Exposure of children to heavy metals from artisanal gold mining in Nigeria: evidences from bio-monitoring of hairs and nails
2020-09-13AdeniyiJohnPaulAdewumiTemitopeAyodejiLaniyanTangfuXiaoYizhangLiuZengpingNing
Adeniyi JohnPaul Adewumi · Temitope Ayodeji Laniyan · Tangfu Xiao ·Yizhang Liu · Zengping Ning
Abstract In recent times, there had been reported cases of Pb poisoning in Anka gold mining area, Northwest Nigeria.Therefore, this study was carried out to determine the extent of bioaccumulation of heavy metals in the hairs and nails of children in the area. Forty samples (twenty nails and twenty hairs) samples were collected from ten boys and ten girls of ages 5-9 residing in the area. To ascertain the sources of heavy metals in children, 15 soils samples,15 groundwater samples, 5 samples of mine tailings, and 5 plants samples were collected. Hair and nails of the subjects were collected using internationally acceptable techniques. All samples were kept in uncontaminated ziplock bags prior to laboratory preparation and analysis. The samples were cleaned using nonionic detergent (triton X-100) and deionized water. The hairs and nails were digested with 10 mL of 6:1 mixture of nitric acid and perchloric acid. The soils, mine tailings, and plants were air-dried at room temperature, sieved, and chemically digested using the aqua regia method. The concentrations of metals in all the samples were determined using highperformance liquid chromatography-inductively coupled plasma-mass spectrometry. Statistical analysis was employed to unravel potential sources of metals in the media. Results showed that heavy metals in children of the area are above results from similar studies and pathological ranges for heavy metals in hairs and nails. Also, heavy metals in environmental media are above the recommended standards. Multivariate analysis showed that the metals are mainly from mining and other anthropogenic sources.Results of correlation between heavy metals in hairs and nails with those in geological samples revealed that heavy metal that bioaccumulates in the children of this area are mostly from contaminated environmental media. It is recommended that complete remediation and effective health education be carried out in the area.
Keywords Anka · Bioaccumulation · Children · Heavymetals · Hairs · Nails
1 Introduction
Heavy metals present in high proportion in geological media such as soils, water, rocks, and plants have high potential to endanger human health through ingestion,inhalation and dermal contacts (NRC 2000; Ogola et al.2002; Wasserman et al. 2004; Arogunjo 2007; Carr et al.2008; Singh et al. 2009; Cao et al. 2010; Kabata-Pendias and Pendias 2011; Momoh et al. 2013; Khan et al. 2013;Bortey-Sam et al. 2016; Kamunda et al. 2016; Emmanuel et al. 2018). Mining and mineral processing are major anthropogenic activities that release heavy metals into the environment (UNEP 2002; Bradl 2005; Wuana and Okieimen 2011; Zhang et al. 2012; Enkhzaya et al. 2016;Fashola et al. 2016; Ngole-Jeme and Fantke 2017) and the consequent environmental impacts of the metals are of high concern (Rajaganapathy et al. 2011; Oyebamiji et al.2018).
Activities of artisanal miners have led to the destruction of valuable farmlands in many parts of Africa and are triggered by supports from powerful politicians and security agents (Van-Bockstael 2019). Only a few governments of developing nations have shown interests in formalizing artisanal and small-scale mining (ASM) involving lowtech, labor-intensive mineral extraction, and processing(Ferring et al. 2016; Salo et al. 2016; Hilson et al. 2017;Hilson and Maconachie 2017; Siwale and Siwale 2016;Maconachie 2017; McQuilken and Hilson 2017) and this is a major setback to the development of the mining industry in many countries in Africa. However, through the formation of legal ‘ASM zones’ and cooperative societies processes of formalization is gradually picking up (De-Haan and Geenen 2016; Huggins et al. 2017).
In developing nations inhabitants of settlements around active and abandoned artisanal mining areas are exposed to heavy metals emanating from mineral exploitation and processing. These metals have contaminated soils in Ecuador (Schudel et al. 2019), Nigeria (Fashola et al. 2016;Oyebamiji et al. 2018). It has also impacted negatively fluvial sediments in Zamora River basin a sub-basin of the Amazonian basin (Mora et al. 2019) and the Puyango-Tumbes River in Ecuador (Schudel et al. 2018). It has negatively affected surface water in parts of Ghana (Nukpezah et al. 2017; Ansah et al. 2018). These had led to many health issues in the areas where these anthropogenic activities are carried out. In Ghana, variations in thyroid hormone have been linked with elevated blood mercury levels in miners working in different artisanal mining pits(Afrifa et al. 2018).
In many Africa countries and other developing nations of the world, children are engaged in artisanal mining to increase household income and serve as a means of vocational training (Potter and Lupilya 2016; Maconachie and Hilson 2016). Insights into family dynamics as revealed in a study by Andre and Godin (2014) showed that children from lower-class background engage more in artisanal mining to maintain the family income and are thus more exposed to environmental contaminants from mining and mineral processing activities than children in the upperclass background. It is also believed that child labor is widespread in many of the continent’s small-scale mining communities is attributed to combinations of cultural issues, household-level poverty, and rural livelihood diversification which are far above the perception of international organizations and policymakers (Hilson 2010, 2012). More than envisioned, many kids from the very early age of seven are working with immediate contact to mercury and are exposed to mercury and other metal intoxication from artisanal gold mining activities in many parts of Africa (Bose-O’Reilly et al. 2008). In over 50 countries children live in small-scale gold mining areas and are exposed in a similar way to mercury (Bose-O’Reilly et al. 2008).
Epidemiologic studies have revealed that children are more susceptible to heavy metal exposure with higher health risk than adults (Perera et al. 2005; Were et al. 2008;Tang et al. 2009), because they are prone to unintentional exposures via inhalation and hand-mouth ingestion (Hornung et al. 2009; Adal 2018), especially in areas where mining activities are prominent. For example, children absorb as much as 50% Pb in ingested dose but only 10%for adults (Adal 2018).
Bioaccumulation can be considered as a particular type of biosorption in which metals are incorporated inside living biomass (Fomina and Gadd 2014). This incorporation relies mostly on active uptake, but passive uptake canal so be involved (Fomina and Gadd 2014). Blood,urine, nails, teeth, and hair are the most easily accessible bioindicators in humans (Mehra and Juneja 2005; Surkan et al. 2007; Rashed and Hossam 2007; Shan and Ikram 2012; Rakib et al. 2013; Abdelrazig et al. 2014). Blood metal levels reflect transient levels whereas hair metal levels show long-term retention, which may be accounted for a long period of exposure (Petering et al. 1971; Hopps 1977; Laker 1982; Mehra and Juneja 2005). Nails also indicate metal body burden (Chaudhary et al. 1995). The presence of toxic and trace elements in biological tissues like hair and nails can be a measure of the amount absorbed by a person (Mehra and Juneja 2005; Abdulrahman et al.2012; Al-Awadeen et al. 2014). Hair is an attractive tissue for analysis because obtaining a sample is non-invasive(Druyan et al. 1998). Concentrations of metals in hair and nails reflect their mean levels in the body during a longer period as compared to body fluids (Mehra and Juneja 2005).
In Northwest Nigeria, arsenic (As), cadmium (Cd),nickel (Ni), lead (Pb), copper(Cu), zinc (Zn), cobalt (Co)and mercury (Hg) are associated with gold mineralization and is of high environmental concerns (Da-Silva et al.2004). It was reported that the Pb poisoning associated with artisanal gold mining caused the death of more than 400 children in Anka area (Liang 2010). Previous studies for local metal pollutions focused on water (Nuhu and Hassan 2014; Hammuel et al. 2014), soils (WHO 2011; Buba and Aboyeji 2015; Tsuwang et al. 2014) and resident blood(Ogabiela et al. 2011). However, for a liable and full understanding for metal exposures of local residents, little was known on metal bioaccumulation in hairs and nails of local children and associated source. Therefore, the aim of this study is to determine the bioaccumulation of heavy metals in hairs and nails of children in Anka area and unravel their sources using multivariate statistical methods.The findings would help to determine the degree of children exposure to heavy metals in the area and further unravel their potential sources into their bodies.
2 Materials and methods
2.1 Study area
Anka is located in Zamfara State, Northwestern Nigeria(12°15′27.05′′N5°51′27.01′′E) (Fig. 1). Villages in this area are Abare, Dareta, Tungar Kudaku, Tungar Dauda,Bagega, Kawaye, Babaram, Waramu, Mallamawa, and Sunke. Samples were collected from Kawaye and Bagega villages. Both villages are inhabited of 15,536 (Nigeria Pollution Commission 2006) and cover a landmass of 5537 km2. The area is underlain by the Proterozoic Schist belt of Nigeria known as the Anka schist belt (ASB)located to the western half of the generalized schist belt of Nigeria sharing boundary with Maru schist belt to the east.
Geologically, the Anka area is underlain by Precambrian migmatitic-gneisses and metasediments of the ASB which are intruded by Older Granites and amphibolites (Danbatta et al. 2009) (Fig. 2). According to Holt et al. (1978) and Holt (1982), the lithology in the area include metaconglomerates, phyllites and acid volcanic. Phyllites were typically observed in Bagega, Tungar Kudaku, and Bidan Zaki. Acid volcanic typically outcrops around Dareta,Abare, east of Anka town and Tungar Daji. Metaconglomerates in the area form units of up to 200 m and are interbedded with feldsphatic metasandstones which contain rounded to angular fragments of quartz, granites, phyllites,quartzite, and volcanics. This belt consists chiefly of poorly exposed, homogenous still water argillites which are associated with coarse clastics and acid intermediate volcanic and intrusive rocks (Fitches et al. 1985).To the east of this belt, coarse clastics represented by green and purple are predominant lithology (Turner 1983). Gold in this area is hosted by schist, phyllites, and quartzites which is related to the Anka Fault System (AFS)and metaconglomerate(Garba 2003; Russ 1957). According to Danbatta et al.(2009), gold mineralizing fluids in the area are of metamorphic origin. The ores of gold are very rich in Pb(galena) and Cu (chalcopyrite) as observed during the fieldwork. Fractures which trend mostly in the North-North-East to South-South-West (NNE-SSW), Northeast-Southwest (NE-SW) and Northwest-Southeast (NW-SE)were observed. According to Adewumi et al. (2017), most of the fractures in schist belts are associated with the Pan-African Orogeny.

Fig. 1 Location map showing the sampling sites
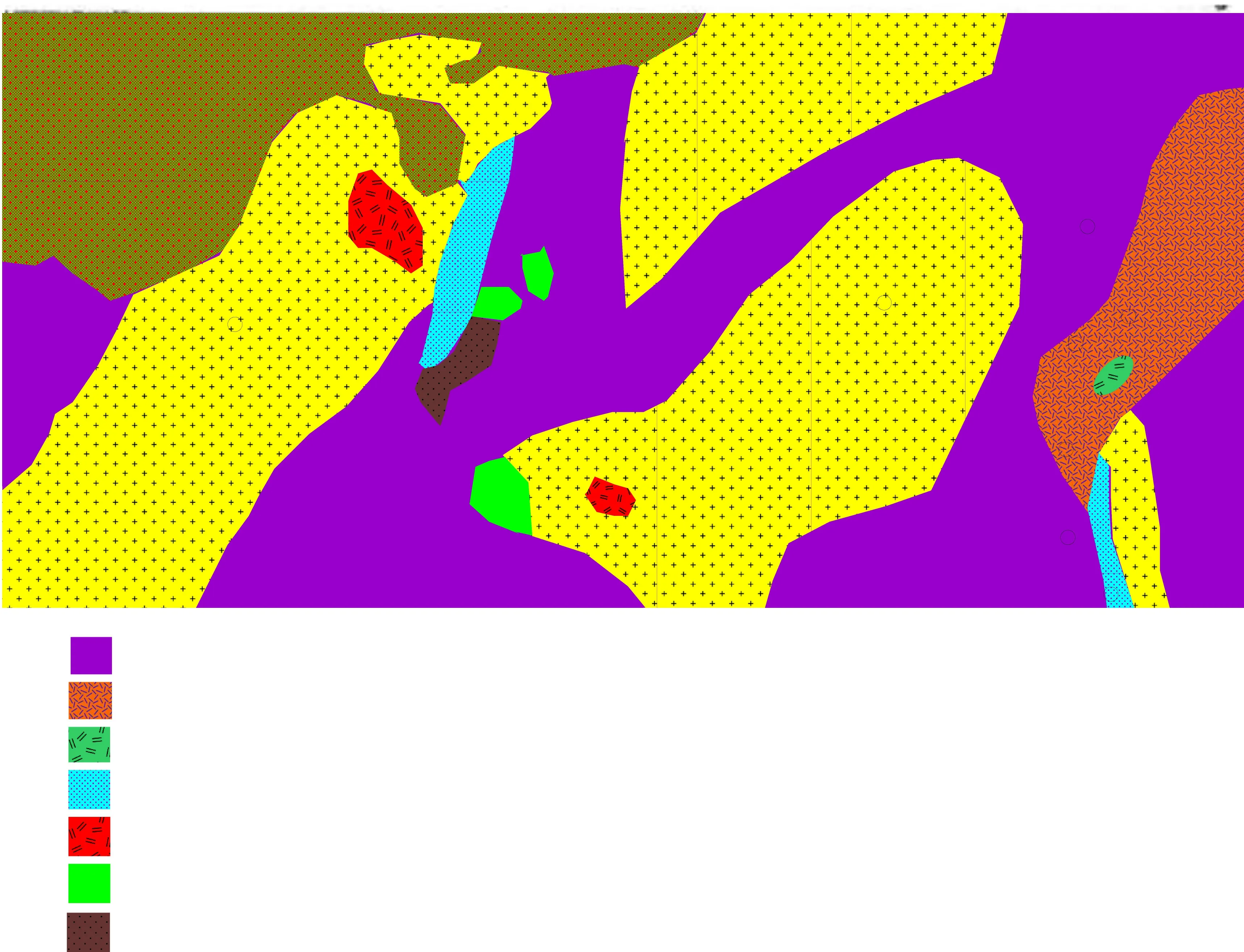
Fig. 2 Geological Map of Anka Area (After Danbatta et al. 2009)
Anka area is characterized by high and undulating topography reaching it speak at 420 meters above the sea level. Major human activities in the area are farming and artisanal mining activities. The major river in the area is the Anka River running from east to west and linking up with river Sokoto in the west. Dendritic streams are common in the area. This area is also characterized by tropical climate(Obasi 1965; Lamb 1983; Adejuwon et al. 1990; Adejuwon 2012). The average temperature in the area is 31.8 °C with the highest temperature of the year observed of 38 °C in April and the lowest temperature of 24 °C observed in January. The mean precipitation in the area is 71.83 mm.
2.2 Sampling and analysis
A total of forty samples (twenty nails and twenty hairs)were collected from twenty children (10 boys and 10 girls)of ages between 5 and 9 from the Kawaye and Bagega villages in 2017. Limited numbers of samples were collected due to traditional and religious beliefs. Two control samples were collected for each sex from a boy and girl residing in areas where mining activities do not take place.Hair samples (4-5 cm long), of the subjects, were collected from the nape of the scalp by cutting approximately 2 mm from the scalp using a pair of sterilized stainless steel scissors washed with ethanol, a neutral solvent, to remove external contamination, if any, and dried (Williams et al.1998). All hair samples were sealed in plastic bags prior to analysis. Samples taken weighed about five gram.
Following Mehra and Juneja (2005) sampling technique for nail, the selected children were asked to wash their hands thoroughly with double distilled water and medicated soap devoid of metal contamination, followed by drying with a clean towel or tissue paper to remove external contamination that may be present. Nails were cut from fingers with sterilized stainless steel scissors. All nail samples were also sealed in plastic bags prior to analysis.
The hair samples were cut into pieces (1 cm) so as to ensure feasible and fast digestion of the samples in the digesting solution to prepare a water-clear solution. Samples, pre-washed with nonionic detergent, were soaked in distilled water for 10 min. This was followed by soaking in acetone to remove external contamination followed by washing with distilled water. Subsequently, they were dried in an oven at 80 °C for 1 h and kept in a desiccator and stored for later mineralization (Chatt and Katz 1988).
For washing of nails, the nail samples scrapped and cleaned of dust particles with nonionic detergent (triton X-100) following the Gammelgaard and Veien (1990)washing technique. This was followed by soaking in acetone to remove external contamination, rinsing five times with deionized water and drying in an oven at 110 °C for 30 min and stored in a desiccator.
The dried hair and nail samples were digested with 10 mL of 6:1 mixture of concentrated nitric acid and concentrated perchloric acid kept overnight at room temperature and consequently heated at 110-120 °C until complete evaporation to obtaining a crystalline white dry deposit or a water-clear solution. It was then diluted with 0.1 M nitric acid. Digested samples were subsequently diluted at a ratio of 1:50 and kept in a refrigerator at a temperature of 3-5 °C.
To ascertain the sources of heavy metals in children, 15 topsoils samples, 15 groundwater samples, 5 samples of mine tailings, and 5 plants samples were collected using internationally acceptable techniques. Four maize (Zea mays) plants and one sorghum (Sorghum bicolor) plant were sampled in this study. Groundwater samples were collected in 1-l plastic bottles which were pre-washed with ultrapure water and diluted nitric acid. Water samples were kept in a refrigerator at a temperature of 4 °C prior to chemical analysis. Soils, mine tailings, and plants were dried in the laboratory at room temperature, pulverized,and sieved using an electronic sedimentological sieve. A fine-grained portion of the particles was collected and digested using aqua regia method.
The concentration of metals was determined using Agilent high-performance liquid chromatography-inductively coupled plasma-mass spectrometry (HPLC-ICPMS) at the State Key Laboratory of Environmental Geochemistry, Institute of Geochemistry, Chinese Academy of Sciences, Guiyang, China. Series of internal standards were prepared in deionized water for instrumental calibration by diluting commercial standards containing 1000 ppm of the metals. All reagents were of analytical grade. To ensure good quality control, standards were measured prior to and after analysis of ten samples. All analysis conformed to the standards set by the Institute. The detection limit for the metals are: Cr: 1 μg/g; Co: 0.1 μg/g; Ni: 0.2 μg/g; Cu:0.2 μg/g; Zn: 2 μg/g; As: 0.2 μg/g; Cd: 0.02 μg/g; Tl:0.02 μg/g and Pb: 0.5 μg/g. The analytical precision,determined by quality assurance/quality control procedures, using duplicates, reagent blanks, and internal standards, was better than ± 10%.
2.3 Statistical analysis
Statistical analyses of hair and nail concentration were carried out using the Statistical Package for Social Sciences(SPSS) version 22. Values of metal concentrations in hairs and nails were compared to pathological ranges of heavy metals in human beings (Blaurock-Busch et al. 2014) and similar studies across the world. To unravel the potential sources of heavy metals bioaccumulation in the children,bivariate correlation, factor, hierarchical cluster, and t test analysis were used.
3 Results and discussion
3.1 Heavy metal concentrations in hairs and nails
The concentration of metals in hairs and nails of boys living in the mining areas of Bagega and Kawaye of Anka were listed in Table 1. The concentration of heavy metals in hairs of the boys were: Cr: 1.61-9.52 μg/g; Co:0.26-1.42 μg/g; Ni: 2.94-18.54 μg/g; Cu: 5.79-39.91 μg/g; Zn: 53.02-125.55 μg/g; As: 0.10-0.40 μg/g; Cd:0.07-0.54 μg/g; Tl: 0.02-0.08 μg/g; Pb: 47-49.58 μg/g.Compared with the concentration of heavy metals in the control sample, metals in the hairs of boys in Anka area was greater than those in the control sample. Cr, Zn, As,and Pb concentrations were far above the results obtained in a similar study in Japan (Sera et al. 2002); Italy(Senofonte et al. 2000); Brazil (Miekeley et al. 1998) and China (Gang et al. 2017). However, Cr, Ni, and Cu concentrations were lower than results obtained by Onuwa et al. (2012) in a similar study in Nigeria. The concentration of heavy metals in nails of the boys were: Cr:2.66-6.82 μg/g; Co: 0.45-2.63 μg/g; Ni: 4.48-7.57 μg/g;Cu: 3.11-133.63 μg/g; Zn: 40.94-213.95 μg/g; As:0.15-0.73 μg/g; Cd: 0.10-2.96 μg/g; Tl: 0.04-0.08 μg/g;Pb: 16.34-335.64 μg/g.

T a b l e 1 H e a v y m e t a l c o n c e n t r a t i o n i n h a i r s a n d f i n g e r n a i l s o f c h i l d r e n i n A n k a a r e a, N o r t h w e s t N i g e r i a I n d i a(9*)I r a n (F N)(8*)P R M(F N)(7*)H B (μ g/g)(6*)N i g e r i a(H) (5*)C h i n a(H) (4*)B r a z i l(H) (3*)I t a l y(H) (2*)B o y s (n = 1 0; A v e r a g e a g e: 8) G i r l s (n = 1 0; A v e r a g e a g e: 8) J a p a n(H) (1*)C S F N C S H C S F N C S H M e t a l s(μ g/g)--4.0 0-0.4 0 0.0 6--1 2.0 0--1 8.2 3-6 8.4 6 1 5.4 8 1.1 8-1 5.1 6>1.4>0.2 6>0.8 7>1 7.4>2 2 0>0.8 7>0.1 4>0.0 2>2---0.0 0 0 5 7 0.0 0 7 9-0.0 0 0 0 5-0.0 0 0 9 4 5.7 7 5.5 9 5 0.9 6 4 6-----0.7 8---0.1 3 0.7 7--1.5 6<0.3 0---<0.0 4<0.0 6--1 2.5 0.9 9---0.0 9 0.2 3--7.1 1 1.2 9 0.3 1 0.9 6 -1.8 1 -3.1 1 -0.0 9 -0.1 2 -0.0 2 -3.1 8 4.8 0 2.9 9 0.9 2 5.4 4 0.2 2 0.1 4 0.0 5 1.0 7 0.1 6 1.1 6 3.7 2 1 9.0 2 0.0 9 0.0 3 0.0 2 5.1 8 1.6 3 6.7 3 3 8.6 6 0.3 6 0.2 6 0.0 5 7 4.1 1 1 0.2 7 1 7.6 6 1.0 3 0.4 2 3.1 9 3.1 1 0.2 1 0.0 8 0.0 3 8.0 3 4.5 1 1.1 0 5.9 1 3 4.6 1 1 4 0.3 7 1 3.8 2 1 5 5.6 6 2 5.1 7 4 2.8 2 1 0.9 2 0.2 3 0.3 6 0.7 3 0.0 6 9 0.1 5 1.0 7 0.1 6 1.1 6 3.7 2 0.0 9 0.0 3 0.0 2 4.5 9 0.7 6 7.5 6 1 4.4 7 8 8.0 8 2 5.1 0.2 6 0.2 3 0.0 5 2 9.8 2 1 0.2 C r C o N i C u Z n A s C d T l P b C S: c o n t r o l s a m p l e (1*)-S e r a e t a l. (2 0 0 2), (2*)-S e n o f o n t e e t a l. (2 0 0 0), (3*)-M i e k e l e y e t a l. (1 9 9 8), (4*)-G a n g e t a l. (2 0 1 7), (5*)-O n u w a e t a l. (2 0 1 2), (6*)-O g a b i e l a e t a l. (2 0 1 1),(7*)-B l a u r o c k-B u s c h e t a l. (2 0 1 4), (8*)-P a r i z a n g a n e h e t a l. (2 0 1 4), (9*)-B l a u r o c k-B u s c h e t a l. (2 0 1 4)H B h u m a n b l o o d, P R M p a t h o l o g i c a l r a n g e s o f m e t a l s, F N f i n g e r n a i l s, H h a i r s
Compared with the concentration of heavy metals in the control sample, all metals in the nails of boys in Anka area were greater than those in the control sample. Cr, Co, Ni,As, Cd, Tl, and Pb concentrations were far above the pathological ranges of heavy metals in nails (Blaurock-Busch et al. 2014). Also, Ni, As, and Cd concentrations were lower compared to results of a similar study in Iran(Parizanganeh et al. 2014) while Zn and Pb were greater than the results in such study in Iran by Parizanganeh et al.(2014). Ni, As, Cd, and Pb in nails of boys in this area were higher than results obtained from a similar study in India by Blaurock-Busch et al. (2014).
The concentration of metals in hairs and nails of girls of ages 5 to 9 living in the Mining areas of Bagega and Kawaye of Anka are shown in Table 1. The concentration of heavy metals in hairs of the girls were: Cr:1.93-15.28 μg/g; Co: 0.22-5.88 μg/g; Ni: 3.76-13.96 μg/g; Cu: 8.30-265.47 μg/g; Zn: 62.34 -271.31 μg/g; As:0.07-0.92 μg/g; Cd: 0.10-0.56 μg/g; Tl: 0.03-0.07 μg/g;Pb: 17.09-171.99 μg/g. Metal concentrations in the hairs of girls in the area were greater than those in the control sample (Table 1). Cr, Zn, and Pb concentrations were above the results obtained in a similar study in Japan (Sera et al. 2002); Italy (Senofonte et al. 2000); Brazil (Miekeley et al. 1998) and China (Gang et al. 2017). As concentration in hairs of girls in this area was greater than those obtained in Italy, Brazil, and Japan. Ni and Cu concentrations in the hair samples were lower in concentration than those reported by Onuwa et al. (2012) in Nigeria while Co in hairs of girls in Anka area was greater than those obtained by the same author.
The concentrations of heavy metals in nails of the girls were: Cr: 2.02-4.35 μg/g; Co: 0.33-1.75 μg/g; Ni:2.19-19.22 μg/g; Cu: 3.39-119.13 μg/g; Zn:22.7-58.28 μg/g; As: 0.09-0.37 μg/g; Cd: 0.09-0.25 μg/g;Tl: 0.02-0.07 μg/g; Pb: 8.21-49 μg/g. All the metals analyzed in nails of girls in the area had concentrations greater than the control sample. Cr, Co, Ni, Cu, Cd, Tl, and Pb concentrations were far above the pathological ranges of heavy metals in nails (Blaurock-Busch et al. 2014). Only As and Zn concentrations were below the pathological ranges. Ni, Zn, As, and Cd concentrations were lower compared to results of related study in Iran (Parizanganeh et al. 2014) while only the Pb concentration was greater than the results in such study in Iran by Parizanganeh et al.(2014). Ni, Cd, and Pb concentrations in the nails of girls in this area were higher than results obtained from a similar study in India by Blaurock-Busch et al. (2014) while As concentration was lower in the samples compared to results of the same study. It had been noted that girls bioaccumulate heavy metals more than boys in some parts of the world (Gang et al. 2017). This research uncovered that boys in the area are exposed to mining/mineral processing than girls and may have contributed greatly to heavy metals bioaccumulation in nails of the boys than the girls. Also,prevailing high environmental conditions such as high wind movement in the area which the children are exposed to might have highly contributed to the accumulation of heavy metals in the kids. This is because wind as a major transporter of potentially toxic metals (PTE) increases the risk of their bioaccumulation through oral ingestion and contact with skins. Studies had revealed that elemental composition in human hair is affected by race and are greater in people of black origin (Rutherford and Hawk 1907; Taylor 1986).
3.2 Heavy metals in soils, groundwater, mine tailings and plants
Mean concentrations of heavy metals in groundwater of the area are presented in Table 2. Cr has a mean concentration of 0.44 mg/L, Co has a mean concentration of 0.07 mg/L, Ni has a mean concentration of 0.32 mg/L, Cu has a mean concentration of 6.21 mg/L while Zn has a mean concentration of 5.42 mg/L. Also, As and Cd have a mean concentration of 0.04 mg/L while Tl and Pb a have mean concentration of 0.05 and 1.09 mg/L respectively. All heavy metals assessed in the groundwater from this area are above the WHO (2010) and NSDWQ (2007) standards, which give clear indications that subsurface water in the area, are contaminated by artisanal mining activities in the area.
The average concentration of heavy metals in soils of the study area is as shown in Table 2. The mean concentrations are: Cr: 49.50 μg/g; Co: 11.79 μg/g; Ni: 18.40 μg/g; Cu: 331.37 μg/g; Zn: 646.3 μg/g; As: 0.44 μg/g; Cd:0.037 μg/g; Tl: 0.33 μg/g and Pb: 550.33 μg/g. The mean concentrations of heavy metals in mine tailings are shown in Table 2. The mean concentrations are: Cr: 89 μg/g; Co:22.5 μg/g; Ni: 55.75 μg/g; Cu: 1874 μg/g; Zn: 98.25 μg/g;As: 9.72 μg/g; Cd: 1.00 μg/g; Tl: 8.13 μg/g and Pb:6500 μg/g. The mean concentrations of heavy metals in plants of the area are: Cr: 24.96 μg/g; Co: 7.14 μg/g; Ni:11.13 μg/g; Cu: 11.42 μg/g; Zn: 26.51 μg/g; As: 0.79 μg/g and Pb: 33.07 μg/g. Cr, Cu, As, Cd and Pb are above the USEPA (2002) standard for heavy metals in both the soils and tailings and thus reflect possible contamination from mining activities while Cr, Co, Ni, Cu, Zn, As, and Pb concentrations were higher in plants of the area than the FAO (2011) standard reflecting that crops in this area are also contaminated by mining/mineral processing.
3.3 Sources of heavy metals exposure in children
Multivariate analysis (‘‘Appendix’’ Tables 3-14) showed that metals which bioaccumulate in the kids are from mixed anthropogenic sources such as mining, mineral processing, and consumption of contaminated water and plants. Major human activities in the study area were mining, mineral processing, and agricultural activities.Plots of components of the factor analysis for heavy metal in hairs and nails of boys are shown in Fig. 3. The plotshowed that while other heavy metals in nails and hairs of boys are possibly from mining activities, Cd, Pb, Zn in hairs and Ni and Zn in nails are from mixed anthropogenic sources which might be a mix of mining, mineral processing, agricultural practices, and consumption of contaminated food. Similar plot was generated for heavy metals in girls in this area, the result showed that Cu, Co,Cd, Tl, Zn, and As in nails of the girls might have originated from mixed anthropogenic sources especially via the consumption of heavy metals laden plants while other metals in hairs and nails might have come from mining and mineral processing activities (Fig. 4).

Table 2 The mean concentration of heavy metals in groundwater, soils and mine tailings in Bagega and Kawaye areas of Anka
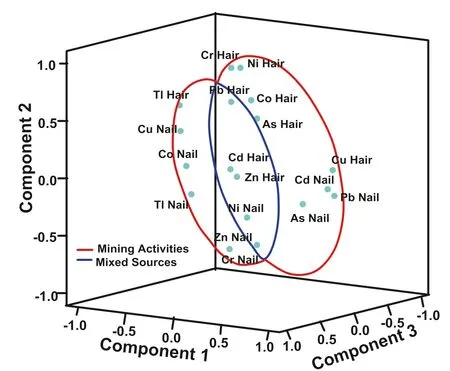
Fig. 3 Plot of factors 1, 2 and 3 depicting the sources of heavy metals in nails and hairs of boys in Anka area

Fig. 4 Plot of factors 1, 2 and 3 depicting the sources of heavy metals in nails and hairs of girls in Anka area
Hierarchical cluster analysis (HCA) revealed that Tl, As,Co, Cd, Ni in hairs and nails and Cu in hairs of boys are from similar sources (Fig. 5). These metals might have originated from mining and mineral processing activities in the area. However, Pb and Zn in nails and Cu in nails might have originated from both agricultural and mining activities. A similar scenario played out in the HCA of heavy metals in the hair and nails of girls in the area (Fig. 6). Tl,Cd, As, Co, Cr, and Ni in hair with Pb in nails are from the similar anthropogenic source while Cu and Zn in nails and Cu, Zn, and Pb in hairs are from mixed sources.
The results of the correlation between heavy metals in groundwater and nails in boys of this area showed that Cr,Co, Ni, Cu, Cd, and Pb have strong and positive correlation which revealed these metals which bioaccumulate in the boys might have been introduced in their bodies via oral intake of the contaminated groundwater and plants. In the girls, the result showed that Co, Ni, Cu, Tl, and Pb in the nails might have also originated from the drinking of polluted groundwater. Zn in nails of the girls might have originated other media than groundwater. Also, the correlation between the heavy metals in groundwater and hairs of the boys showed that there is a positive and strong correlation between Cr, Co, Ni, Cu, Cd, and Pb which also revealed that they might have been introduced into their bodies through the consumption of contaminated water. Arsenic (As) in hairs of the boys might have originated from other sources than groundwater. In girls, Ni, Cu, Zn, As, and Tl in hairs originated from groundwater of the area.

Fig. 5 Hierarchical cluster analysis of heavy metals in hairs and nails of boys in Anka area

Fig. 6 Hierarchical Cluster Analysis of Heavy Metals in Hairs and Nails of Girls in Anka Area
Similarly, the results of the correlation between the metals in soils and nails of the Children showed that Co,Ni, Cu, As, Cd, and Pb in nails of the boys might have originated from the inhalation, ingestion and dermal contact with contaminated soils in the area while Co, Zn, As,Cd, Tl, and Pb in nails of the girls might have originated from contaminated soils. Correlation of heavy metals in soils and hairs of children in the area showed that Cr, Ni,Zn, As, Cd, and Pb might have come from polluted soils in the area. Furthermore, the correlation between heavy metals in soils and hairs of girls showed that Cr, Co, Ni,Cu, Zn, As and Pb in them might have their source as the contaminated soils.
Chromium, Co, Ni, Cu, Zn, As, Cd, and Pb in nails of boys in this area might have originated from contacts with mine tailings. Tl in nails of boys in this area might have originated from media other than mine tailings. In nails of the girls, Cr, Cu, Zn, As, Cd, Tl, and Pb might have originated from mine tailings while Co and Ni might have come from other media apart from mine tailings. Also, in hairs of the boys, Co, Ni, Cu, Zn, As, Cd, and Pb might have originated from the mine tailings while Cr and Tl in their hairs might have originated from other media. Similarly, Cu, Zn, As, Tl, and Pb in hairs of girls in this area might have originated from other sources.
Correlation analysis between heavy metals in plants and hairs of boys in the area revealed that Co, Ni, Cu, Zn, As,and Pb are from similar sources while Cr in the crops and hairs are of different origins. However, heavy metals in hairs of girls in the area are from similar sources. For boys,heavy metals in plants and nails are from a similar source while Cr in plants and nails of girls are from dissimilar sources. This showed that metals in plants in this area mainly from soils contaminated by mining and mineral processing. The study further revealed that oral ingestion of contaminated water and plants have greatly contributed more to heavy metals bioaccumulation although inhalation of contaminated soils and mine tailings also played major roles. It also unraveled that mining and mineral processing were the main human activities contributing to metal accumulation in children in this area.
Lead (Pb), Cd, Zn, and Cu in hairs and nails of the boys and girls are higher than these heavy metals in the blood of children (ages 1-10) in the area reported by Ogabiela et al.(2011). This showed that hairs and nails of the children bioaccumulate heavy metals more than blood. This study further showed that children residing in active mining areas are more prone to heavy metals than children in areas where mining activities does not occur. This may lead to high mortality ratios (Filler et al. 2017; Linos et al. 2011).Bioaccumulation of toxic metals in the human body and plants may reduce the life span of humans by half (Arif et al. 2015).
Health issues related to heavy metals have been greatly discussed by several researchers (Singh and Kalamdhad 2011; Tchounwou et al. 2012; Roels et al. 1981; Roels et al. 1983). High cadmium levels in children are attributed to the participation of children at the gold processing which may cause lung and kidney problems in them (Majid et al.1999; Ogabiela et al. 2011; Filler et al. 2017). Children can be exposed to Co in the same ways as adults (ATSDR 2004). Kids living or working near hazardous waste sites such as mining sites, may experience systemic, immunological, neurological, reproductive, developmental, genotoxic, carcinogenic effects and eventually death. Acute exposures of children in Kawaye and Bagega area to potentially toxic metals are likely linked to the death of over 400 children in Anka in 2010 as reported by Liang(2010). Also, they may be exposed to arsenic by eating soil which may increase the risk of cancer in the liver, bladder,and lungs (ATSDR 2007a, b). Long-term exposure to inorganic arsenic in children may result in lower IQ scores(ATSDR 2007a, b).
Gold mining is one of the major sources of Pb in environmental media. Children are more vulnerable to Pb poisoning than adults and are exposed to lead all through their lives (Needleman 2004; Lanphear et al. 2005). Babies and children can swallow and breathe leading dirt, dust, or sand while they play on the floor or ground (ATSDR 2007a, b). It affects the blood, development, and behavioral pattern of children (Cory-Slechta et al. 1983). Exposure of kids to lead can cause mental retardation and physical growth (ATSDR 2007a, b) and delinquent behaviours(Needleman et al. 1996).
4 Conclusions
This research was carried out to determine the extent of heavy metal bioaccumulation in children in Anka mining area, Northwest Nigeria using hair and fingernails from children. However, this work did not evaluate the concentration of heavy metals in other bio-indicators such as urine and other vital organs such as liver and kidney. From this study, high levels of Cr, Co, Ni, Cu, Zn, As, Cd, Tl,and Pb in children between the ages of 5 and 9 years in Anka area, Nigeria were recorded. The study showed that heavy metals bioaccumulate more in nails and hairs of children in the area than in the blood as reported by Ogabiela et al. (2011). The study further showed that heavy metals in hairs and nails of the children originated from mining, mineral processing, and agricultural activities in the area. However, mining and mineral processing contributed more to the accumulation of toxic metals in children of this area. The study also revealed that oral ingestion, of contaminated groundwater and plants contributed significantly to heavy metal bioaccumulation in the children. It is recommended that total remediation program should be carried out by the Federal Government of Nigeria and related international organizations to stall untimely death of children in the area while proper disposal of mine wastes should be encouraged amongst artisanal miners. Inhabitants of this area should be educated on the health problems associated with heavy metal bioaccumulation in human beings.
Acknowledgements This research was sponspored by research opening fund of State Key Laboratory of Environmental Geochemistry, Guiyang, Guizhou Province, China with grant No.SKLEG2017910. The authors are indebted to the Ministry of Environment of Zamfara State, Nigeria, the Emir of Anka and Village Heads of Bagega and Kawaye villages in the Anka Emirate. We also appreciate the management of Achievers University, Nigeria for giving the first author study leave to the State Key Laboratory of Environmental Geochemistry, Guiyang, Guizhou Province, China.Our heartfelt thanks go to Oladoyin Ayodeji Felix and Haske Muhammed for their assistance during the sample collection. Xiao Long, Atta Ratsool, Ma Liam and Oyebamiji Abiola are appreciated for their assistance during the laboratory analysis of sample. We are grateful to all the anonymous reviewers for sparing their precious time in making this manuscript of great quality.
Compliance with ethical standards
Conflict of interest On behalf of all authors, the corresponding author states that there is no conflict of interest.
Appendix
See Tables 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 and 14.
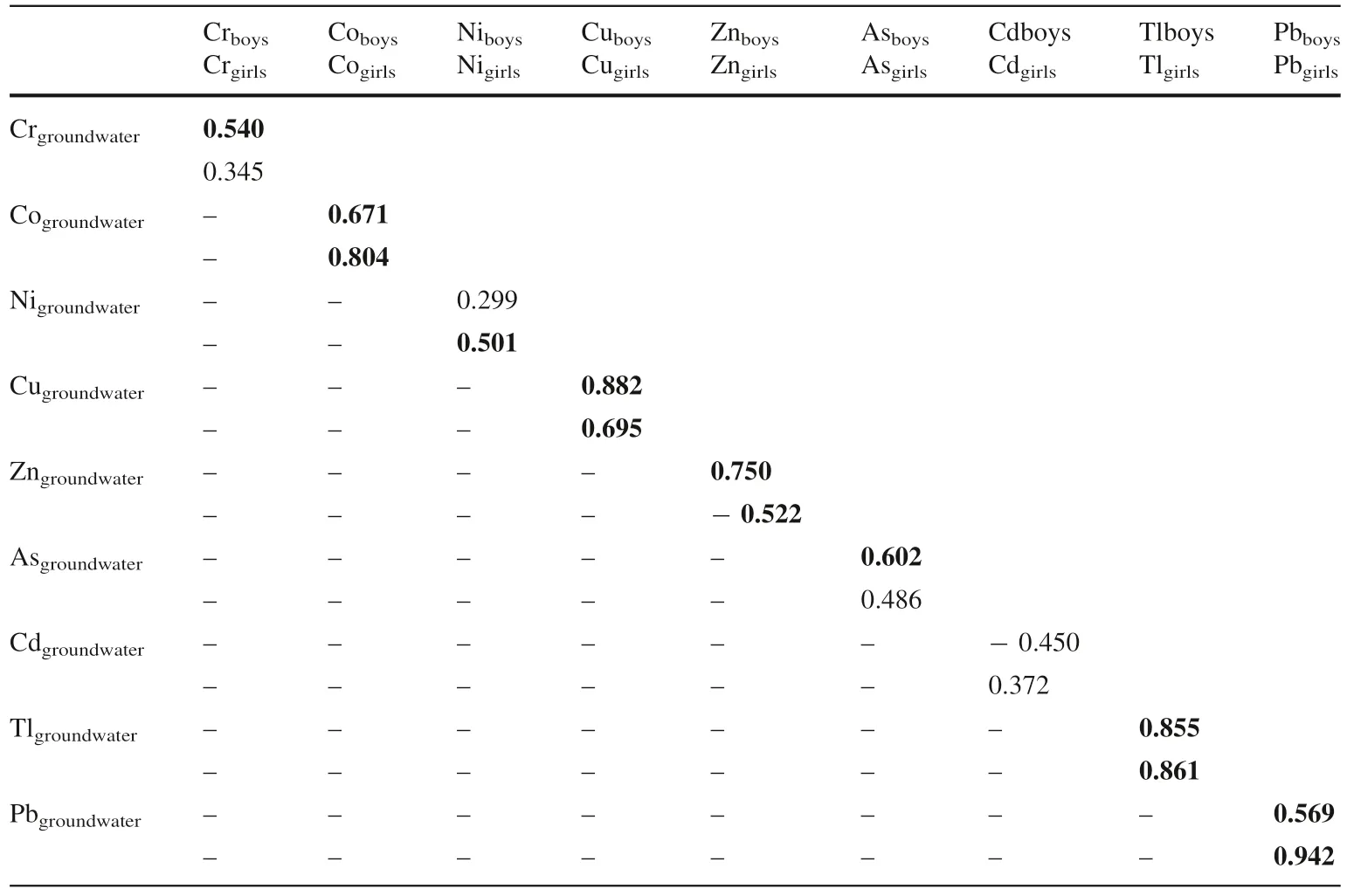
Table 3 Correlation of heavy metals in groundwater and nails of children
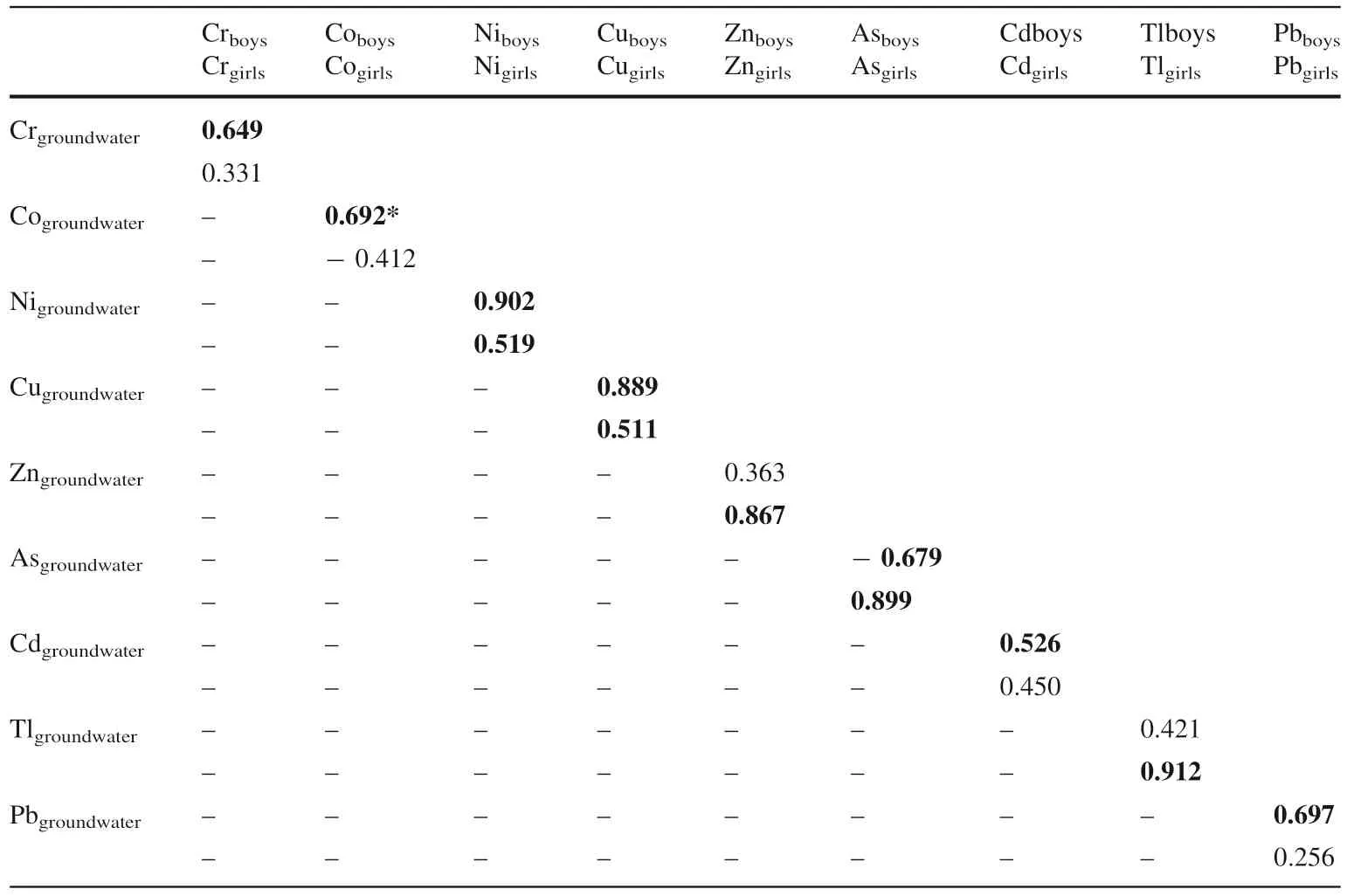
Table 4 Correlation of heavy metals in groundwater and hairs of children

Table 5 Correlation of heavy metals in soils and nails of children

Table 6 Correlation of heavy metals in soils and hairs of children

Table 7 Correlation of heavy metals in mine tailings and nails of children

Table 8 Correlation of heavy metals in mine tailings and hairs of children
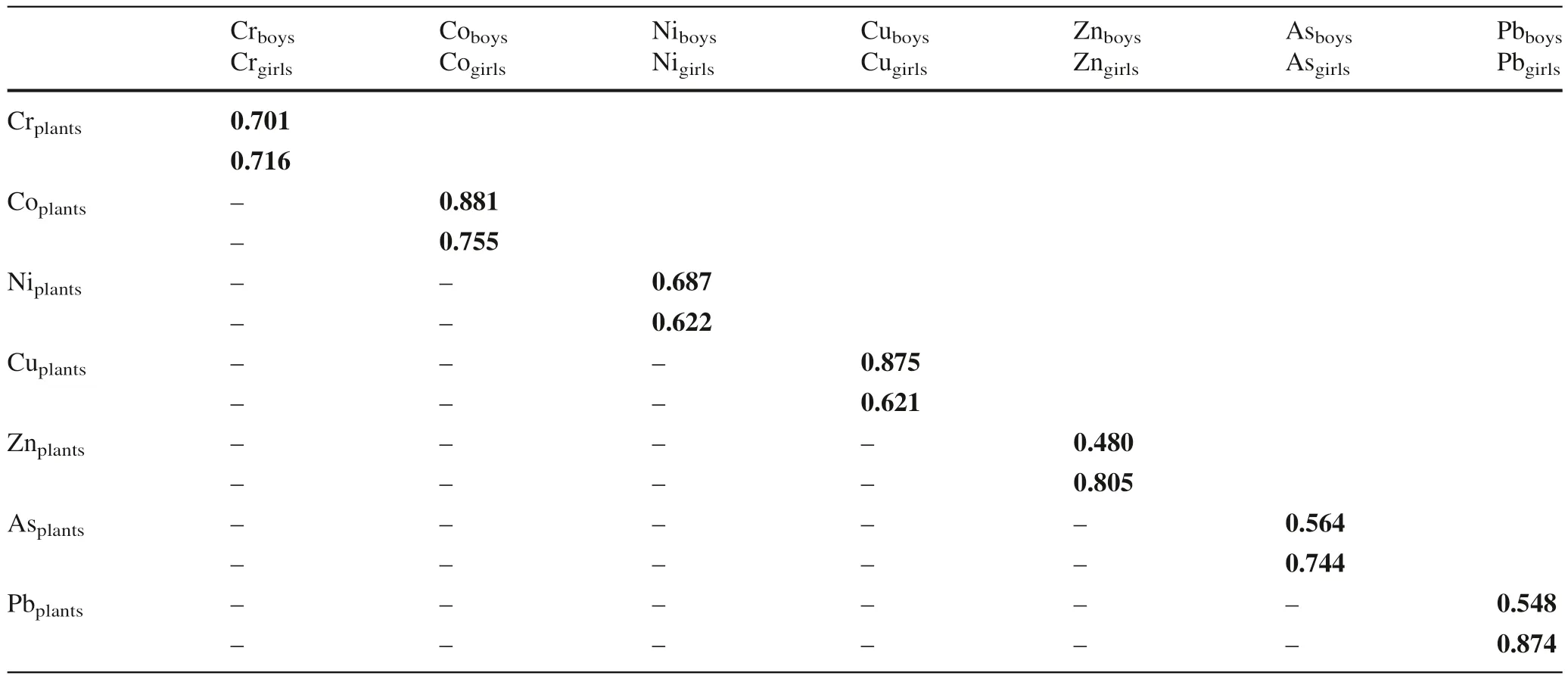
Table 9 Correlation of heavy metals in plants and nails of children

Table 10 Correlation of heavy metals in plants and hairs of children
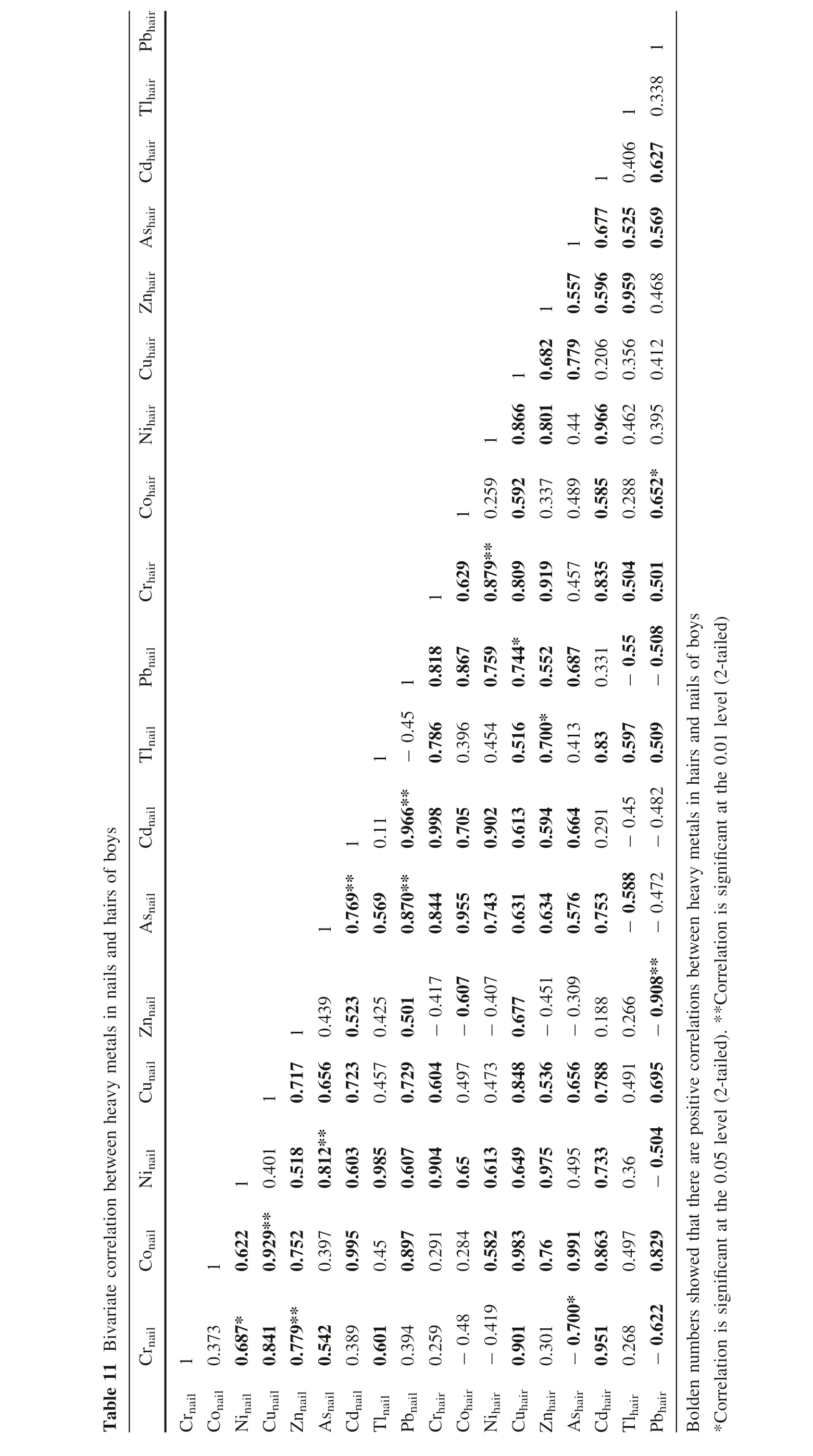
T a b l e 1 1 B i v a r i a t e c o r r e l a t i o n b e t w e e n h e a v y m e t a l s i n n a i l s a n d h a i r s o f b o y s P b h a i r N i h a i r C u h a i r Z n h a i r A s h a i r C d h a i r T l h a i r C o h a i r C r h a i r P b n a i l T l n a i l C d n a i l A s n a i l C u n a i l Z n n a i l N i n a i l C o n a i l C r n a i l 0.7 7 9 0.5 5 7 1 1 0.8 6 6 1 0.8 0 1 0.6 8 2 1 0.4 4 0.9 6 6 0.2 0 6 0.5 9 6 0.6 7 7 1 0.4 6 2 0.3 5 6 0.9 5 9 0.5 2 5 0.4 0 6 1 1 0.5 9 2 0.3 3 7 0.4 8 9 0.5 8 5 0.2 8 8 0.6 5 2* 0.3 9 5 0.4 1 2 0.4 6 8 0.5 6 9 0.6 2 7 0.3 3 8 1 1 0.6 2 9 0.8 7 9** 0.2 5 9 0.8 0 9 0.9 1 9 0.4 5 7 0.8 3 5 0.5 0 4 0.7 4 4*0.8 6 7 0.6 8 7 0.3 3 1 0.7 5 9- 0.5 5- 0.5 0 8 0.5 0 1 1 0.3 9 6 0.7 8 6 0.4 5 4 0.5 1 6 0.7 0 0* 0.5 5 2 0.4 1 3 0.8 3 0.5 9 7 0.7 6 9** 1 0.1 1 0.9 9 8 0.9 0 2 0.7 0 5 0.6 1 3 0.5 9 4 0.6 6 4 0.2 9 1 1 0.5 6 9 0.8 7 0** 0.9 6 6** - 0.4 5 1 0.8 1 8 0.8 4 4 0.9 5 5 0.7 4 3 0.6 3 1 0.6 3 4 0.5 7 6 0.7 5 3- 0.5 8 8 - 0.4 5 1 0.7 1 7 1 0.7 2 3 0.5 2 3 0.4 5 7 0.4 2 5 0.7 2 9 0.5 0 1 0.6 0 4 - 0.4 1 7 0.4 9 7 - 0.6 0 7 0.4 7 3 - 0.4 0 7 0.8 4 8 0.6 7 7 0.5 3 6 - 0.4 5 1 0.6 5 6 - 0.3 0 9 0.7 8 8 0.1 8 8 0.4 9 1 0.2 6 6 1 0.5 1 8 0.8 1 2** 0.6 5 6 0.4 3 9 0.6 0 3 0.9 8 5 0.6 0 7 0.9 0 4 0.6 5 0.6 1 3 0.6 4 9 0.9 7 5 0.4 9 5 0.7 3 3 0.3 6- 0.5 0 4 0.6 9 5 - 0.9 0 8** - 0.4 7 2 - 0.4 8 2 0.5 0 9 1 0.6 2 2 0.9 2 9** 0.4 0 1 0.7 5 2 0.3 9 7 0.9 9 5 0.4 5 0.8 9 7 0.2 9 1 0.2 8 4 0.5 8 2 0.9 8 3 0.7 6 0.8 6 3 0.4 9 7 0.8 2 9 1 0.6 8 7*0.6 0 1 0.2 5 9- 0.4 1 9 0.2 6 8 C r n a i l C o n a i l 0.3 7 3 N i n a i l C u n a i l 0.8 4 1 Z n n a i l 0.7 7 9**A s n a i l 0.5 4 2 C d n a i l 0.3 8 9 T l n a i l P b n a i l 0.3 9 4 C r h a i r C o h a i r - 0.4 8 N i h a i r C u h a i r 0.9 0 1 Z n h a i r 0.3 0 1 A s h a i r - 0.7 0 0* 0.9 9 1 C d h a i r 0.9 5 1 T l h a i r P b h a i r - 0.6 2 2 B o l d e n n u m b e r s s h o w e d t h a t t h e r e a r e p o s i t i v e c o r r e l a t i o n s b e t w e e n h e a v y m e t a l s i n h a i r s a n d n a i l s o f b o y s*C o r r e l a t i o n i s s i g n i f i c a n t a t t h e 0.0 5 l e v e l (2-t a i l e d). **C o r r e l a t i o n i s s i g n i f i c a n t a t t h e 0.0 1 l e v e l (2-t a i l e d)

T a b l e 1 2 B i v a r i a t e c o r r e l a t i o n b e t w e e n h e a v y m e t a l s i n n a i l s a n d h a i r s o f g i r l s P b h a i r C d h a i r T l h a i r A s h a i r Z n h a i r N i h a i r C u h a i r C o h a i r C r h a i r P b n a i l T l n a i l C d n a i l A s n a i l C u n a i l Z n n a i l C o n a i l N i n a i l C r n a i l 1 0.4 6 1 1 0.6 9 5 0.8 1 9** 0.9 0 6 0.8 6 1 1 0.6 2 8 0.8 5 8** 1 0.9 1 3 0.6 9 3 0.8 3 3 1 0.8 3 9 0.8 6 6** 0.8 2 9** 0.6 1 6 0.9 8 8 0.4 7 8 - 0.2 7 1 0.4 0.4 8 0.5 1 8 0.3 7 4 0.4 2 5 0.5 3 5 0.9 6 0.8 5 9** 0.7 8 3** 1 0.8 7 9** 1 0.3 8 3 0.5 5 2 0.4 5 4 1 0.7 5 6** 0.5 9 6 0.7 9 0.3 9 4 0.6 4 1*1 0.5 9 9 0.5 3 2 0.8 0 7 0.9 1 5** 0.6 6 2 0.5 9 8 0.8 2 3 0.3 5 3 0.3 4 4 0.6 4 0* 1 0.8 2 5 0.5 9 9 0.4 6 7 0.2 4 4 0.9 8 5 0.4 6 4 0.7 2 6 0.4 3 8- 0.4 8 0.5 4 0.4 3 8 1 0.7 6 6 0.9 2 6 0.6 1 4 0.9 8 1 0.8 1 8 0.7 6 0.9 3 7 0.5 9 0.3 3 7 1 0.4 9 2 0.8 7 0.6 6 6 0.4 4 9 0.4 0 6 0.8 3 9 0.9 8 3 0.5 8 3 0.3 9 3- 0.6 2 0.8 0 8 0.8 2 6 0.9 0 8 0.8 1 9 0.7 2 6 0.3 6 8 1 0.6 5 7 0.8 3 6 0.4 8 6 0.5 3 2 0.9 1 8** 0.6 7 7 0.6 9 2 0.9 4 8 0.5 1 4 0.6 7 7 0.3 2 9 0.6 8 2 0.5 6 7 0.8 4 9 - 0.3 5 0.9 4 0.6 4 3 0.2 9 8 0.4 9 1 0.6 0 5 0.7 7 1 1 0.4 4 0.5 1 6 0.7 0 8 0.8 8 1 1 0.4 0.4 5 9 0.9 0 6 0.9 9 1 0.3 9 1 0.6 2 4 0.6 7 9 0.3 2 9 0.9 6 9 0.5 4 0.5 2 8 0.6 2 7 0.7 8 4 0.4 3 3 0.5 5 5 0.7 8 6 0.4 3 5 0.9 7 8 0.4 5 7 0.4 4 3 0.6 4 4 0.9 4 6 0.3 2 7 0.5 3 7 0.9 1 4 0.4 9 1 0.6 6 1*- 0.5 8 1 0.4 0 7 0.8 4 5 0.5 6 0.5 1 2 0.5 0 2 0.8 1 5** 0.7 2 5 0.4 3 6 0.4 2 3 C r n a i l C o n a i l 0.9 8 3 N i n a i l C u n a i l 0.9 9 1 Z n n a i l 0.7 1 6 A s n a i l 0.5 4 6 C d n a i l 0.5 1 T l n a i l P b n a i l C r h a i r C o h a i r 0.5 6 3 N i h a i r C u h a i r 0.6 6 4 Z n h a i r 0.4 9 9 A s h a i r 0.8 8 5 C d h a i r 0.8 8 7 T l h a i r P b h a i r B o l d e n n u m b e r s s h o w e d t h a t t h e r e a r e p o s i t i v e c o r r e l a t i o n s b e t w e e n h e a v y m e t a l s i n h a i r s a n d n a i l s o f g i r l s T h e s y m b o l a s t e r i s k s h o w e d t h a t c o r r e l a t i o n i s s i g n i f i c a n t a t t h e 0.0 5 l e v e l (2-t a i l e d) w h i l e t h e s y m b o l d o u b l e a s t e r i s k s h o w e d t h a t c o r r e l a t i o n i s s i g n i f i c a n t a t t h e 0.0 1 l e v e l (2-t a i l e d)

Table 13 Factor analysis of heavy metals in hairs and nails of boys
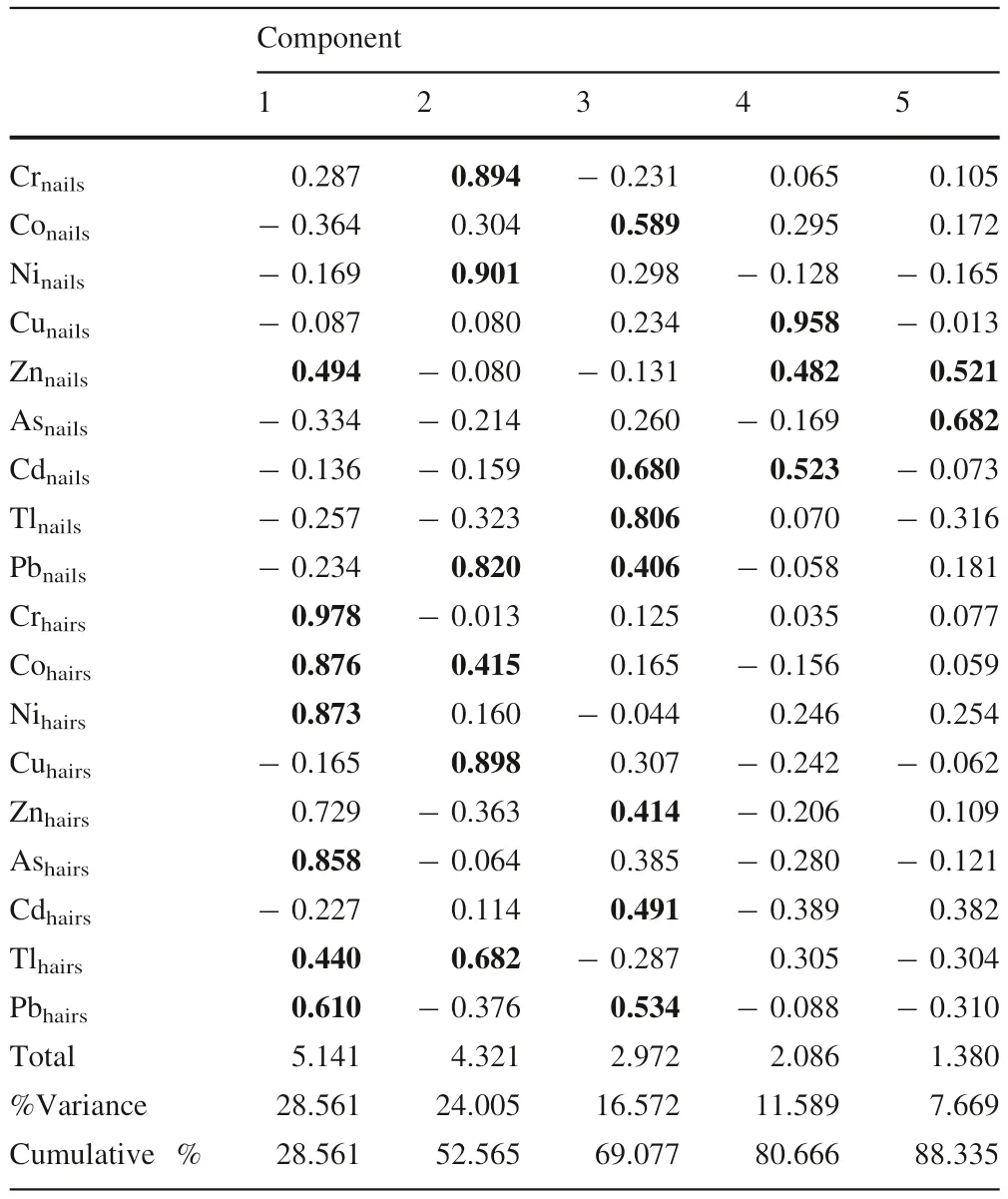
Table 14 Factor analysis of heavy metals in hairs and nails of girls
杂志排行
Acta Geochimica的其它文章
- Development of in-situ Micro-Raman spectroscopy system for autoclave experimental apparatus
- Constraints on granite-related uranium mineralizationin the Sanjiu uranium ore field, SE China provided by pyrite mineralogy, major and trace elements, S-He-Ar isotopes
- Studying DDTs in agricultural soils of selected rural communities of Armenia
- Fluid inclusion and H-O isotope study of the Jiguanshan porphyry Mo deposit, Xilamulun Metallogenic Belt: implications for characteristics and evolution of ore-forming fluids
- REE geochemistry of core sediments of Cauvery delta, India for provenance studies
- CO2 flux of soil respiration in natural recovering karst abandoned farmland in Southwest China
