In-situ formation of perylene in lacustrine sediments and its geochemical significance
2020-09-13JianyangGuoHaiqingLiao
Jianyang Guo · Haiqing Liao
Abstract To identify the possible sources and formation mechanism of perylene in sediments, temporal trends of perylene and other polycyclic aromatic hydrocarbons, as well as the organic matters, were analyzed in sediment cores from Dianchi Lake and Sihailongwan Maar Lake in China. Significantly high concentrations of perylene were observed in both cores and increased gradually with the sediment depth. Source identification suggested that perylene in both cores were of primarily diagenetic origin,other than the anthropogenic inputs. Both terrigenous and aquatic organic matters could be the source of perylene in sediments and diagenetic formation of perylene in sediment is kinetically controlled by the microbial activities under anaerobic conditions. All evidence points to the fact that the formation of perylene in sediment should be an index of the microbial activities and environmental conditions, other than the source of specific organic matters or precursors. In addition, we should be cautious when using any geochemical indicators involving the microbial alternation.
Keywords Sediment core · Source identification ·Formation mechanism · Organic matters
1 Introduction
Perylene is a pentacyclic aromatic hydrocarbon and is frequently found in ancient sediments of freshwater(Wakeham et al. 1980; Tan and Heit 1981) and marine systems (Venkatesan 1988). Identification of the precursor and formation mechanism of perylene in sediments have attracted continuous interests because they are still intriguing mysteries. Although trace or small amounts of perylene can be found in products of combustion and/or abnormal thermal exposure of organic materials (OMs)(Jenkins et al. 1996; Simoneit and Fetzer 1996), the predominance of perylene in ancient sediments clearly indicates that perylene is originated primarily from in situ formation via diagenesis under anoxic conditions, other than transported and deposited into the sediment (Orr and Grady 1967; Wakeham et al. 1979; Gschwend et al. 1983;Silliman et al. 2000; Grice et al. 2009; Fan et al. 2011).A variety of materials has been suggested as the precursors of perylene in sediments, yet the origin of perylene remains to be unclear. Early on, it has been believed that perylene was formed from a terrigenous precursor under anoxic condition (Aizenshtat 1973; Ishiwatari et al. 1980),while others linked the formation of perylene to aquatic precursors (Wakeham et al. 1979; Hites et al. 1980;Wakeham et al. 1980). Silliman et al. (1998, 2000, 2001)have carefully investigated the relationship between the formation of perylene and the source of OMs in sediment.They concluded that perylene can be formed from more than one precursor (both terrigenous and aquatic could be the possibility) and hypothesized that the formation of perylene is controlled by in situ microbial processing of non-specific OMs rather than the availability of a specific precursor. In addition, Silliman et al. (2001) also hypothesized that the formation of perylene can only be significant in sediments after the reactive OMs have been consumed, based on a study conducted in Green Bay of Lake Michigan, USA. However, the product-precursor(wood degrading fungi) relationship is still reported even very recently (Jiang et al. 2000; Grice et al. 2009). The formation mechanism of perylene remains contentious and needs further clarification.
In the present work, sedimentary records of perylene in Dianchi Lake (DC, an urban lake in Southwest China) and Sihailongwan Maar Lake (SHLW, a remote lake in Northeast China) were reported. The aims of the present work are to (1) identify the possible source and formation mechanism of perylene in DC and SHLW, (2) confirm whether the formation of perylene can only be significant in sediment after the reactive type of OMs have been consumed, and (3) probe into the possible geochemical significance of perylene formation in sediment.
2 Sampling and methods
2.1 Study area
Dianchi Lake (DC) and Sihailongwan Maar Lake (SHLW)are selected for investigation (Fig. 1). DC (24°40′-25°03′N, 102°37′-102°48′E) is a typical urban lake in Southwest China. The anthropogenic activities are extensive in its catchment within recent decades. SHLW(42°17′N, 126°36′E) is a remote Maar Lake in Northeast China, where human activity is very limited, and the lake is far from any pollution sources. The basic information for both lakes is given in Table 1. One reason for selecting DC and SHLW as the studied area is that the sediments from both lakes were rich in OMs and have been investigated carefully. The other reason is that the source of OMs are quite different between them, due to their totally different sedimentary environments and the morphometric characteristics (Fang et al. 2009, 2010; Wang et al. 2009). Plentiful perylene in sediments in both lakes can be expected,thus it was possible to check the relationship between the formation of perylene and the source of OMs.
Fig. 1 Map of the sampling sites (SHLW: Sihailongwan maar Lake;DC: Dianchi Lake)
2.2 Sampling
The sediment cores were collected at the deepest site of each lake using a gravity corer and were sectioned at 0.5 or 1 cm intervals according to the assumed or known sedimentation rates and sample mass requirement for chemical analyses (Chu et al. 2005; Guo et al. 2013). The depths of the collected cores in DC and SHLW were 55 cm and 28 cm, respectively. Upon collecting, samples were wrapped in baked (450 °C) aluminum foil and transported to the laboratory, where they were stored at - 20 °C until further treatment.
The sedimentation rate of SHLW has been investigated using137Cs and210Pb and the sedimentation rate of 0.11 cm/a achieved from the previous investigation was used in the present work (Chu et al. 2005). The dating of sediment core in DC was using137Cs and the detailed information has been presented in our previous work (Guo et al. 2013). Based on the peak of137Cs corresponding to 1963, an average sedimentation rate of 0.28 cm/a was obtained (Guo et al. 2013).
2.3 Extraction and instrumental analyses
Extraction procedures were based on a previously reported method (Guo et al. 2010). Briefly, an aliquot (~5 g dry weight) of freeze-dried and homogenized sample, spiked with a mixture of denudated PAHs (including naphthalened8, acenaphthene-d10, phenanthrene-d10, chrysene-d12, and perylene-d12) as recovery surrogates, then Soxhlet extracted with a mixture of hexane and acetone (1:1, v:v) for 48 h. Approximate 2 g of activated copper was added for desulphurization. Cleanup and fractionation were performed on alumina/silica gel columns. PAHs fraction was eluted with 70 mL dichloromethane/hexane (3:7, v:v), then concentrated to 500 μL under a gentle flow of nitrogen.The internal standards, 2-fluorobiphenyl and p-terphenyld14, were added prior to instrumental analysis.
Analyses of PAHs were performed on an Agilent 6890 gas chromatograph system equipped with an Agilent 5975B mass selective detector operating in selective ion monitoring mode using a DB-5 capillary column (60 m length × 0.25 mm i.d. × 0.25 μm film thicknesses).Splitless injection of 1.0 μL of sample was conducted with an auto-sampler. The GC oven temperatures wereprogrammed from 90 to 180 °C at a rate of 10 °C min-1, to 220 °C at a rate of 2 °C min-1, and then to 290 °C at a rate of 8 °C min-1(hold for 30 min). The quantified compounds included perylene and sixteen priority PAHs proposed by US EPA.

Table 1 Morphometric and other characteristics of DC and SHLW
2.4 Quality control and quality assurance
Surrogate recoveries in all samples were 64.1 ± 8.6% for naphthalene-d8, 82.6 ± 9.6% for acenaphthene-d10,84.1 ± 11.6% for phenanthrene-d10, 84.4 ± 11.8% for chrysene-d12, and 75.8 ± 13.5% for perylene-d12. For method quality control, procedural blanks, triplicate spiked blanks, and triplicate spiked matrices were analyzed for each batch of samples. Mean recoveries of perylene and sixteen target PAHs ranged from 75.8 ± 4.9% to 114 ± 8.2% in triplicate spiked blanks and from 60.8 ± 1.1% to 121 ± 9.1% in triplicate spiked matrices.Only trace amounts of targets were detected in blanks and were subtracted from the analyzed data. Detection limits were 0.01-0.02 ng/g dry weight for PAHs (defined as S/N >3). All results were expressed on a dry weight basis and corrected for surrogate recoveries.
2.5 Element analysis
Element analysis was performed on an elemental analyzer(Vario EL III, Germany). After removing the inorganic carbon with superfluous 1 M HCl overnight, an aliquot(~1 g dry weight) of the freeze-dried and homogenized sample was rinsed with deionized water twice and dried in an oven at 60 °C for 12 h. Known amounts of sulfanilamide (C6H8N2O2S) were used to calibrate the instrument and quantify the amount of C and N in samples. Total organic carbon (TOC) was calculated on a whole-sediment basis. Organic carbon/organic nitrogen (Corg/Norg) ratios were calculated on an atomic basis.
3 Results
3.1 Down core variation of perylene in sediments
Down-core variations of perylene in sediment cores from DC and SHLW were shown in Fig. 2. As expected, high contents of perylene were found in both cores, even in the surface sediments. Based on the sedimentary rates of both cores (Chu et al. 2005; Guo et al. 2013), the analyzed sediments present the depositional years of approximately one century. Within this period, content of perylene in sediments from SHLW ranged from 184 to 2694 ng/g,increased successively as the sediment-depth increased. As for DC, within the range of 189-1901 ng/g, concentrations of perylene were also increased as the increasing depth in the upper sediments (0-14 cm) but presented a flat pattern in the deeper layers (14-35 cm). The vertical profiles of perylene in DC and SHLW were not only different from that in a mountain pond in Maine, USA (Gschwend et al.1983) and a mountain lake in Taiwan (Fan et al. 2011), but also different from that in high altitude lakes in Europe(Grimalt et al. 2004). This implied that the sedimentary record of perylene was highly site-specific, probably due to the different sedimentary environments.
If only the increasing stage of perylene was taken into account, then the content of perylene was alm ost linearly related to the age of sediments in SHLW (R2= 0.99), and to a less extent in DC (R2= 0.90). This indicated that the increasing rates of perylene in both cores were almost constant, and the formation of perylene in sediments seems to be a first-order reaction, as suggested by an early research work (Gschwend et al. 1983). A similar situation was also found in Lake Erhai and Lake Qinghai (Unpublished data). As indicated in Fig. 3, the yearly increasing rate of perylene in DC was 38.0 ng/g, which is more than twice that in SHLW (17.6 ng/g). This indicated that the formation rate of perylene in SHLW is approximately two times that in DC.

Fig. 2 Vertical profiles of perylene and TOC (perylene:open cycle; TOC: solid cycle)
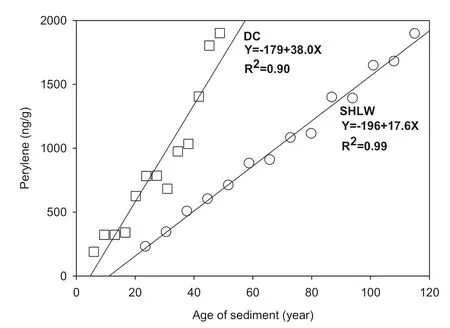
Fig. 3 Increasing rate of perylene in sediment cores from SHLW and DC
3.2 In-situ formation of perylene in both cores
Generally, a sedimentary record of PAHs is indicative of anthropogenic activities, especially for the record of high molecular weight forms (Hites et al. 1977; Wilcke 2007).For example, BbF is a product of high-temperature combustion (Mai et al. 2003), while INP and BghiP are the tracers of vehicular emission (Harrison et al. 1996; Miguel and Pereira 1989). As shown in Fig. 4, the vertical profiles of the anthropogenic sources of PAHs (including BaP,BbF, INP, and BghiP) in SHLW were totally different from the corresponding vertical profile of perylene. This is also the case in DC, except that the declined trends of anthropogenic PAHs in the surface layers. This suggested that perylene in both cores probably stemmed from another source, other than the anthropogenic source.
Evidence on the non-anthropogenic source of perylene can also be indicated by the low ratio of perylene to sum of pentacyclic-aromatic isomers (Rperylene/Σ5rings-PAHs) (Baumard et al. 1998; Zander 1983) and the low ratio of pyrene/perylene (Rpyrene/perylene) in the sediment (Venkatesan 1988). As displayed in Fig. 5, Rperylene/Σ5rings-PAHswere in the range of 0.2-0.98 in SHLW and 0.23-0.98 in DC,indicating that perylene in both lakes were primarily diagenetic in origin (Jenkins et al. 1996; Simoneit and Fetzer 1996), even at the surface sediments. As to the values of Rpyrene/perylenein SHLW (0.12-2.15) and DC (0.01-1.06),they were also substantially lower than that of combustion source of PAHs (9-15) (Venkatesan 1988), which further substantiates that most of the perylene in both cores were not anthropogenic in origin. Overall, all the evidence points to the fact that perylene in both lakes was originated primarily from in situ formation via diagenesis.
3.3 Contents and characteristics of TOC in both cores
TOC is a proxy of summed inputs of OMs from aquatic production, riverine transport/surface runoff, and atmospheric deposition into the sediment (Silliman et al. 2000).In SHLW, TOC contents were relatively consistent during the whole core, with a mean value of 5.96 ± 0.49% and a slightly high value between the 1950s and 1980s (Fig. 2).This is consistent well with its relatively stable sedimentary environment. As to DC, TOC contents in deeper sediments(before the 1960s) were generally low and constant, with the mean value of 0.87 ± 0.12%, but quickly increased in the upper sediments (from 0.98 to 8.77%, after 1960s)(Fig. 2).
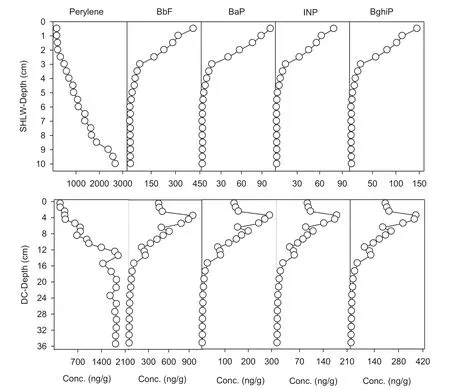
Fig. 4 Vertical profiles of perylene and other PAHs in both cores

Fig. 5 Down core variations of the ratio of Perylene/Σ5ring-PAHs in both cores
Different sources of OMs in sediments usually have the characteristic ratio of organic C to organic N (RCorg/Norg).RCorg/Norgless than 10 is characteristic of lacustrine algae,whereas vascular land plants have RCorg/Norgusually greater than 20 (Meyers 1994; Meyers and Lallier-Verge´s 1999). In SHLW, RCorg/Norgin sediments was little varied(12.0-13.4) with the mean value of 12.8 ± 0.38. This indicated that the composition of TOC in SHLW sediments was relatively constant and the sources of TOC were the mixture of terrigenous and aquatic origin. In fact, the source of TOC in SHLW has been carefully investigated using multi-indicators, including aliphatic hydrocarbons,fatty alcohols, fatty acids and GDGTs, as well as δ13Corgand δ15Ntotal(Fang et al. 2015). The results indicated that TOC in SHLW was primarily the land-derived OMs. While in DC, TOC in sediments were totally different between the deeper sediments and the upper sediments. In detail,TOC in deeper sediments (before the 1960s) is characterized by the low RCorg/Norg(7.8-9.9, 8.9 ± 0.7), a typical value of lacustrine algae source of OMs. While in the upper sediments (after the 1960s), RCorg/Norgincreased successively with a mean value of 13.8 ± 2.0. This suggested that along with the increase in TOC content, an increasing proportion of land-derived OMs was mixed in the upper sediments in DC.
Overall, the content and composition of TOC in SHLW sediments were relatively constant, the same as the deeper sediments in DC. While in the upper sediments in DC, both content and composition of TOC were significantly varied.
4 Discussion
As mentioned above, TOC in deeper sediments of DC was of primarily lacustrine origin, whereas TOC in deeper sediments of SHLW was mainly of terrigenous origin.However, a significant amount of perylene was found in deeper sediments of both cores. In previous investigations,either terrigenous or aquatic precursors for perylene have been suggested and have been summarized by Silliman et al. (1998, 2001). As indicated in the present work, perylene in sediment does not have a specific precursor and both aquatic and land-derived OMs are the probabilities.This consolidates the hypothesis that the formation of perylene is primarily mediated microbially rather than the availability of a specific precursor material (Silliman et al.2000, 2001).
Theoretically, it can be expected that there was a correlation between TOC and perylene, no matter TOC in sediment is aquatic or land-derived origin. But this phenomenon rarely occurs in reality. In fact, the contents of perylene in sediments depend on the formation rate of perylene and the depositional time, and perylene formation in sediment is likely still continuing in the modern depositional environments. In the present work, successive decrease in contents of perylene in sediments from the deeper layers to the surface layers clearly indicated that the formation of perylene in SHLW sediment is still ongoing.This is also the case for perylene in the upper sediments in DC. This revealed that the abundance of perylene is not necessarily related to TOC content in sediments, especially in modern depositional environments. Similar results were also found elsewhere (Fan et al. 2011; Silliman et al. 1998).It is unreasonable to link perylene in sediment to the corresponding TOC (either terrigenous or aquatic origin)unless the formation of perylene in the sediment has completed. When discussing the relationship between the vertical profile of perylene and the source information of OMs in sediments, previous investigations were seldom taking the depositional time into account. This often leads to unexpected or uncertain, even contradictory deductions,because the abundance of perylene in sediments is usually time-dependent, while the source information of OMs in sediments is less time-dependent. In other words, it is possible to find a certain correlation between the contents of perylene and the sources of OMs, only when the precursor converted to perylene was complete. Nevertheless, it is not easy to judge when perylene formation in sediment will cease.
Interestingly, it was found that the contents of TOC and perylene were both relatively constant in the deeper sediments in DC. This could result from the possible precursor of perylene in sediment being exhausted and the cease of perylene formation. If this assumption is true, the TOC normalized contents of perylene must be constant. The TOC-normalized content of perylene in the deeper sediments in DC was 226 ± 21.1 μg/g, with a standard deviation of 9.3 %. Considering the errors in measurement for perylene and TOC, it can be judged that the assumption is tenable. Another thing is that the formation rates were relatively constant in both sediment cores, although their formation rates were quite different. This indicated that the formation of perylene in sediments is likely to be a firstorder reaction and the vertical profiles of perylene in sediment core were kinetically controlled (or time-dependent)in the modern depositional environments. In addition,although the contents of TOC in sediments in SHLW were generally higher than in DC, the formation rate of perylene in SHLW was almost half that in DC. This suggested that the formation rate of perylene in sediment depends on the different environmental settings, and the abundance of perylene in sediment is more likely controlled by the microbial activities, other than the abundance of precursor materials in sediment.
One of the purposes of the work design is to verify an early hypothesis that perylene formation can only be significant in sediments after the reactive type of OMs has been consumed (Silliman et al. 2001). In the present work,significant levels of diagenetic perylene were found in the surface sediments in both lakes. A similar situation was also found in Chini Lake, Malaysia (Bakhtiari et al. 2009).This indicated that perylene can be formed in very recent sediments and the pervious hypothesis could be a specific case, other than a common phenomenon. Previous investigations confirmed that the formation of perylene in sediment is usually under anaerobic conditions. SHLW is a typical deep lake in Northeast China, with a maximum depth of 50 m and the annual ice-covered period of approximately 5 months (Chu et al. 2005). As to DC, it has been suffered from heavy eutrophication for several decades (since the 1980s). Therefore, an anaerobic condition in surface sediments in both lakes can be expected. This is probably the main reason for the abundance of perylene in surface sediments in both lakes.
4.1 Scenarios for perylene formation in sediment
Based on the information provided by the previous investigations and the present work, possible scenarios on the formation of perylene in sediment can be summarized as follows: (1) perylene in sediment can be derived from terrigenous and aquatic OMs. Although either terrigenous or aquatic OMs have been suggested as the sources of perylene in sediment, the formation of perylene in sediment is primarily microbial-mediated rather than the availability of specific precursor material. In fact, both terrigenous and aquatic OMs are probability; (2) vertical profiles of perylene in sediments are kinetically controlled in modern depositional environments. We should be cautious when linking the abundance of perylene to the source information of OMs in sediments; (3) formation rate of perylene in sediment is likely to be a first-order reaction and the abundance of perylene in sediment is more likely controlled by the microbial activities, other than the abundance of precursors in sediment; and (4) perylene can be significant in very recent sediments, depending on the sedimentary settings. Diagenetic perylene abundant in surface sediments is possible, provided that there is an anaerobic condition.
4.2 Geochemical significance of perylene formation in sediment
As described above, the formation of perylene in sediment can be derived from both terrigenous and aquatic OMs and is kinetically controlled by the microbial activities under anaerobic condition. Thus, the geochemical significance of perylene in sediment is not an index of the sources of OMs,but an index of the microbial activities and the environmental conditions in sediment. Moreover, if the formation of perylene in sediment is greatly influenced by the microbial activities, it is speculated that the chemical and isotopic information in sediment may be substantially altered by the microbial activities under certain circumstances. This reminds us that caution should be paid when developing lake palaeoclimate using δ13C of carbonate in sediment (Zhu, et al. 2011). The significance of δ13C of carbonate to palaeoclimate is based on the response of δ13C of carbonate to lake palaeoclimate (Leng and Marshall 2004; Li and Ku 1997). However, δ13C of carbonate may be altered by the microbial activities in sediment. For example, the methanation process mediated by microbial communities in lacustrine sediments can lead to the enrichment of13C in dissolved inorganic carbon, resulting in the abnormal positive δ13C values of carbonate in sediment (Lamb et al. 2007). This could be the major reason for the abnormal positive δ13C values of carbonate (23.1‰) in sediment from lake Chaohai, southwest China (Zhu et al. 2011). Though there was no direct evidence to support this, similar situation was also found in Lake Tilo(Lamb et al. 2000) and lake Bosumtwi (Talbot and Kelts 1986). This implied that we should be cautious when using any geochemical indicators involving the microbial alternation. At least, we should be sure that microbial alternation is negligible or can be described accurately.
5 Conclusions
High contents of perylene were found in sediments from DC and SHLW, even in the surface sediments. Perylene in both cores was primarily originated from in situ formations via diagenesis, other than the anthropogenic inputs. The formation of perylene in sediment can be derived from both terrigenous and aquatic OMs and is kinetically controlled by the microbial activities under anaerobic conditions. The geochemical significance of perylene in sediment should be an indicator of the microbial activities and environmental conditions, other than the specific OMs or precursors. In addition, we should be cautious when using any geochemical indicators involving the microbial alternation.
Acknowledgements This work is financially supported by the Chinese NSF Projects (Nos. 41877406 and 91647205), and the jointed project of National Natural Science Foundation and Guizhou Karst Scientific Research Center (U1612441).
杂志排行
Acta Geochimica的其它文章
- Development of in-situ Micro-Raman spectroscopy system for autoclave experimental apparatus
- Exposure of children to heavy metals from artisanal gold mining in Nigeria: evidences from bio-monitoring of hairs and nails
- Constraints on granite-related uranium mineralizationin the Sanjiu uranium ore field, SE China provided by pyrite mineralogy, major and trace elements, S-He-Ar isotopes
- Studying DDTs in agricultural soils of selected rural communities of Armenia
- Fluid inclusion and H-O isotope study of the Jiguanshan porphyry Mo deposit, Xilamulun Metallogenic Belt: implications for characteristics and evolution of ore-forming fluids
- REE geochemistry of core sediments of Cauvery delta, India for provenance studies
