Preparation, characterization and health benefit functions of unripe apple polyphenols-chitooligosaccharides microcapsule
2020-09-21ZhengHuzheChoiJihyeSeongGiunChungShinkyo
Zheng Huzhe, Choi Jihye, Seong Giun, Chung Shinkyo
Preparation, characterization and health benefit functions of unripe apple polyphenols-chitooligosaccharides microcapsule
Zheng Huzhe1,2, Choi Jihye2, Seong Giun2, Chung Shinkyo2※
(1.,,, 223003,; 2.,,, 41566,)
In order to improve unripe apple polyphenols (APP) and chitooligosaccharides (COS) multifunctional effect, unripe apple polyphenols-chitooligosaccharides microcapsule (APCM) was prepared by spray-drying method. The microcapsule particle size, distribution, and the binding characters of APCM were detected. And the effects of simulated gastrointestinal digestion model on functional compounds release and health benefit functions were also evaluated. The results showed that the mean particle size of APCM was 32.98m. In addition, APCM exhibited the smallest span value of 1.19, which meant APCM was more homogeneous than COS and APP. The results of binding characters showed APP had a sharper peak at 1 237 and 1 194 cm-1, which was not observed in APCM. However, APCM had similar absorption bands to the APP, which could be inferred that the combination of the APCM was due to hydrogen bonding, electrostatic, and van der Waals interactions between COS and APP. The simulated gastrointestinal digestion model results showed that the release of polyphenols from APCM occurred within 60 min. In simulated gastric fluid (SGF) treatment, the amount of total phenolic content (TPC) released from APCM ranged from 25.6% to 76.5%, and in the continuous incubation period in simulated intestinal fluid (SIF) treatment, the TPC released from 31.3% to 97.6%. The results ofantioxidant and reactive oxygen species (ROS) scavenging activity indicated that APCM exhibited more excellent radical scavenging activity compared with that of APP and COS toward oxygen radical absorbance capacity (ORAC), 1,1-diphenyl-2-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP), hydroxyl radical (•OH), superoxide anion radical (O2•-), and hydrogen peroxide radical (H2O2) scavenging activity, respectively. Furthermore, APCM exhibited not only higher aldose reductase inhibition activity (<0.05), but also better Angiotensin I converting enzyme (ACE) inhibition activity (<0.05) compared with APP and COS. The results suggest that APCM has greater potential for future research and development in the field of functional food or drugs.
agricultural products; oxidation resistance; apple polyphenols-chitooligosaccharides microcapsule; simulated gastrointestinal digestion model; release ratio; aldose reductase; angiotensin I converting enzyme
0 Introduction
Unripe apples lie scattered about the orchards because of manually thinning out or falling, however, they contain abundant amounts of polyphenol compounds[1-2]that have been reported to exert a variety of biological actions such as free radical scavenging activity[3], anti-allergic activity[4], and anti-arteriosclerosis activity[5].Over the past few years, not only by-products, but also a number of other agricultural wastes of plant origin have attracted considerable attention as potential sources of bioactive phytochemicals, which can be used for various purposes in the pharmaceutical, cosmetic and food industry. However, in many cases, there has been a rather significant lack of appropriate feasibility studies on the extraction and exploitation of such bioactive compounds, since extraction and deep processing techniques have been often regarded as bottlenecks in the food processing industry[1-2].
Chitosan is a linear polysaccharide composed of randomly distributed-(1-4)-linked D-glucosamine and N-acetyl-D-glucosamine[6]. Due to the nontoxic, antioxidant, anti-mutagenetic ability, biodegradable and biocompatible properties of chitosan provide potential for many biotechnological applications[7]. Chitosan used as drug carrier can improve drug absorption and release[8-9]. Unfortunately, the application of chitosan is limited to the heavy molecular weight and insolubility, especially in human body[10]. Chitooligosaccharides (COS), which is obtained through the decomposition of chitosan, is deacetylated chitin oligosaccharides, and can be dissolved in water easily to give versatile bioactive functions such as antioxidant and aldose reductase inhibition activity[6]. In addition, compared with native chitosan, COS has been reported to possess many superior functional properties, such as enhancing antimicrobial activity[10], enhancing bioavailability and delivery of drugs to cancer cells, gelation into hydrogel beads for encapsulation applications[11], complexation with functional compounds to formulate advanced nano-carrier for drugs and nutrients[12]. However, COS has a little limitation, such as narrow range of application, and less powerful, etc.[13].To overcome this drawback, COS was modificated with functional groups obtained derivatives, such as COS-Nisin conjugate[10], cinnamol-COS polymer[13], chlorogenic acid-COS conjugate[14], COS-coated tea polyphenols nanoparticles[15],tea catechins-COS conjugation[16], salicylic acid-grafted COS[17], etc. The health benefits functions through mixing various functional groups with COS are improved significantly.
This study focuses on the preparation and characterization of health benefit functions of unripe apple polyphenols-chitooligosaccharides microcapsule (APCM) by spray drying, Fourier transform infrared spectroscopy (FTIR) analysis, laser particle size analysis techniques, anddigestion experiment. This study will provide experimental data to elucidate the APCM and the development of foods and medicines with more health care efficacy.
1 Materials and methods
1.1 Materials, reagents and instruments
Unripe apples (cv. Fuji) were collected on the 85thdays after full bloom from the orchard of Kyungpook National University in Daegu, Korea, and stored in a freezer until the experiment. Viscozyme L (from, 100 fungal-glucanase U/mL, Bagsvaerd, Denmark). Folin Ciocalteu reagent, 1,1-diphenyl-2-picrylhy drazyl (DPPH) reagent, xanthine oxidase (90 U/mg), and caffeic acid were obtained from Sigma Co. (St. Louis, MO). COS (MV, 3.4×104Da) was purchased from Kitto Life Co. (Pyongtaeg, Korea). All organic solvents and other chemicals were analytical grade and purchased from Duksan Co. (Seoul, Korea). Laser particle size analyzer (N5/SL-13320, Beckman Coulter Inc., Pasadena, CA, USA), FTIR (GX model RXI, PerkinElmer Inc., CT, USA), Mini spray-dryer (Büchi B-191, Flavil, Switzerland), Multiable counter (Victor 3 1420, PerkinElmer Inc., CT, USA) were used in this study.
1.2 Preparation of apple polyphenols
Ten kilograms of whole unripe apples were blanched at 85 ℃ for 15 min for the inhibition of polyphenol oxidase, crushed, and homogenized with equal volume of water. After addition of 1.95% Viscozyme L, the homogenized solution was incubated at 47.1 ℃, for 12.5 h[2], filtered, and concentrated to apple crude polyphenols with a rotary evaporator. And then the sample was applied onto Amberlite XAD-7 column (100 cm × 7 cm), washed out with deionized water, and eluted with 95% ethanol. The eluted solution was collected, concentrated, and finally unripe apple polyphenols (APP) powder was achieved by spray-drying process with the same method of preparation APCM.
1.3 Preparation of APCM
According to our previous study, APP and COS powder was dissolved in 20 times distilled water, respectively, and mixed at a ratio of 2.5:1 (w/w), thoroughly stirred before the spray drying process. Spray-drying was done using Büchi B-191 spray dryer with the inlet temperature of 150 ℃, pump setting of 40%, air flow rate of 125 m3/h, aspirator set at 60% and outlet temperature of 65 ℃, the solution flow rate was 250 mL/h. As a contrast, COS was performed the same spray-drying process.
1.4 Characterizations of APCM
For particle size distribution, a laser particle size analyzer was performed using a Beckman Coulter equipment. The distribution ratio, average particle diameter, and sphericity degree were determined using an image processing program. The mean, mode and median were measured and the span was calculated according to Bruna et al.[18].
Span = (90−10) /50(1)
where90,50, and10are the volume diameters (m) at 90%, 50%, and 10% of the cumulative volume, respectively.
The FTIR was carried out using FTIR Nicolet Avatar 360 (32 scans, Resolution 4.000, Wave number range 4 000-400 cm-1). The samples were prepared by KBr pellet method.
1.5 Antioxidant and reactive oxygen species (ROS) scavenging activities
Antioxidant activities were determined by oxygen radical absorbance capacity (ORAC), DPPH and ferric reducing antioxidant power (FRAP). ORAC was determined according to the method of Ou et al.[19]ORAC value was expressed as mmol trolox equivalents per gram of sample (mmol/g). The scavenging activity for DPPH radicals was determined according to Liu et al.[20]. The activity was expressed in mmol trolox equivalents per gram of sample (mmol/g). FRAP was performed according to previously reported by Kandola et al.[21]. The results were expressed in mmol trolox equivalents per gram of sample (mmol/g). Hydroxyl radical (•OH), superoxide anion radical (O2•-), and hydrogen peroxide radical (H2O2) scavenging activities were determined as ROS activities. •OH scavenging activity was determined by the method of described by Kandola et al.[21]. The results were expressed in mmol quercetin equivalents per gram of sample (mmol/g). O2•-radical scavenging activity was determined using the method of Krawitzky et al.[22]The results were expressed in mmol quercetin equivalents per gram of sample (mmol/g). H2O2scavenging activity was determined according to the method described by Ou et al.[19]. The results were expressed in mmol quercetin equivalents per gram of sample (mmol/g). Total phenolic content (TPC) was determined by the Folin-Ciocalteu method[22], and expressed as gallic acid equivalents (mg/g).
Caffeic acid(250mol/L) was used for the positive control in the above antioxidant tests.
1.6 Release ratio of polyphenols from APCM in simulated gastrointestinal digestion model
During a certain period, extraction of TPC in a simulated gastrointestinal digestion model was defined as release ratio of polyphenols. The release ratio was likely to affect absorption and utilization in the human body[23].release ratio of polyphenols from APCM was carried out in simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) by the method of Pasukamonsetet al. with a modification[23]. Briefly, the sample was treated with SGF (1.0 g of NaCl, 12 mol/L HCl, 3.5 mL, pH value 1.68) at 37 ℃ with agitation for 0-30 min. Then, the pH value was adjusted to SIF (0.2 mol/L NaOH, 0.2 mol/L KH2PO4, pH value 6.8), followed by incubation at 37 ℃ with agitation for 0-30 min. APP as a control was performed. The samples as a pair were simultaneously divided into 6 parts, each of which was prepared for each subsequent experiment, respectively. And then, the solution was used todetermine TPC and antioxidant activities.
1.7 Aldose reductase inhibition activity
Crude aldose reductase was prepared from rat lenses. The lenses were removed from the eyes of 8-week-old male sprague-dawley rats, each weighing 100-150 g, and were homogenized in 12 volumes of 135 mmol/L Na, K-phosphate buffer (pH value 7.0) containing 0.5 mmol/L phenylmethylsulfonyl fluoride and 10 mmol/L 2-mercaptoethanol. The homogenate was centrifuged at 10 000 r/min for 30 min, and the resulting supernatant was retained as an enzyme preparation. All procedures were carried out at 4 ℃. The protein content (6.67 mg/mL) of the enzyme preparation was determined using Coomassie blue reagent (Bio-Rad) with bovine serum albumin as a standard. Aldose reductase inhibition activity was assayed by the method described in a previous paper[24]. The luorescence was measured using multiable counter at an excitation wavelength of 360 nm and an emission wavelength of 460 nm.

whereis sample absorbance, CB is absorbance of control blank (enzyme and sample free), SC is negative control absorbance (sample free).
1.8 Angiotensin I converting enzyme (ACE) inhibition activity
Crude ACE was prepared from rabbit lung acetone powder. Briefly, 2 g of rabbit lung acetone powder was homogenized in 10 mL of 100 mmol/L sodium borate buffer (pH value 8.3) containing 300 mmol/L NaCl, and stirred in 4 ℃ for 24 h. The homogenate was centrifuged at 15 000 r/min in 4 ℃ for 60 min, and the supernatant was retained as an enzyme preparation. ACE inhibition activity was assayed by a modification of the method of Zhang et al.[4]. The absorbance at 228 nm was measured to calculate the ACE inhibition activity.

whereis sample absorbance, SB is absorbance of sample blank,is control absorbance, and CB is absorbance of control blank.
1.9 Statistical analysis
The data were presented as means ± SD. The values were evaluated by analysis of variance (ANOVA), followed by Duncan’s multiple range test.<0.05 was considered as significant difference.
2 Results and Analysis
2.1 Yield of unripe apple polyphenols with Viscozyme L extraction
Since apple polyphenols are mainly contained in the peel and seeds, most of the polyphenols are left in the pomace during juice production[5]. Therefore, the enzyme-aided extraction of unripe apple polyphenols using Viscozyme L was attempted in this study. After enzyme-aided extraction, XAD-7 adsorption was introduced to the process, and the increment profiles of the polyphenols contents was showed in Table 1. The results indicate that APP and TPC extraction yield increase by about 2.5 and 2 times, respectively, compared with the control treatment, indicating the occurrence of more active hydrolysis reaction under the enzyme-aided condition.

Table 1 Yield of apple polyphenols and total phenolic content extracted with Viscozyme L-aided extraction
Note: a. Without enzyme treatment; b. Ratio of Viscozyme L to substrate 1.95%; pH 3.7; 47.12℃; 12.52 h.
2.2 Microcapsule particle size and distribution measurement
As a drugs and nutrients carrier, COS helps overcome certain adverse characteristics of drugs and nutrients such as insolubility and hydrophobicity[25]. However, the disadvantage of the narrow scope of COS limits its application as an active functional food[13]. In recent years, the interactions between COS and green tea extract[26], strawberry extract[27], thyme extract[28], and apple peel extract[29]have been studied respectively. These days, a spray-drying technique has been developed for drug making associated with COS[30]. Spray-drying is a well-established method for processing solutions, emulsions, and suspensions into powders, efficiently controlling the size, powder density, and morphology of the particles[31]. In addition, spray-dried powders that exhibit sustained drug release properties may be generated through the inclusion of drug release modifiers[18]. In this study, APCM was produced by spray-drying technique to improve bioavailability and delivery of apple polyphenols to target cells by drug carrier COS. Table 2 shows the results of the size distribution of COS, APP, and APCM in terms of average size and heterogeneity of size distribution (span). The size distribution range were from 0.5m to 150m. The mean particle size of COS, APP, and APCM was 31.33, 36.98, and 32.98m, respectively. The skewness results show that the distribution is distorted relative to the right side of the normal distribution, and kurtosis results show that the distribution is sharp compared with the normal distribution. Moreover, the microcapsule presented some heterogeneity (span ranged from 1.19 to 1.26), confirming the results of morphology. In our case, among the products, APCM exhibited the smallest span value, which means APCM is more homogeneous than COS and APP. These results were supported by Bruna et al.[18]who studied chitosan-encapsulatedextract, and affirmed that smaller particles had a greater surface area exposed, resulting in a faster degradation of sensitive compounds. Therefore, it is expected that APCM can better stabilize health benefit functions compounds of the release than COS and APP.

Table 2 Particle size and distribution analysis of COS, APP, APCM microcapsules by spray-drying
Note: Means with different superscript letters in the same column are significantly different (<0.05).
2.3 FTIR analysis
FTIR spectroscopy was applied to identify the functional groups by their characteristic vibration, and the most significant vibrations, occurred in the parochial extent of 4000-400 cm-1. The FTIR spectra of COS, APP, and APCM are presented in Fig.1. The spectrum of COS exhibited typical characteristic bands due to the amino and amide groups present in its structure, stretching vibrations of the C=O drawing (amide I), N-H stretching (amide II) and C-N bending (amide III) were visible at 1 632, 1 528 and 1 296 cm-1[12,32], respectively. The basic characteristics of APP were shown at 3 488 cm-1(strong and wide O-H stretch), 1 439 cm-1(C=C stretch), 1 272 cm-1(C=O stretch)[14,27]. However, The FTIR of APP showed a sharper peak at 1 237 and 1 194 cm-1[3], which was not observed in APCM. The loss of the APP bands was due to polyphenol bindings to polymer C=O, C-N and N-H groups by hydrophilic interaction during APCM encapsulation[14]. It was in agreement with the reported spectra[16], who pronounced that tea catechin were conjugated to chitosan by bind nanoparticles via hydrophilic, hydrophobic, and H-bonding contacts with polyphenols. Furthermore, a new band at 1 439 cm-1was observed in APCM when compared with COS, indicating an electrostatic interaction between the protonated amino groups of COS and the dissociated carboxylate groups of APP[29,32]. Besides, the absorbance at 855 cm-1in APP and APCM may be due to the band attributed to the =C-H groups in polyphenols in APP. These results were supported by Dou et al.[3]who showed the similar FTIR pattern to that of thinned young apple extraction. In addition, some of these observations were also reported for the reactions between COS and strawberry polyphenols[27], resveratrol[33], chlorogenic acid[14], respectively.
Furthermore, APCM had similar absorption bands to the APP, and these modifications to the bands intensities indicate that there are minor interactions between the COS and APP after spray-drying. It could be inferred that the vast majority of APCM is obtained by physical combining, due to hydrogen bonding, electrostatic, and van der Waals interactions between COS and APP[12].

Fig.1 FTIR spectrum of APCM prepared by spray-drying technique
2.4 Stability of antioxidant and ROS scavenging activities
Recent evidence indicates that the low molecular COS can scavenge free radical[20]. However, very few attempts have been made to evaluate the antioxidant activity of COS in polyphenols-rich materials. Therefore, the effects of APCM on antioxidant and ROS scavenging activities were compared in this work. For antioxidant activities, APCM (8 532.22, 2 717.05 mmol/g, and 2 493.04 mmol/g) exhibited the highest radical scavenging activity, followed by APP (8 251.42, 2 424.01 and 2 341.85 mmol/g), and COS (63.18, 34.71 and 50.53 mmol/g) toward ORAC, DPPH and FRAP, respectively. For ROS scavenging activities, APCM (1 764.51, 1 208.47, 1 864.15 mmol/g) exhibited the highest scavenging activities, followed by APP (1 404.02, 975.61, 1 467.63 mmol/g), and COS (51.02, 66.33, 87.94 mmol/g) toward •OHscavenging activity, O2•-scavenging activity, and H2O2scavenging activity, respectively (Table 3). In addition, antioxidant and ROS scavenging activities of samples were compared with that of the major component of unripe apple polyphenol[2], caffeic acid (250mol/L). Caffeic acid also exhibited high antioxidant and ROS scavenging activity, comparable with that of APCM and APP (Table 3). Interestingly, APP exhibited higher antioxidant and ROS scavenging activities than caffeic acid, respectively.These results indicated that high antioxidant and ROS scavenging activities corresponded to a great extent to high TPC as well (Table 1). Here, APCM exhibited excellent scavenging activity, which fact is attributable to its synergy effect of COS and polyphenols.

Table 3 Effects of COS, APP and APCM on antioxidant and ROS scavenging activities
Note: Mean±SD (=3), means with different superscript letters in the same column are significantly different (<0.05).
2.5 Release ratio of polyphenols from APCM in simulated gastrointestinal digestion model
To further understand the polyphenols release ratio from APCM on human body, it is essential to determine their release ratio. However, there is little information on polyphenols, how digestion process affects polyphenols, and their solubility and antioxidant stability from APCM. Thus, studies intended to encapsulation of COS with polyphenols to abtain the release of polyphenolsfound that the process might be reversible. The TPC of the APP and its encapsulation APCM in SGF (Fig.2a) and SIF (Fig.2b) digestion are demonstrated in Fig.2. The results showed that the release of polyphenols from APP and APCM occurred within 60 min. In SGF treatment, the amount of TPC released from APCM ranged from 25.6% to 76.5% during the incubation periods. However, the continuous incubation in SIF treatment, the TPC released from APCM ranged from 5.7% to 21.1%. According to He et al.[34]in an acid medium, increasing COS solubility higher than basic medium, in our case, SGF treatments exhibited higher TPC release percentage than SIF treatment at the same incubation periods, may be due to the decreased cross-linking density of the particles by acidity fluids, and enhance COS solubility. This enhanced APCM solubility will lead to higher release of mixed polyphenols. In addition, as a control treatment, the APP showed good TPC release capacity both in SGF and SIF solutions. However, the solubility in SGF was a little better than SIF solution. Recently, many researches indicate that after release in stomach and small-intestine, unreleased polyphenols could be further released in large-intestine for prolonged periods[4,23,25]. Since, further studies on the release and absorbed by human body are required.
2.6 Aldose reductase inhibition activity
Aldose reductase is the rate-limiting enzyme in the polyol pathway which was found to be enhanced during hyperglycemia converting excess glucose into sorbitol[35]. Hence, inhibit of aldose reductase activity has been considered a potential treatment of diabetic complications[36]. It has been well acknowledged that plant-derived extracts and phytochemicals such as flavonoids and polyphenols are potential alternatives to synthetic inhibitors against aldose reductase[24]. Recently, Zheng et al.[37]reported that COS could reduce the level of blood glucose in patients with type Ⅱdiabetes mellitus. However, relatively little work has been done on aldose reductase inhibition activity of apple polyphenols-COS complex. Therefore, in this study, aldose reductase inhibition activity of APCM was determined and compared with COS and APP. Fig.3 showed at the concentration of 0.05, 0.1, and 0.2 mg/mL, APP showed 36.36%, 50.27%, and 72.98% inhibition against aldose reductase, respectively, whereas APCM showed 45.03%, 63.48%, and 83.64%, respectively. These results indicate that APP and APCM have excellent inhibitory effectsagainst aldose reductase. Our results were supported by Veeresham et al.[38]who reported that inhibition of aldose reductase in apples extraction because of abundant polyphenolic compounds in apple fruit. Genaro-Mattos et al.[39]investigated caffeic acid has anti-inflammatory, antimutagenic, and anti-carcinogenic properties, which could be linked to its antioxidant and ROS scavenging activities. As a major component of unripe apple polyphenols[2], caffeic acid was selected to test aldose reductase inhibition activities compared with COS, APP, and APCM (Fig.3). At caffeic acid concentration of 0.5 mmol/mL exhibited 32.39% inhibition against aldose reductase. However, at the concentration of 1 and 2 mmol/mL exhibited significant inhibition activity, which was similar activity of APP at the concentration of 0.5 and 1.0 mg/mL, respectively. Our results were similar to Caroline et al.[40]who have announced that caffeic acid acting as a diabetic inhibitor by inhibiting aldose reductase activity. Besides, COS only showed very weak aldose reductase inhibition activity at all concentrations (Fig.3). Yu et al.[41]reported that the antidiabetic effect of COS is mediated via inhibition of intestinal-glucosidase and glucose transporters. Inflammation is one of the important pathogenesis of type Ⅱdiabetes. Lin et al.[42]findings suggested COS could directly decrease the levels of many inflammation factors such as TNF-via the JNK/NF-kB pathways in the type Ⅱdiabetes model. Thus, in our case, though aldose reductase inhibition activity of COS was very weak, however, APCM was approximately 1.25 times higher than APP, which was probably due to the immune-stimulating effects as well as drug carriers of COS or synergy effect.
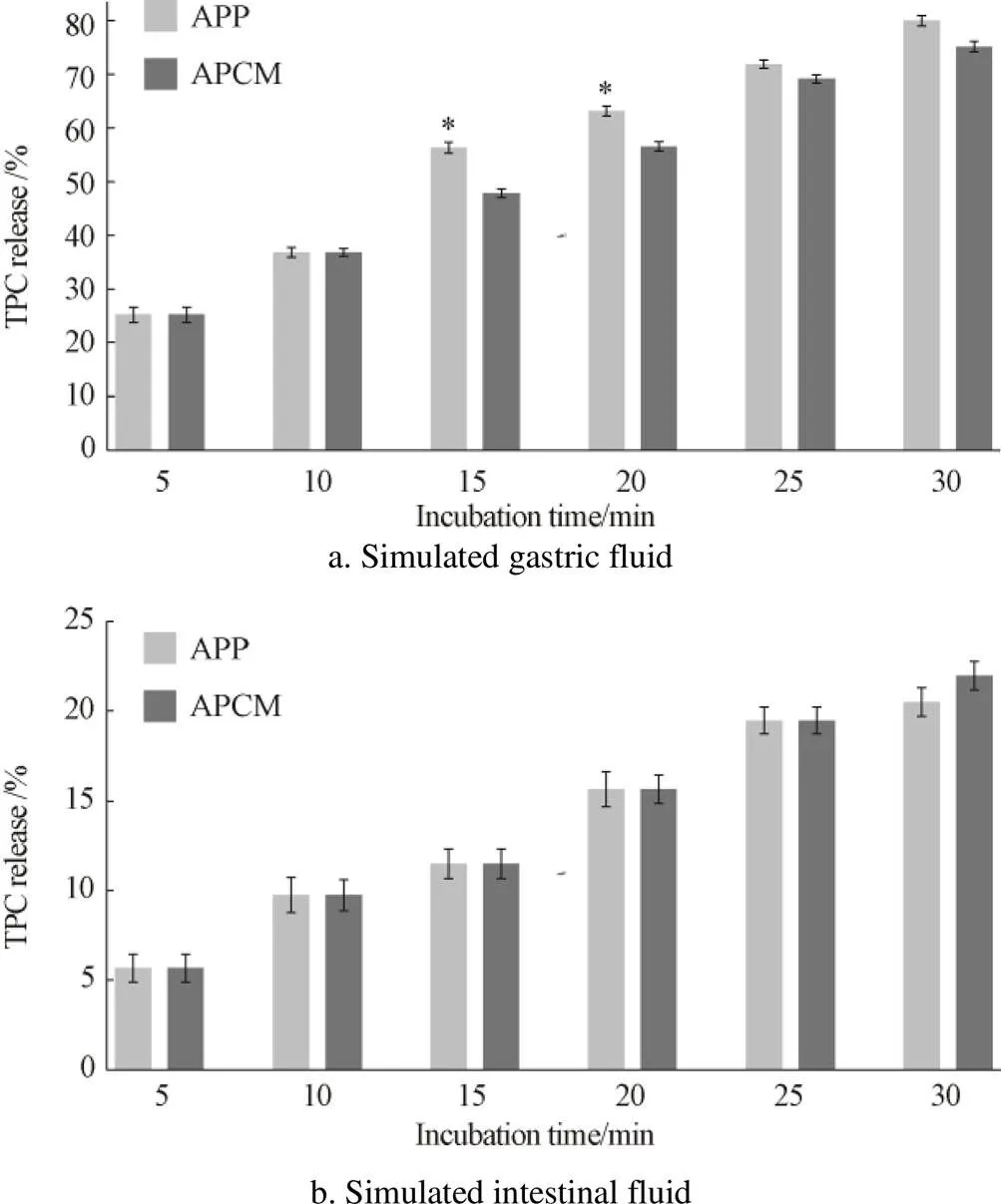
Note: Results were expressed as mean ± SD per group. *P<0.05, APCM group compared with APP group.

Note: Results were expressed as mean ± SD per group. *P<0.05, APCM group compared with COS group; #P<0.05, APCM group compared with APP group; ※P<0.05, APCM group compared with caffeic acid (CA) group. The same below.
2.7 ACE inhibition activity
ACE is potentially of great importance for controlling blood pressure by virtue of renin-angiotensin system[4]. Since the discovery of ACE inhibitors in snake venom, researches focused on the safe and economical food sources as ACE inhibitor has increased. Recently, several studies have shown associations between the regular consumption of polyphenols-rich foods and a decreased risk for cardiovascular disease by induce reductions in blood pressure and inhibit ACE activity[43]. Recently, COS has been documented to exhibit multiple biological properties such as anti-inflammatory and ACE inhibitory activities[44]. In our case, a significant reduction of ACE activities was observed when treated with COS, APP, and APCM from 0.05 mg/mL to 0.2 mg/mL, respectively. However, APP and APCM showed stronger inhibitory effect than COS at all concentrations (Fig.4). It means that, at the same concentration, APCM inhibition activity of ACE was approximately 2.5 and 1.5 times higher than COS and APP, respectively. So far, although no studies have been reported on the inhibitory effect of the polyphenols and COS complex on ACE inhibition activity, from the results of APCM exhibited higher inhibition activity than COS and APP, it is likely that the synergistic effect of polyphenols-COS complex plays a key role. In addition, the inhibition activities were compared with caffeic acid. Caffeic acid at 0.5 mmol/mL showed a little inhibition activity (13.45%) produced against ACE, however, it exhibited significant higher inhibition activity (54.95%) at 2 mmol/mL. Meanwhile APP at 0.2 mg/mL showed similar activity (52.22%). The results were supported by Nileeka et al.[45]who also reported that inhibition of ACE in flavonoid-rich apple peel extract.

Fig.4 Angiotensin I converting enzyme inhibition activity of COS, APP, APCM and CA
3 Conclusion
In the present study, a quick, simple, and effective method to prepare apple polyphenols-chitooligosaccharides microcapsule (APCM) is established by spray-drying process and characterized using laser particle size analyzer and Fourier transform infrared spectroscopy investigations. In simulated gastrointestinal digestion model, it is showed that APCM exhibited not only much higher release ratio of polyphenols (<0.05), but also better antioxidant and reactive oxygen species scavenging activities, and even the ability to inhibit aldose reductase and Angiotensin I converting enzyme are stronger than that of apple polyphenols or chitooligosaccharides (<0.05). These results suggest that APCM, as bioactive microcapsule of unripe apple polyphenols can be processed into tablets, sticks, capsules etc, and it is expected to be variously used as a functional material in the food industry.
[1] Wang O, Wei Y, Zhang D, et al. Optimization of combined extraction and separation of pectins and polyphenols from apple pomace[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CASE), 2012, 28(23): 286-292. (in Chinese with English abstract)
[2] Zheng H Z, Hwang I W, Kim B K, et al. Phenolics enrichment process from unripe apples[J]. Journal of the Korean Society for Applied Biological Chemistry, 2014, 57(4): 457-461.
[3] Dou J, Meng Y H, Liu L, et al. Purification, characterization and antioxidant activities of polysaccharides from thinned-young apple[J]. International Journal of Biological Macromolecules, 2015, 72(6): 7231-7240.
[4] Zhang Y, Chang S K. Comparative studies on ACE inhibition, degree of hydrolysis, antioxidant property and phenolic acid composition of hydrolysates derived from simulatedgastrointestinal proteolysis of three thermally treated legumes[J]. Food Chemistry, 2019, 281(6): 154-162.
[5] Barreira J C, Ana A A, Isabel C F. Bioactive and functional compounds in apple pomace from juice and cider manufacturing: Potential use in dermal formulations[J]. Trends in Food Science & Technology, 2019, 90(4): 76-87.
[6] Khan B M, Masroor A K, Husna S, et al. Chitosan and its ligosaccharides, a promising option for sustainable crop production-a review[J]. Carbohydrate Polymers, 2020, 227(5): 1-17.
[7] Xue H Y, Zhang Y, Zhang B Y, et al. Preparation characterization and bacteriostatic properties of punicalagin reducing chitosan/nano silver sol[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CASE), 2018, 34(4): 306-314. (in Chinese with English abstract)
[8] Xia W S, Liu P, Zhang J L, et al. Biological activities of chitosan and chitooligosaccharides[J]. Food Hydrocolloids, 2011, 25(2): 170-179.
[9] Ngo D H, Vo T S, Ngo D N, et al. Biological effects of chitosan and its derivatives[J]. Food Hydrocolloids, 2015, 51(10): 200-216.
[10] Zheng X J, He Y, Zhou H, et al. Preparation, characterization and antimicrobial activity of chitosan oligosaccharide-nisin conjugate[J]. Journal of Chinese Institute of Food Science and Technology, 2019, 19(11): 108-115. (in Chinese with English abstract)
[11] Luo Y, Teng Z, Wang X, et al. Development of carboxymethylchitosan hydrogel beads in alcohol-aqueous binary solvent for nutrientdelivery applications[J]. Food Hydrocolloids, 2013, 31(2): 332-339.
[12] Hu Q B, Luo Y C. Polyphenol-chitosan conjugates: Synthesis, characterization, and applications[J]. Carbohydrate Polymers, 2016, 151(20): 624-639.
[13] Yue L, Sun D, Imran M K, et al. Cinnamyl alcohol modified chitosan oligosaccharide for enhancing antimicrobial activity[J]. Food Chemistry, 2020, 309(9): 1-6.
[14] Rui L Y, Xie M H, Hu B, et al. Enhanced solubility and antioxidant activity of chlorogenic acid-chitosan conjugates due to the conjugation of chitosan with chlorogenic acid[J]. Carbohydrate Polymers, 2017, 170(15): 206-216.
[15] Liang J, Li F, Fang Y, et al. Synthesis, characterization and cytotoxicity studies of chitosan-coated tea polyphenols nanoparticles[J]. Colloids and Surfaces B: Biointerfaces, 2011, 82(2): 297-301.
[16] Chanphai P, Tajmir-Riahi H A. Conjugation of tea catechins with chitosan nanoparticles[J]. Food Hydrocolloids, 2018, 84(11): 561-570.
[17] Wang X Y, Zhang L, Wei X H, et al. Molecular dynamics of paclitaxel encapsulated by salicylic acid-grafted chitosan oligosaccharide aggregates[J]. Biomaterials, 2013, 34(7): 1843-1851.
[18] Bruna R P C, Paula M O, Guilherme M G, et al. Improving stability of antioxidant compounds from(jabuticaba) fruit peel extract by encapsulation in chitosan microparticles[J]. Journal of Food Engineering, 2018, 238(11): 195-201.
[19] Ou B, Hampsch-Woodill M, Prior R L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe[J]. Journal of Agricultural and Food Chemistry, 2001, 49(10): 4619-4626.
[20] Liu J, Wen X Y, Lu J F, et al. Free radical mediated grafting of chitosan with caffeic and ferulic acids: structures and antioxidant activity[J]. International Journal of Biological Macromolecules, 2014, 65(4): 97-106.
[21] Kandola K, Bowman A, Birch-Machin M A. Oxidative stress-a key emerging impact factor in health, ageing, lifestyle and aesthetics[J]. International Journal of Cosmetic Science. 2015, 37(10): 1-8.
[22] Krawitzky M, Arias E, Peiro J M, et al. Determination of color, antioxidant activity, and phenolic profile of different fruit tissue of Spanish verde doncella apple cultivar[J]. International Journal of Food Properties, 2014, 17(10): 2298-2311.
[23] Pasukamonset P, Kwon O, Adisakwattana S. Alginate-based encapsulation of polyphenols from Clitoria ternatea petal flower extract enhances stability and biological activity under simulated gastrointestinal conditions[J]. Food Hydrocolloids, 2016, 61(12): 772-779.
[24] Pankaj G, Vivek J, Ashutosh P, et al. Evaluation of effect of alcoholic extract of heartwood of Pterocarpus marsupium onantioxidant, anti-glycation, sorbitol accumulation and inhibition of aldose reductase activity[J]. African Journal of Traditional, Complementary and Alternative Medicines, 2017, 7(3): 307-314.
[25] Lee D S, Woo J Y, Ahn C B, et al. Chitosan-hydroxy cinnamic acid conjugates: Preparation, antioxidant and antimicrobial activity[J]. Food Chemistry, 2014, 148(8): 97-104.
[26] Liu J, Liu Y J, Pan J X, et al. Effect of tea polyphenols appended with chitosan on the shelf life of salmon[J]. China Tea Processing, 2019, 4(4): 66-71.
[27] Rama P, Caroline M, Ratul K D, et al. Encapsulation and release studies of strawberry polyphenols inbiodegradable chitosan nanoformulation[J]. International Journal of Biological Macromolecule, 2016, 88(7): 171-178.
[28] Kata T T, Nikola Z M, Verica B D, et al. Chitosan microbeads for encapsulation of thyme (L. ) polyphenols[J]. Carbohydrate Polymers, 2014, 111(13): 901-907.
[29] Riaz A, Lei S C, Muhammad H S, et al. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols[J]. International Journal of Biological Macromolecule, 2018, 114(15): 547-555.
[30] Dila A, Mine G, Aysun Y, et al. Stirred-type yoghurt incorporated with sour cherry extract in chitosan-coated liposomes[J]. Food Hydrocolloids, 2020, 101(2): 1-10.
[31] Francesca S, Patrizia P, Teresa M, et al. Technological properties and enhancement of antifungal activity of a Paeonia rockii extract encapsulated in a chitosan-based matrix[J]. Journal of Food Engineering, 2014, 120(1): 260-267.
[32] Muxika A, Etxabide A, Uranga J, et al. Chitosan as a bioactive polymer: Processing, properties and applications[J]. International Journal of Biological Macromolecules, 2017, 105(2): 1358-1368.
[33] Muhammad A K, Chun Y, Zheng F, et al. Alginate/chitosan- coated zein nanoparticles for the delivery of resveratrol[J]. Journal of Food Engineering, 2019, 258(10): 45-53.
[34] He M J, Zeng J Y, Zhai L, et al. Effect ofsimulated gastrointestinal digestion on polyphenol and polysaccharide content and their biological activities among 22 fruit juices[J]. Food Research International, 2017, 102(12): 156-162.
[35] Chethan S, Shengmin S, Mohamed A.andinhibition of aldose reductase and advanced glycation end products by phloretin, epigallocatechin 3-gallate and [6]- gingerol[J]. Biomedicine & Pharmacotherapy. 2016, 84(12): 502-513.
[36] Hou G Y, Lu W, Shu L, et al. Inhibitory effect of eleven herbal extracts on advanced glycation end-products formation and aldose reductase activity[J]. Chinese Chemical Letters, 2014, 25(7): 1039-1043.
[37] Zheng J P, Yuan X B, Cheng G, et al. Chitosan oligosaccharides improve the disturbance in glucose metabolism and reverse the dysbiosis of gut microbiota in diabetic mice[J]. Carbohydrate Polymers, 2018, 190(2): 77-86.
[38] Veeresham C, Rama R A, Asres K. Aldose reductase inhibitors of plant origin[J]. Phytotherapy Research, 2014, 28(10): 317-333.
[39] Genaro-Mattos T C, Maurício  Q, Rettori D, et al. Antioxidant activity of caffeic acid against iron-induced free radical generation-a chemical Approach [J]. PLoS One, 2015, 10(11): 1-12.
[40] Caroline M S, Renata P A, Iguatemy L B, et al.methods to determine the antioxidant activity of caffeic acid[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2019, 219(4): 358-366.
[41] Yu S Y, Kwon Y I, Lee C, et al. Antidiabetic effect of chitosan oligosaccharide (GO2KA1) is mediated via inhibition of intestinal alpha-glucosidase and glucose transporters and PPARγ expression[J]. BioFactors, 2017, 43(1): 90-99.
[42] Lin C W, Chen L J, Lee P L, et al. The inhibition of TNF-a-induced E-selectin expression in endothelial cells via the JNK/NF-kB pathways by highly N-acetylated chitooligosaccharides[J]. Biomaterials, 2007, 28(7): 1355-1366.
[43] Karunrat S, George S, Izabela K. Composition and inhibitory activities towards digestive enzymes of polyphenolic-rich fractions of Davidson’s plum and quandong[J]. LWT- Food Science and Technology, 2014, 57(1): 366-375.
[44] Amin S, Hu Z H, Teh S S, et al. Antioxidant and functional properties of protein hydrolysates obtained from squid pen chitosan extraction effluent[J]. Food Chemistry, 2017, 227(15): 194-201.
[45] Nileeka B, Vasantha R H. Antihypertensive properties of flavonoid-rich apple peel extract[J]. Food Chemistry, 2012, 135(4): 2320-2325.
苹果多酚-壳寡糖微胶囊的制备表征及释放特性
郑虎哲1,2,Choi Jihye2,Seong Giun2,Chung Shinkyo2※
(1. 江苏食品药品职业技术学院健康学院,淮安 223003,中国;2. 庆北大学食品科学与工程学部,大邱 41566,韩国)
为了有效提高未熟苹果多酚(Apple Polyphenols,APP)和壳寡糖(Chitooligosaccharides,COS)多功能协调效应,该研究采用喷雾干燥法研制未成熟的苹果多酚-壳寡糖微胶囊(Apple Polyphenols-Chitooligosaccharides Microcapsule,APCM),并测定了APCM的微胶囊粒度和分布,以及结构表征,并评价了模拟胃肠道消化模型对总多酚(Total Phenolic Content,TPC)释放和健康益处功能的影响。激光粒度分析结果表明,APCM的平均粒径为32.98m。跨度值最小为1.19,这意味着APCM比COS和APP更均匀、颗粒度更小。APP在1 237和1 194 cm-1处观察到了清晰的峰形,在APCM中同一位置处未观察到。但是,APCM与APP具有相似的吸收带,这意味着APP与COS也可能通过范德华力和分子间氢键的方式形成APCM。模拟胃肠消化模型结果表明,APCM中多酚的释放发生在60 min以内。在模拟胃液消化系统(Simulated Gastric Fluid,SGF)处理中,APCM释放的TPC从25.6%到76.5%不等,而在模拟肠液消化系统(Simulated Intestinal Fluid,SIF)持续处理中,TPC释放量达到31.3%到97.6%。体外抗氧化活性和活性氧(Reactive Oxygen Species,ROS)清除活性结果表明,相比与APP和COS,APCM对抗氧化能力指数、清除DPPH自由基、铁离子还原抗氧化力、•OH清除活性、O2•-清除活性和H2O2清除活性表现出更出色的清除自由基活性。此外,与APP或COS相比,APCM不仅表现出更高的糖还原酶抑制活性(<0.05),而且具有更好的血管紧张素I转换酶(Angiotensin I Converting Enzyme,ACE)抑制活性(<0.05)。结果表明,APCM今后可在功能性食品或药物领域更大的研发潜力。
农产品;抗氧化;苹果多酚-壳寡糖微胶囊;模拟胃肠消化系统;释放率;醛糖还原酶;血管紧张素Ⅰ转换酶
Zheng Huzhe, Choi Jihye, Seong Giun, et al. Preparation, characterization and health benefit functions of unripe apple polyphenols-chitooligosaccharides microcapsule[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2020, 36(14): 281-289. (in English with Chinese abstract)doi:10.11975/j.issn.1002-6819.2020.14.034 http://www.tcsae.org
郑虎哲,Choi Jihye,Seong Giun,等.苹果多酚-壳寡糖微胶囊的制备表征及释放特性[J]. 农业工程学报,2020,36(14):281-289. doi:10.11975/j.issn.1002-6819.2020.14.034 http://www.tcsae.org
2020-03-09
2020-07-05
Agriculture and fishery, Gyeongsangbukdo (No. GBTA 2016-04), Korea; Qinglan project (Su Teach 2019Ⅲ) in Jiangsu Province, China.
Zheng Huzhe, Doctor, Associate professor, major in food science and biotechnology. Email:huzhezheng@163.com
Chung Shinkyo, Doctor, Professor, major in food science and biotechnology. Email:kchung@knu.ac.kr
10.11975/j.issn.1002-6819.2020.14.034
TS255.1
A
1002-6819(2020)-14-0281-09
