38]。Koutsidis等[39]采用GC-MS技术对牛肉成熟期间生成的代谢产物进行研究,发现苯丙氨酸、蛋氨酸、赖氨酸、亮氨酸和异亮氨酸等均逐渐增加,其中甲硫氨酸丰度增长近7倍。Consolo等[40]也发现牛肉成熟21 d后代谢产物丰度增加了近1/3,其中大部分代谢物质对肉的风味有促进作用。另外,还有学者指出牛肉成熟期间缬氨酸、亮氨酸和异亮氨酸与成熟时间具有良好的相关性[41]。因此,基于代谢组学技术,有望预测肉牛宰后的成熟时间。
成熟方式也会影响肉的风味,有学者研究发现与湿法成熟相比,干法成熟会提高生鲜肉特有风味[42]。为了进一步明确这一原因,Kim等[43]对比了牛肉在不同成熟方式下的代谢产物。发现相较于湿法成熟,干法成熟会提高牛肉中谷氨酸等多种氨基酸等的含量,但IMP的含量却低于湿法成熟牛肉,这一结果也初步表明可能是由于干法成熟方式下蛋白水解程度更高所致。Mungure[44]也发现干法成熟会促进鹿肉中某些呈味氨基酸的产生。但干法成熟由于过多的汁液损失和修整损失也使得其出品率较湿法成熟低。为了改善这一不足,Zhang[45]尝试采用逐步成熟的方式(先干法成熟7d,后湿法成熟14 d),发现这一成熟方式可提高牛肉中磷脂酰胆碱、磷脂酰乙醇胺和谷氨酸等代谢产物的含量,在改善风味的同时提高了出品率。可见借助代谢组学技术能有效监测期间呈味小分子物质的变化,并为成熟方式的优化提供理论参考。
2.3 代谢组学在肉品货架期预测中的应用
微生物过度增殖导致的肉类腐败也一直是困扰肉类工业的重要问题[46]。贮藏期间,致腐微生物会优先利用肉中的葡萄糖作为能量物质,当葡萄糖消耗殆尽时其他物质(如乳酸、丙酮酸、氨基酸、核苷酸等)也会被分解代谢[47]。由此,借助代谢组学技术可以通过监测产生的初级代谢产物及多种腐败气味分子(醇类、醛类等)预测肉类的腐败程度[48]。如表2所示,Argyri等[49]研究了不同包装及贮藏条件下碎牛肉的代谢产物,发现有机酸可以作为标志牛肉腐败程度的潜在物质。Frank等[11]也发现乳酸为真空包装牛肉冰温贮藏期间的典型代谢产物。
肉品贮藏期间由微生物代谢产生的挥发性物质(Volatile Organic Compounds,VOCs)主要包括醇类、醛类、酯类、挥发性脂肪酸、硫化物等[47]。近年来相关学者对这些VOCs进行广泛的研究,并确定了多个与肉品腐败相关的生物标志物(表2)。其中,对于包装方式而言,不同气体成分会影响肉的菌群结构,并激发特定腐败菌(Specific Spoilage Organisms,SSOs)启动相应的代谢通路,导致肉品腐败时释放出特征VOCs。众多学者研究发现透氧托盘包装条件下,2,3-丁二醇、2-丁酮、二乙酰、乙偶姻等为潜在的腐败标志物[12-13,15],主要归因于假单胞菌、热杀索丝菌等优势腐败菌的生长代谢;真空包装条件下,乙酸、丁酸、戊酸等有机酸为典型的VOCs[13-14,48],主要与乳酸菌等微生物密切相关;高氧气调包装下乙偶姻和己酸等为多种优势腐败菌产生的特征VOCs[13-14]。另外,VOCs也受基质中营养物质可利用程度等内在因素及微生物污染状况等外在因素的影响,使得不同研究结果稍有差异。但基于目前的研究,关于肉中致腐微生物代谢活动与其腐败(感官)之间的关系仍未完全阐明,且肉中的内源酶及SSOs均能催化代谢反应生成VOCs[54],使得据此对肉品货架期进行准确评估还存在一定困难。
2.4 代谢组学在肉制品加工中的应用
目前,代谢组学技术也被广泛地用于监控、预测肉制品不同加工阶段品质变化(表3)。García-García等[55]分析了西班牙发酵干香肠加工期间代谢产物变化,发现加工初期乳酸、肌酸/磷酸肌酸/肌肽信号占据主导;发酵过程中(2 d)-葡萄糖、-葡萄糖含量降低,但乙醇、乳酸、乙酸以及氨基酸等含量逐渐增加,随着干燥成熟的进行,乙醇、乙酸和甲酸的含量显著降低(<0.05),氨基酸信号增强,有效实现了发酵香肠生产过程监控。
酱卤制品是中国传统熟肉制品的典型代表,不同的加工工艺赋予了不同产品独特风味。但目前传统手工作坊式生产仍占有较大比重。基于代谢组学技术,有助于改进其加工技术,实现酱卤制品的规模化生产。Yang等[56]发现多种氨基酸、蔗糖、-葡萄糖、醋酸和肌酐等均随卤猪肘熟制时间(0~90 min)逐渐增加,其中熟制60 min和90 min时代谢产物丰度及感官品质较高。Yang等[57]研究了150~300 MPa高压腌制对于卤肉制品代谢物的影响,发现高压处理可提高大部分代谢产物含量,但压力水平对于丙氨酸、乳酸、醋酸、甲酸、延胡索酸等物质影响不显著,最终优选出150 MPa高压腌制为最经济的改善卤肉风味的加工方式。依据代谢组学的研究结果,周楠楠等[58]发现相比于干糟,湿糟过程对糟鸭的滋味化合物影响更显著。
较长的加工周期是形成干腌火腿独特风味的关键,而这主要归因于加工期间所生成的多种小分子代谢产物。明确干腌火腿的呈味机制,将有助于缩短加工时间,提高产业效率。Zhang等[59]分析了干腌火腿不同加工阶段代谢产物的变化。大部分代谢产物的含量随加工时间逐渐增加,其中多种氨基酸,有机酸和核苷酸衍生物(次黄嘌呤氨基酸)等有助于最终产品风味的改善。Sugimoto等[60]指出日本干腌火腿成熟540 d时鸟嘌呤和核苷酸含量以及谷氨酰胺和天冬酰胺在总氨基酸中占比最高,整体感官评分最佳。此外,Shi等[61]基于代谢组学探究了大河乌猪干腌火腿风味及其形成机制,发现己醛、3-甲基丁醛、壬醛、辛醛为其特征风味物质,主要来源于脂肪酸的氧化及氨基酸的降解。

表2 不同贮藏条件下肉及肉制品中(潜在)腐败生物标志物
注:TSQ为三重四极杆;PRT为质子转移反应;SPME为固相微萃取;TOF为飞行时间质谱仪;FDA为多因素判别分析;HAC为层次聚类;NMDS为非度量多维尺度分析;MS/O为质谱/嗅觉;PROC MIXED为混合线性模型。
Note:TSQ is Triple-Stage Quadrupole; PRT is Proton-Transfer-Reaction; SPME is Solid Phase Micro-Extraction; TOF is Time of Flight Mass Spectrometer; FDA is Factorial Discriminant Analysis; HAC is Hierarchical Agglomerative Clustering; NMDS is Non-Metric Multidimensional Scaling Analysis; MS/O is Mass Spectrometry/Olfactometry; PROC MIXED is mixed model.
如上所述,利用代谢组学技术能够有效表征肉制品加工期间呈味物质的变化,为其加工工艺的优化奠定良好的理论基础。另外,中国传统肉制品种类繁多,采用该技术测定传统肉制品的特征成分,有助于传承优良的加工工艺,预期将会为中国传统肉制品产业的发展带来良好契机。
2.5 代谢组学在肉及肉制品产地溯源中的应用
产地溯源不仅是食品产业链风险监测的有效措施,也是地理标志产品保护的必要手段。不同产地肉及肉制品由于动物饲喂、产品加工方式等的不同最终会导致其代谢产物存在差异(表3)。Shintu等[62]对来自5个国家的牛肉干进行代谢组学分析,发现脯氨酸、苯丙氨酸、谷氨酸/谷氨酰胺、琥珀酸等可作为区分不同产地的特征代谢产物。此外,Jung等[63]分析了来自4个国家市售牛肉的代谢产物,发现琥珀酸、异亮氨酸、蛋氨酸、酪氨酸和缬氨酸在不同产地牛肉间差异显著。除以上代谢产物外,脂质在肉品中的分布也具有地域性特征,目前也已成功作为肉品产地溯源的靶标。程碧君[64]基于脂肪酸图谱,筛选出a-C18:3、C14:0、C17:0和MUFA作为我国四大牛肉主产区产地溯源的指标体系并建立了溯源判别模型,整体判别率可达83.6%。Mi等[65]对中国不同地域猪肉进行了脂质代谢组分析,共鉴定出100种差异脂质代谢产物,并建立了猪肉来源的定性辨别模型,正确辨别率可达91.1%。Vasilev等[66]也利用脂肪酸指纹图谱实现了北马其顿不同牧区羊肉的溯源。另外,稳定同位素、矿物元素指纹图谱技术等是也在肉品溯源研究中应用较广[70]。有学者将代谢组学与上述检测技术联合用于牛肉产品鉴别,有效提高了检测准确度[71]。
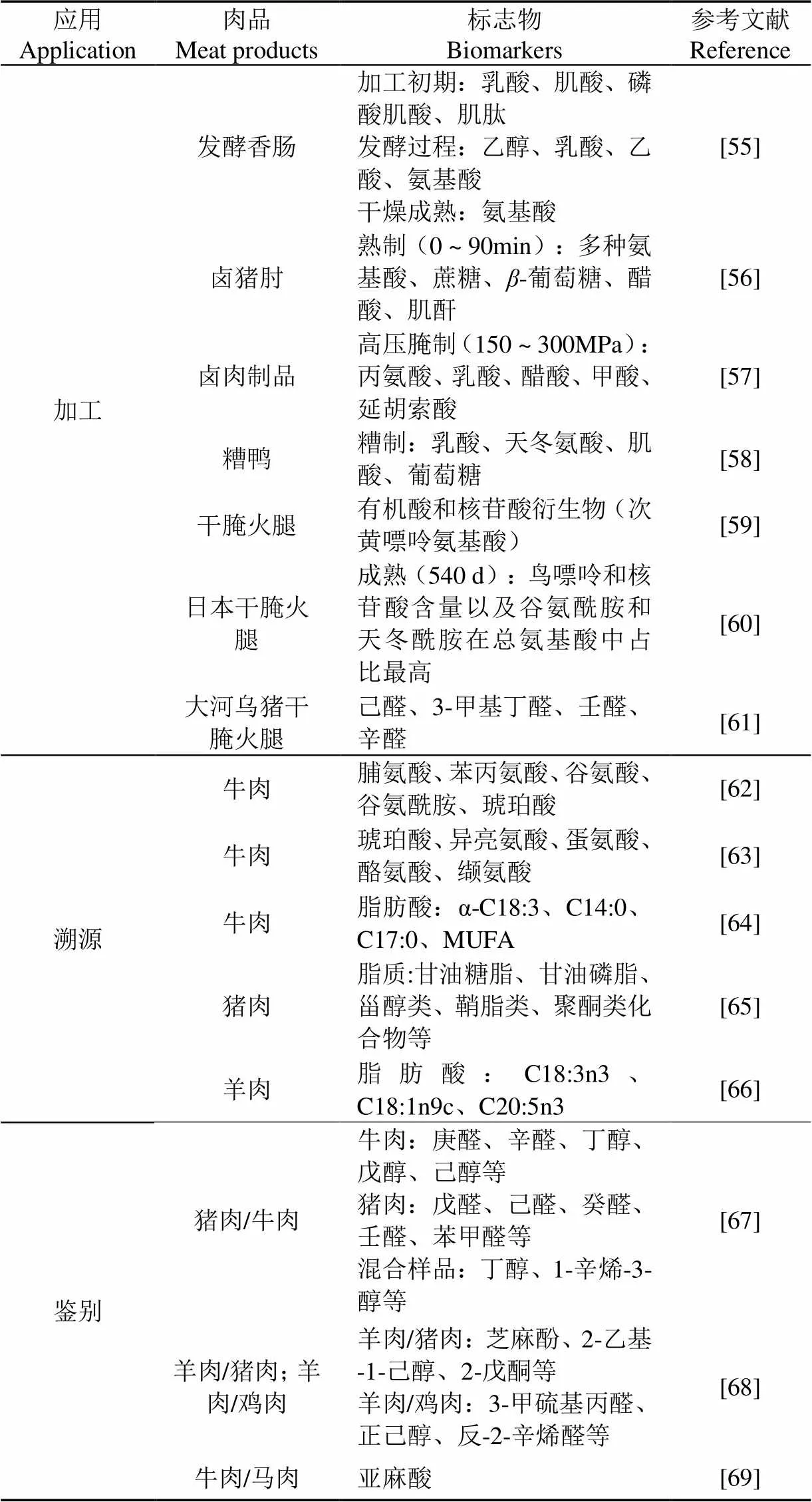
表3 代谢组学在肉制品加工、产地溯源及鉴别中的应用
注:-C18:3为a-亚麻酸;C14:0为肉豆蔻酸;C17:0为十七烷酸;MUFA为单不饱和脂肪酸;C18:3n3为-亚麻酸甲酯;C18:1n9c为油酸甲酯;C20:5n3为二十碳五烯酸。
Note:-C18:3 is a-linolenic acid; C14:0 is myristic acid; C17:0 is heptadecanoic acid; MUFA is monounsaturated fatty acids; C18:3n3 is methyl linolenate; C18:1n9c is methyl oleate; C20:5n3 is eicosapentaenoic acid.
由此可见,代谢组学技术在肉品产地溯源研究中具有良好的应用前景。但肉品代谢产物复杂,且受外界多种因素影响,使得该方法的重复性受到一定限制。因此,为提高甄别准确度,后续研究中可将鉴别出的特异性代谢产物基于靶向代谢组学技术进一步定量分析,有助于提高模型鉴别能力;或将该技术与其他分析技术联合应用于肉品产地溯源。
2.6 代谢组学在肉及肉制品鉴别中的应用
随着肉类需求增多,在经济利益的驱使下食品产业链中掺杂掺假、以次充好问题时有发生,严重破坏了市场秩序,损害了消费者的利益。目前,基于不同肉中的特征代谢产物的差异,已经成功实现了牛肉、羊肉、猪肉等的有效鉴别(表3)。Pavlidis等[67]基于挥发性代谢产物对猪肉、牛肉及其混合肉进行鉴别,发现庚醛、辛醛、丁醇等与牛肉样品存在相关性;戊醛、己醛、癸醛等与猪肉样品存在相关性;而2-丁醇、1-辛烯-3-醇等则是混合样品典型的代谢产物。孟新涛等[68]建立了基于气相离子迁移谱(GC Ion Mobility Spectrometry,GC-IMS)的羊肉掺假鉴别的新方法,发现当羊肉中掺入猪肉比例大于5%时,芝麻酚、2-乙基-1-己醇、2-戊酮等5种特征风味物质含量减少;当羊肉中鸡肉掺入比例达到10%时,3-甲硫基丙醛、正己醇、反-2-辛烯醛等46种特征风味物质含量减少。另外,Jakes等[69]通过检测牛肉和马肉样品中甘油三酯图谱,发现马肉中含有更高比例的不饱和脂肪酸,尤其是亚麻酸,依据代谢组学结果这两种肉均可被有效的鉴别。目前,基于代谢组学技术对肉及肉制品的鉴别多基于对样品小分子物质整体轮廓进行筛选。虽然该技术针对非特定目标物的检测优势明显,但其特异性相对其他检测技术(蛋白质组学技术、DNA法、传感器法等)较差。而且,代谢组学技术也存在耗时长且操作复杂等缺陷,以至于在肉品掺杂掺假检测中的应用潜力仍受到一定限制。
3 结论与展望
代谢组学作为一种新兴技术,弥补了基因组学、蛋白质组学等研究中的不足,在肉品科学领域展现出巨大的潜力和优势。基于代谢组学技术有助于我们深入理解动物的品种、年龄、饲喂及宰前管理方式等宰前因素及宰后条件对肉品品质的影响,并为生鲜肉货架期预测、肉制品加工工艺优选等提供新的角度。
但目前关于肉品代谢组学研究仍存在一些问题。
1)与药物学、医学等领域相比,代谢组学在肉品科学中的应用仍处于发展阶段。同时,肉品代谢产物复杂多样,使得经现有检测技术识别出的代谢产物依然十分有限。因此,为提高代谢组学技术在肉品科学中的应用潜力,多平台集成联合将会是未来的发展方向。
2)虽然现有的研究已经获取了大量涉及肉品品质的差异代谢产物,但这些研究多是基于非靶向代谢组学技术对小分子代谢产物整体轮廓进行筛选,虽然对非特定目标物优势明显,但实验的重复性较差。因此在肉品产地溯源、真伪鉴别等方面的应用受到一定限制。此外,不同研究采用的检测技术、分析方法等有所不同,使得研究结果有所差异。因此,今后可将识别出的差异代谢产物进行定量分析,或通过验证实验进一步明确特征代谢产物的影响机制。
3)目前肉品相关代谢物数据库还不完善,以至于未能准确建立代谢生物标志物与肉品品质之间的相关性。
另外,针对上述问题,今后也需在提高代谢组学检测技术分辨率的基础上,建立统一的数据分析方法;将代谢组学与其他组学(基因组学、蛋白质组学等)整合分析,有助于生物信息挖掘和系统生物学集成,这也将为肉品品质监测及改善提供新的契机。
[1] Li S, Tian Y, Jiang P, et al. Recent advances in the application of metabolomics for food safety control and food quality analyses[J]. Critical Reviews in Food Science and Nutrition, 2020, 4: 1-22.
[2] Miggiels P, Wouters B, Westen Van G, et al. Novel technologies for metabolomics: More for less[J]. Trends in Analytical Chemistry, 2019, 120: 1-9.
[3] Muroya S, Ueda S, Komatsu T, et al. MEATabolomics: Muscle and meat metabolomics in domestic animals[J]. Metabolites, 2020, 10: 1-12.
[4] Liu X, Locasale J W. Metabolomics: A Primer[J]. Trends in Biochemical Sciences, 2017, 42(4): 274-284.
[5] Wishart D S. NMR metabolomics: A look ahead[J]. Journal of Magnetic Resonance, 2019, 306: 155-161.
[6] Takis P G, Ghini V, Tenori L, et al. Uniqueness of the NMR approach to metabolomics[J]. Trends in Analytical Chemistry, 2019, 120: 1-9.
[7] Purslow P P. New Aspects of Meat Quality[M]//Bertram H C. NMR Spectroscopy and NMR Metabolomics in Relation to Meat Quality. Cambridge: Woodhead Publishing, 2017: 355-371.
[8] 秦泽宇,王浩,温荣欣,等. 基于核磁共振的代谢组学技术在肉品科学中的应用[J]. 食品工业科技,2019,40(2):312-315.
Qin Zeyu, Wang Hao, Wen Rongxin, et al. Application of metabolomics based on nuclear magnetic resonance (NMR) in meat science[J]. Science and Technology of Food Industry, 2019, 40(2): 312-315. (in Chinese with English abstract)
[9] 李娟,任路静,孙冠男,等. 气相色谱-质谱联用技术及其在代谢组学中的应用[J]. 生物工程学报,2013,29(40):434-446.
Li Juan, Ren Lujing, Sun Guannan, et al. Gas chromatography-mass spectrometry (GC-MS) and its application in metabonomics[J]. Chinese Journal of Biotechnology, 2013, 29(40): 434-446. (in Chinese with English abstract)
[10] 刘畅,罗玉龙,窦露,等. 亚麻籽饲喂对苏尼特羊肉风味品质的影响[J]. 农业工程学报,2019,35(21):304-311.
Liu Chang, Luo Yulong, Dou Lu, et al. Effect of feeding flaxseed on meat flavor quality of Sunit lambs[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2019, 35(21): 304-311.
[11] Frank D, Hughes J, Piyasiri U, et al. Volatile and non-volatile metabolite changes in 140-day stored vacuum packaged chilled beef and potential shelf life markers[J]. Meat Science. 2020, 161:108016.
[12] Argyri A A, Mallouchos A, Panagou E Z, et al. The dynamics of the HS/SPME-GC/MS as a tool to assess the spoilage of minced beef stored under different packaging and temperature conditions[J]. International Journal of Food Microbiology, 2015, 193: 51-58.
[13] Ercolini D, Ferrocino I, Nasi A, et al. Monitoring of microbial metabolites and bacterial diversity in beef stored under different packaging conditions[J]. Applied and Environmental Microbiology, 2011, 77: 7372-7381.
[14] Jääskeläinen E, Hultman J, Parshintsev J, et al. Development of spoilage bacterial community and volatile compounds in chilled beef under vacuum or high oxygen atmospheres[J]. International Journal of Food Microbiology, 2016, 223: 25-32.
[15] Storia A L, Ferrocino I, Torrieri E, et al. A combination of modified atmosphere and antimicrobial packaging to extend the shelf-life of beefsteaks stored at chill temperature[J]. International Journal of Food Microbiology, 2012, 158: 186-194.
[16] 李娟,任路静,孙冠男,等. 气相色谱-质谱联用技术及其在代谢组学中的应用[J]. 生物工程学报,2013,29(4):434-446.
Li Juan, Ren Lujing, Sun Guannan, et al. Gas chromatography-mass spectrometry (GC-MS) and its application in metabonomics[J]. Chinese Journal of Biotechnology, 2013, 29(4): 434-446. (in Chinese with English abstract)
[17] 林艳萍,司端运,刘昌孝. 液相色谱和质谱联用技术结合化学计量学应用于代谢组学的研究进展[J]. 分析化学,2007,35(10):1535-1540.
Lin Yanping, Si Duanyun, Liu Changxiao. Advances of liquid chromatography/mass spectrometry combined with chemometric approaches applied to metabonomics[J]. Chinese Journal of Analytical Chemistry, 2007, 35(10): 1535-1540. (in Chinese with English abstract)
[18] England E M, Matarneh S K, Oliver E M, et al. Excess glycogen does not resolve high ultimate pH of oxidative muscle[J]. Meat Science, 2016, 114: 95-102.
[19] Yu Q, Tian X, Shao L, et al. Targeted metabolomics to reveal muscle-specifc energy metabolism between bovine longissimus lumborum and psoas major during early postmortem periods[J]. Meat Science, 2019, 156: 166-173.
[20] 齐小城,章弘扬,梁琼麟,等. 液质联用技术及其在代谢组学研究中的应用[J]. 中成药,2009,31(1):106-112.
[21] Jacyna J, Kordalewska M, Markuszewski M J. Design of experiments in metabolomics-related studies: An overview[J]. Journal of Pharmaceutical and Biomedical Analysis, 2019, 164: 598-606.
[22] Graham S F, Farrell D, Kennedy T, et al. Comparing GC-MS, HPLC and1H NMR analysis of beef longissimus dorsi tissue extracts to determine the effect of suspension technique and ageing[J]. Food Chemistry, 2012, 134: 1633-1639.
[23] Warner R D, Jacob R H, Rosenvold K. Altered post-mortemmetabolism identified in very fast chilled lamb M. longissimus thoracis et lumborum using metabolomic analysis[J]. Meat Science, 2015, 108: 155-164.
[24] 许彦阳,姚桂晓,刘平香. 代谢组学在农产品营养品质检测分析中的应用[J]. 中国农业科学,2019,52(18):3163-3176.
Xu Yanyang, Yao Guixiao, Liu Pingxiang, et al. Review on the application of metabolomic approaches to investigate and analysis the nutrition and quality of agro-products[J]. Scientia Agricultura Sinica, 2019, 52(18): 3163-3176. (in Chinese with English abstract)
[25] Lu X, Zhao X, Bai C, et al. LC-MS-based metabonomics analysis[J]. Journal of Chromatography B, 2008, 866: 64-76.
[26] Cevallos-Cevallosa J M, Reyes-De-Corcuera J I, Etxeberria E, et al. Metabolomic analysis in food science: A review[J]. Trends in Food Science & Technology, 2009, 20: 557-566.
[27] Gomez J F M. Meat metabolomic pathway of Nellore and crossbred Angus × Nellore cattle [C]// 65thInternational Congress of Meat Science and Technology. Potsdam, Germany. 2019: 833-836.
[28] Straadt I K, Aaslyng M D, Bertram H C. An NMR-based metabolomics study of pork from different crossbreeds and relation to sensory perception[J]. Meat Science, 2014, 96: 719-728.
[29] Wang X,Fang C, He J, et al. Comparison of the meat metabolite composition of Linwu and Pekin ducks using 600 MHz1H nuclear magnetic resonance spectroscopy[J]. Poultry Science, 2017, 96:192-199.
[30] Kim H C. NMR-based metabolomic comparison of chicken meat from different breeds with multivariable analyses[C]// 65thInternational Congress of Meat Science and Technology. Potsdam, Germany. 2019: 797-800.
[31] Wang X, Jiang G, Kebreab E, et al.1H NMR-based metabolomics study of breast meat from Pekin and Linwu duck of different ages and relation to meat quality[J]. Food Research international, 2020, 133: 109126.
[32] Liu C, Pan D, Ye Y, et al.1H NMR and multivariate data analysis of the relationship between the age and quality of duck meat[J]. Food Chemistry, 2013, 141: 1281-1286.
[33] Xiao Z, Ge C, Zhou G, et al.1H NMR-based metabolic characterization of Chinese Wuding chicken meat[J]. Food Chemistry, 2019, 274: 574-582.
[34] Muroya S, Oe M, Nakajima I, et al. CE-TOF MS-based metabolomic profiling revealed characteristic metabolic pathways in postmortem porcine fast and slow type muscles[J]. Meat Science, 2014, 98(4): 726-735.
[35] Antonelo D S, Cônsoloa N R B, Gómez J F M, et al. Metabolite profile and consumer sensory acceptability of meat from lean Nellore and Angus × Nellore crossbreed cattle fed soybean oil[J]. Food Research International, 2020, 132: 109056.
[36] Zawadzki A, Arrivetti L O R, Vidal M P, et al. Mate extract as feed additive for improvement of beef quality[J]. Food Research International, 2017, 99: 336-347.
[37] Baira E, Dagla L, Siapi E, et al. UHPLC-HRMS-based tissue untargeted metabolomics study of naringin and hesperidin after dietary supplementation in chickens[J]. Food Chemistry, 2018, 269: 276-285.
[38] 李敬,杨媛媛,赵青余. 肉风味前体物质与风味品质的关系研究进展[J]. 中国畜牧杂志,2019,55(11):1-7.
[39] Koutsidis G, Elmore J S, Oruna-Concha M J, et al. Water-soluble precursors of beef flavour. Part II: Effect of post-mortem conditioning[J]. Meat Science, 2008, 79: 270-277.
[40] Consolo N R B. Meat metabolites profile changed by ageing time[C]// 65thInternational Congress of Meat Science and Technology. Potsdam, Germany. August, 2019: 793-796.
[41] Bischof G. Analysis of aging type and time of beef by1H-NMR spectroscopy[C]// 65thInternational Congress of Meat Science and Technology. Potsdam, Germany. 2019: 824-826.
[42] Warren K E, Kastner C L. A comparison of dry-aged and vacuum-aged beef strip loins[J]. Journal of Muscle Foods, 1992, 3(2): 151-157.
[43] Kim Y H B, Kemp R, Samuelsson L M. Effects of dry-aging on meat quality attributes and metabolite profiles of beef loins[J]. Meat Science, 2016, 111: 168-176.
[44] Mungure T. NMR-based metabolites profiling of wet and dry aged pulsed electric fields treated venison[C]// 65thInternational Congress of Meat Science and Technology. Potsdam, Germany. 2019: 819-821
[45] Zhang R. Effect of step-wise dry-ageing and trimming on the metabolite profiles of dry-aged bull beef[C]//65thInternational Congress of Meat Science and Technology. Potsdam, Germany. 2019: 788-790.
[46] Nychas G J E, Skandamis P N, Tassou C C, et al. Meat spoilage during distribution[J]. Meat Science, 2008, 78: 77-89.
[47] Casaburi A, Piombino P, Nychas G, et al. Bacterial populations and the volatilome associated to meat spoilage[J]. Food Microbiology. 2015, 45: 83-102.
[48] Mansur A R, Seo D H, Song E J, et al. Identifying potential spoilage markers in beef stored in chilled air or vacuum packaging by HS-SPME-GC-TOF/MS coupled with multivariate analysis[J]. LWT - Food Science and Technology, 2019, 112: 1-6.
[49] Argyri A A, Doulgeraki A I, Blana V A, et al. Potential of a simple HPLC-based approach for the identification of the spoilage status of minced beef stored at various temperatures and packaging systems[J]. International Journal of Food Microbiology, 2011, 150: 25-33.
[50] Reis M M, Reis M G, Mills J, et al. Characterization of volatile metabolites associated with confinement odour during the shelf-life of vacuum packed lamb meat under different storage conditions[J]. Meat Science, 2016, 113: 80-91.
[51] Zareian M, Böhner N, Loos H M, et al. Evaluation of volatile organic compound release in modified atmosphere packaged minced raw pork in relation to shelf-life[J]. Food Packaging and Shelf Life, 2018, 18: 51-61.
[52] Nieminen T T, Dalgaard P, Björkroth J. Volatile organic compounds and Photobacterium phosphoreum associated with spoilage of modified-atmosphere-packaged raw pork[J]. International Journal of Food Microbiology, 2016, 218: 86-95.
[53] Lyte J M, Legako J F, Martin J N, et al. Volatile compound characterization of modified atmosphere packaged ground beef held under temperature abuse[J]. Food Control, 2016, 59: 1-6.
[54] Remenant B, Jaffrès E, Dousset X, et al. Bacterial spoilers of food: Behavior, fitness and functional properties[J]. Food Microbiology, 2015, 45: 45-53.
[55] García-Garcíaa A B, Lamichhanec S, Castejónb D, et al.1H HR-MAS NMR-based metabolomics analysis for dry-fermented sausage characterization[J]. Food Chemistry, 2018, 240: 514-523.
[56] Yang Y, Pan D, Sun Y, et al.1H NMR-based metabolomics profiling and taste of stewed pork-hock in soy sauce[J]. Food Research International, 2019, 121: 658-665.
[57] Yang Y, Ye Y, Wang Y, et al. Effect of high pressure treatment on metabolite profile of marinated meat in soy sauce[J]. Food Chemistry, 2018, 240: 662-669.
[58] 周楠楠,楼宵玮,王颖,等.1H核磁共振结合多元统计方法分析糟鸭加工过程中滋味化合物的变化[J]. 食品科学,2019,40(6):233-239.
Zhou Nannan, Lou Xiaowei, Wang Ying, et al. Changes in taste compounds during processing of vinasse-cured duck as studied by 1h nmr combined with multivariate data analysis[J]. Food Science, 2019, 40(6): 233-239. (in Chinese with English abstract)
[59] Zhang J, Yi Y, Pan D, et al.1H NMR-based metabolomics profiling and taste of boneless dry-cured hams during processing[J]. Food Research International, 2019, 122: 114-122.
[60] Sugimoto M, Sugawara T, Obiya S, et al. Sensory properties and metabolomic profiles of dry-cured ham during the ripening process[J]. Food Research International, 2020, 129: 1-9.
[61] Shi Y, Li X, Huang A. A metabolomics-based approach investigates volatile flavor formation and characteristic compounds of the Dahe black pig dry-cured ham[J]. Meat Science, 2019, 158: 1-8.
[62] Shintu L, Caldarelli S, Franke B M. Pre-selection of potential molecular markers for the geographic origin of dried beef by HR-MAS NMR spectroscopy[J]. Meat Science, 2007, 76: 700-707.
[63] Jung Y, Lee J, Kwon J, et al. Discrimination of the geographical origin of beef by1H NMR-based metabolomics[J]. Journal of Agricultural Food Chemistry, 2010, 58: 10458-10466.
[64] 程碧君. 基于脂肪酸指纹分析的牛肉产地溯源研究[D]. 北京:中国农业科学院,2012:40-48.
Cheng Bijun. Study on Beef Geographical Origin Traceability based on Fatty Acid Fingerprint Analysis[D]. Beijing: Chinese Academy of Agricultural Sciences, 2012:
40-48. (in Chinese with English abstract)
[65] Mi S, Shang K, Li X, et al. Characterization and discrimination of selected China’s domestic pork using an LC-MS-based lipidomics approach[J]. Food Control, 2019, 100: 305-314.
[66] Vasilev D, Dimovska N, Hajrulai-Musliu Z, et al. Fatty acid profile as a discriminatory tool for the origin of lamb muscle and adipose tissue from different pastoral grazing areas in North Macedonia - A short communication[J]. Meat Science, 2020, 162: 1-5.
[67] Pavlidis D E, Mallouchos A, Ercolini D, et al. A volatilomics approach for off-line discrimination of minced beef and pork meat and their admixture using HS-SPME GC/MS in tandem with multivariate data analysis[J]. Meat Science, 2019, 151: 43-53.
[68] 孟新涛,张婷,许铭强,等. 基于气相离子迁移谱的羊肉掺伪快速鉴别方法[J]. 新疆农业科学,2019,56(10):1939-1947.
Meng Xintao, Zhang Ting, Xu Mingqiang, et al. Detection of authenticity of mutton with Gas Chromatography - Ion Mobility Spectrometry (GC-IMS) [J].Xinjiang Agricultural Sciences, 2019, 56(10): 1939-1947. (in Chinese with English abstract)
[69] Jakes W, Gerdova A, Defernez M A D, et al. Authentication of beef versus horse meat using 60 MHz1H NMR spectroscopy[J]. Food Chemistry, 2015, 175: 1-9.
[70] Monahana F J, Schmidta O, Moloney A P. Meat provenance: Authentication of geographical origin and dietary background of meat[J]. Meat Science, 2018, 144: 2-14.
[71] Renou J P, Bielicki G, Deponge C, et al. Characterization of animal products according to geographic origin and feeding diet using nuclear magnetic resonance and isotope ratio mass spectrometry. Part II: Beef meat[J]. Food Chemistry, 2004, 86: 251-256.
Application of metabolomics in monitoring the qualities of meat and meat products
Chen Xue1, Luo Xin1,2, Liang Rongrong1, Zhu Lixian1, Yang Xiaoyin1, Han Mingshan3, Cheng Haijian4, Zhang Yimin1※
(1.,,271018,; 2.,210095,;3.,,028100,;4.,,250000,)
Meat and meat products have been the most important sources of proteins in the human diet, particularly on directly linking to public health and welfare. Meat quality has attracted much more attention for the meat industry worldwide, as the meat consumption is increasing in recent years, due to the improvement of living standards. Generally, the meat quality depends highly on pre-slaughter factors, including breed, age, muscle types, as well as the ways of post-slaughter processing, where the alteration of metabolites normally occurs all over the stages during meat production. Thus, the intrinsic mechanism of meat quality at the metabolites level has been a highly relevant issue for improving the nutritional value of meat. As a branch of systems biology, metabolomics mainly focuses on the whole metabolome, metabolites of molecular weight below 1 500 Da, to represent in a biological system, whether it being stimulated or disturbed. Recently, the interest in the application of metabolomics has been extended to the field of meat science with constantly rising studies. This present review systematically summarized the main techniques that used in metabolomics, based on the methodology of recent studies, including the Nuclear Magnetic Resonance (NMR) spectroscopy, Gas Chromatography-Mass Spectroscopy (GC-MS), and High-Performance Liquid Chromatography-Mass Spectroscopy (HPLC-MS), as well as the applied methods for data analysis. Five aspects were also overviewed, according to the recent findings in metabolomics associated with meat quality traits. 1) In pre-slaughter factors, animal breed, ages, muscle types, and diet can be recognized as the most significant indictors of meat quality. Most previous studies confirmed that the metabolomics profiling related to age or breed can contribute to the assessment of meat quality, and thereby to provide theoretical support for the development of high-quality meat resources. Moreover, the different types of muscle in an animal have shown the distinct metabolic characteristics of individual energy. In recent reports, these differences in postmortem muscle metabolites were identified to provide useful theoretical information regarding the biochemistry process of muscle to meat conversion. Additionally, metabolomics has shown a promising potential to distinguish the various feeding regime, and dietary addition of mate extract, such as naringin, hesperidin, further to facilitate the creation of novel management schemes for mitigating limitation in meat quality. 2) Metabolomics can offer a new perspective to predict the post-mortem ageing time, shelf-life of meat and meat products. Previous studies also found that the metabolomics can achieved data information on the flavor and taste that related to metabolites changes, particularly occurring on ageing of meat, predicting ageing time, and differentiating various aging conditions. The reason is that the metabolites variation in meat depended mainly on the ageing time and conditions after post-mortem. The growth and enzymatic activity of microorganisms can cause the meat decomposition and formation of metabolites, resulting in the meat spoilage. Hence, those changes have also been reviewed on critical metabolites that exploited for monitoring the shelf-life of meat. 3) In processed meat products, numerous biochemical and biophysical reactions can pose some influence on the final quality during meat handling and cooking. These changes detected by metabolomics can be contributed to the optimization of the processing technology. 4) A potential technique of metabolomics was applied to identify metabolic markers that selected for the substantiation of the claim, and further to aid in the certification of the geographical origin of meat product. 5) Metabolomics has also been developed as a useful tool for the adulteration detection of meat and meat products, showing the reliable meat identification. Finally, an insightful prospect was made, in order to provide a sound theoretical basis for the further application of metabolomics to meat science.
meat; quality control; metabolomics; shelf life; geographical origin traceability; biomarker
陈雪,罗欣,梁荣蓉,等. 代谢组学在肉及肉制品品质监测中的应用[J]. 农业工程学报,2020,36(17):291-300.doi:10.11975/j.issn.1002-6819.2020.17.034 http://www.tcsae.org
Chen Xue, Luo Xin, Liang Rongrong, et al. Application of metabolomics in monitoring the qualities of meat and meat products[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2020, 36(17): 291-300. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2020.17.034 http://www.tcsae.org
2020-04-02
2020-08-31
现代农业产业技术体系建设专项资金资助(肉牛CARS-37);山东省现代农业产业技术体系创新团队建设专项资金(SDAIT-09-09)
陈 雪,博士生,主要从事肉品科学研究。Email:2019010030@sdau.edu.cn
张一敏,博士,副教授,主要从事肉品科学研究。Email:ymzhang@sdau.edu.cn
10.11975/j.issn.1002-6819.2020.17.034
TS251.1
A
1002-6819(2020)-17-0291-10
