miR-21/PDCD4/AP-1对食管癌细胞增殖、侵袭及迁移的影响
2020-08-27杨鲸蓉吴健叶仕新
杨鲸蓉 吴健 叶仕新
[摘要] 目的 研究miR-21/PDCD4/AP-1對食管癌细胞增殖、侵袭和迁移的影响。 方法 瞬时转染食管鳞癌细胞为实验对象,分组方法为单转染组:空白对照组、miR-21 mimic阴性对照(miR-21 mimic NC)组、miR-21 mimic组、miR-21 inhibitor NC组、miR-21 inhibitor组、siRNA NC组、PDCD4 siRNA组、c-Jun siRNA组;共转染组:miR-21 inhibitor NC+siRNA NC组、miR-21 inhibitor+siRNA NC组、miR-21 inhibitor+PDCD4 siRNA组。分别运用CCK-8、Transwell迁移和侵袭检测miR-21/PDCD4/AP-1反馈环路对食管癌EC9706细胞增殖、迁移和侵袭能力的影响。结果 转染后2 d起,miR-21 mimic组EC9706细胞的增殖能力明显高于空白对照组、miR-21 mimic NC组、miR-21 inhibitor NC组和miR-21 inhibitor组(P < 0.05)。miR-21 mimic组穿过小室的细胞计数多于空白对照组和miR-21 mimic NC组(P < 0.05);miR-21 inhibitor组穿过小室的细胞计数少于miR-21 inhibitor NC组(P < 0.05)。miR-21 mimic组穿透Matrigel胶的侵袭细胞数多于空白对照组和miR-21 mimic NC组(P < 0.05);miR-21 inhibitor组穿透Matrigel胶的侵袭细胞数少于miR-21 inhibitor NC组(P < 0.05)。转染后2 d起,PDCD4 siRNA组EC9706细胞的增殖能力高于空白对照组和siRNA NC组(P < 0.05)。PDCD4 siRNA组穿过小室的细胞计数、穿透Matrigel胶的侵袭细胞数多于空白对照组和siRNA NC组(P < 0.05)。miR-21 inhibitor+siRNA NC组EC9706细胞的增殖能力低于miR-21 inhibitor+PDCD4 siRNA组和miR-21 inhibitor NC+siRNA NC组(P < 0.05)。miR-21 inhibitor+PDCD4 siRNA组穿过小室的细胞计数、穿透Matrigel胶的侵袭细胞数多于miR-21 inhibitor+siRNA NC组(P < 0.05)。c-Jun siRNA组EC9706细胞的增殖能力低于空白对照组和siRNA NC组(P < 0.05)。c-Jun siRNA组穿过小室的细胞计数、穿透Matrigel胶的侵袭细胞数均少于siRNA NC组(P < 0.05)。 结论 miR-21/PDCD4/AP-1反馈环路可调控食管癌细胞的生长和侵袭,该环路为阻止的侵袭、转移提供新的治疗策略。
[关键词] miR-21;PDCD4;AP-1;食管癌
[中图分类号] R735.1 [文献标识码] A [文章编号] 1673-7210(2020)07(b)-0004-06
[Abstract] Objective To study the effect of miR-21/PDCD4/AP-1 on the proliferation, invasion and migration of esophageal cancer cells. Methods Transient transfection of esophageal squamous cell carcinoma cells was used as experimental object. The single transfection group: blank control group, miR-21 mimics negative control (miR-21 mimic NC) group, miR-21 mimic group, miR-21 inhibitor NC group, miR-21 inhibitor group, siRNA NC group, PDCD4 siRNA group, c-Jun siRNA group. Cotransfection group: miR-21 inhibitor NC + siRNA NC group, miR-21 inhibitor + siRNA NC group, miR-21 inhibitor + siRNA NC group inhibitor + PDCD4 siRNA group. CCK-8, Transwell migration and invasion were used to detect the effect of miR-21/PDCD4/AP-1 feedback loop on the proliferation, migration and invasion of EC9706 cells. Results From the 2nd day after transfection, the proliferation ability of EC9706 cells in miR-21 mimic group was significantly higher than that in blank control group, miR-21 mimic NC group, miR-21 inhibitor NC group and miR-21 inhibitor group (P < 0.05). The number of cells passing through the chamber in miR-21 mimic group was more than that in blank control group and miR-21 mimic NC group (P < 0.05); the number of cells passing through the chamber in miR-21 inhibitor group was less than that in miR-21 inhibitor NC group (P < 0.05). The number of invasive cells penetrating Matrigel in miR-21 mimic group was more than that in blank control group and miR-21 mimic NC group (P < 0.05); the number of invasive cells penetrating Matrigel in miR-21 inhibitor group was less than that in miR-21 inhibitor NC group (P < 0.05). From the 2nd day after transfection, the proliferation ability of EC9706 cells in PDCD4 siRNA group was higher than that in blank control group and siRNA NC group (P < 0.05). The number of cells passing through the chamber and invading cells passing through Matrigel in PDCD4 siRNA group was higher than that in blank control group and siRNA NC group (P < 0.05). The proliferation ability of EC9706 cells in miR-21 inhibitor + siRNA NC group was lower than that in miR-21 inhibitor + PDCD4 siRNA group and miR-21 inhibitor NC + siRNA NC group (P < 0.05). The number of cells passing through the chamber and the number of cells penetrating Matrigel in miR-21 inhibitor + PDCD4 siRNA group was more than that in miR-21 inhibitor + siRNA NC group (P < 0.05). The proliferation ability of EC9706 cells in c-Jun siRNA group was lower than that in blank control group and siRNA NC group (P < 0.05). The number of cells passing through the chamber and the number of invasive cells passing through Matrigel in c-Jun siRNA group was less than that in siRNA NC group (P < 0.05). Conclusion miR-21/PDCD4/AP-1 feedback loop can regulate the growth and invasion of esophageal cancer cells, which provides a new therapeutic strategy for preventing invasion and metastasis.
[Key words] miR-21; PDCD4; AP-1; Esophageal cancer
食管癌是高发恶性肿瘤之一,其5年生存率低,为10%~30%。由于食管癌预后差,亟需寻找新的有效的治疗方案。近年来,miRNA和食管癌关系的研究日益深入[1-2]。miR-21作为一种癌基因,参与肿瘤细胞的增殖、转化和转移等多种生物学行为。其中,miR-21可通过PI3K/Akt/mTOR、TGF-β、PI3K/Akt/FOXO1等多条途径直接调控PDCD4[3-4]。PDCD4也可通过调节AP-1和p21、抑制p53活性等方式抑制肿瘤细胞生长。而miR-21存在转录因子AP-1的靶点,活化的AP-1可通过启动子区结合而激活miR-21转录。因此,miR-21/PDCD4/AP-1形成了反馈环路[5]。目前,miR-21/PDCD4/AP-1在食管癌细胞恶性生物表型中的作用和作用机制均不明确。因此,本研究应用CCK-8、Transwell迁移及侵袭实验初步探讨miR-21/PDCD4/AP-1反馈环路对食管癌EC9706细胞增殖、侵袭和迁移的影响,为食管鳞状细胞癌发病机制的研究和诊疗提供新的思路。
1 材料与方法
1.1 药物与细胞
食管癌EC9706细胞为联勤保障部队第九○○医院实验室保存。
1.2 仪器与试剂
超净工作台OptiClean1300(Healforce公司);TDZ4A-WS低速离心机(湖南湘仪公司);IX73倒置显微镜(Olympus);160iCO2培养箱(Thermo公司);BCD-160TMPQ冰箱(海尔公司);DW-86W108-80冰箱(捷盛);CX31-32RFL荧光显微镜(Olympus公司);DMEM细胞培养基、胰酶(Invitrogen公司);胎牛血清(杭州四季青公司);转染试剂Lipofectin 2000购自Invitrogen公司;miR-21 inhibitor、miR-21 mimic、miR-21 mimic negative control(NC)、PDCD4 siRNA、c-Jun siRNA、siRNA NC购自上海吉玛制药技术有限公司。
1.3 细胞培养
食管癌细胞EC9706于含10%胎牛血清、100 U/mL青霉素、100 mg/L链霉素的RPMI1640(Gibco)培养液中,37℃、5%CO2培养箱贴壁培养。每隔2 d用0.3%胰酶进行消化和传代,待细胞生长至对数生长期时用于后续实验。
1.4 细胞转染
细胞转染前1 d在6孔培养板中接种适当数量的细胞,按Lipofectin 2000说明书进行操作。分组方法为单转染组:空白对照组、miR-21 mimic阴性对照(miR-21 mimic NC)组、miR-21 mimic组、miR-21 inhibitor NC组、miR-21 inhibitor组、siRNA NC组、PDCD4 siRNA组、c-Jun siRNA组;共转染组:miR-21 inhibitor NC+siRNA NC组、miR-21 inhibitor+siRNA NC组、miR-21 inhibitor+PDCD4 siRNA组。将培养板置于37℃、5%CO2培养箱中孵育,6~8 h后换液,72 h后检测细胞增殖、迁移和侵袭能力改变。
1.5 CCK-8檢测细胞增殖活性
将对数生长期经转染处理的各组食管癌EC9706细胞接种于96孔板,每孔5000个细胞/100 μL,并将培养板放置于培养箱培养,分别于转染1、2、3、4、5 d后,按CCK-8说明书操作方法加入100 μL含10% CCK-8的培养基,并设置不含细胞的培养基对照组,每组设置3个复孔。在培养箱内孵育培养板1~4 h。用酶标仪测定吸光度(450 nm处)。
1.6 Transwell法检测细胞迁移能力
将Transwell小室置入24孔板中,聚碳酸脂膜上层为无血清培养液,24孔板下室加入完全培养基作为趋化因子。照前述转染方法将EC9706细胞行单转染和共同转染,然后制备细胞悬液。每组3个样本。37℃、5%CO2的条件下培养48 h。取出上室,轻轻擦去上室内面,取膜后使用95%乙醇固定,然后依次清水浸洗、伊红染色和清水浸洗,倒置晾干,用刀片将PC膜取下,封片,最后高倍倒置显微镜下每孔随机选取3个视野拍照,并对穿过小室的细胞计数。
1.7 Transwell法检测细胞侵袭能力
照前述转染方法将EC9706细胞行单转染和共同转染,收集各组细胞,PBS洗涤2次,用无血清培养液重悬;将密度为1×106/mL的各组细胞接种于上室,下室加入0.5 mL完全培养基。以PBS作为对照,每组设3个复孔,培养箱培养24 h后,用湿棉签擦除上室内侧基质胶和未穿过膜的细胞,室温下多聚甲醇固定,结晶紫染色,清水浸洗后晾干,封片。高倍镜下观察穿过小室的细胞数量并拍照、计数。
1.8 统计学方法
采用SPSS 19.0软件进行统计分析。计量资料用均数±标准差(x±s)表示,多组间比较采用单因素方差分析,进一步两两比较采用SNK-q检验。以P < 0.05为差异有统计学意义。
2 结果
2.1 miR-21促进食管癌EC9706细胞的增殖、迁移和侵袭能力
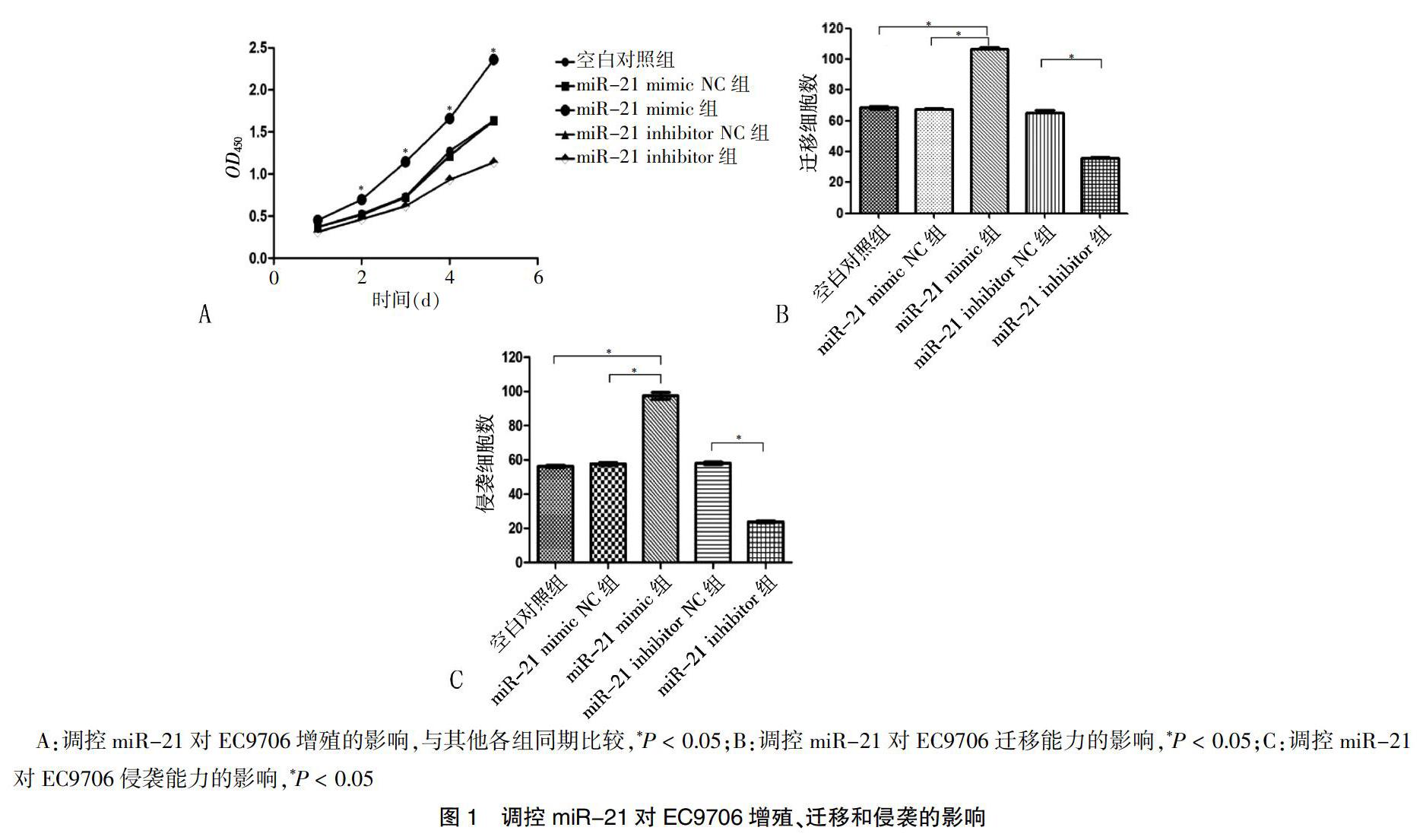
CCK-8检测结果显示,转染后2 d起,miR-21 mimic组EC9706细胞的增殖能力明显高于空白对照组、miR-21 mimic NC组、miR-21 inhibitor NC组和miR-21 inhibitor组,差异有统计学意义(P < 0.05)(图1A)。Transwell迁移实验结果显示,miR-21 mimic组穿过小室的细胞计数多于空白对照组和miR-21 mimic NC组,差异有统计学意义(P < 0.05);miR-21 inhibitor组穿过小室的细胞计数少于miR-21 inhibitor NC组,差异有统计学意义(P < 0.05)(图1B)。Transwell侵袭实验结果显示,miR-21 mimic组穿透Matrigel胶的侵袭细胞数多于空白对照组和miR-21 mimic NC组,差异有统计学意义(P < 0.05);miR-21 inhibitor组穿透Matrigel胶的侵袭细胞数少于miR-21 inhibitor NC组,差异有统计学意义(P < 0.05)(图1C)。Transwell迁移和侵袭实验证实,高表达miR-21可以促进食管癌EC9706细胞的增殖、迁移和侵袭能力;反之,低表达miR-21抑制其恶性生物表型能力。
2.2 PDCD4抑制食管癌EC9706细胞的增殖、迁移和侵袭能力
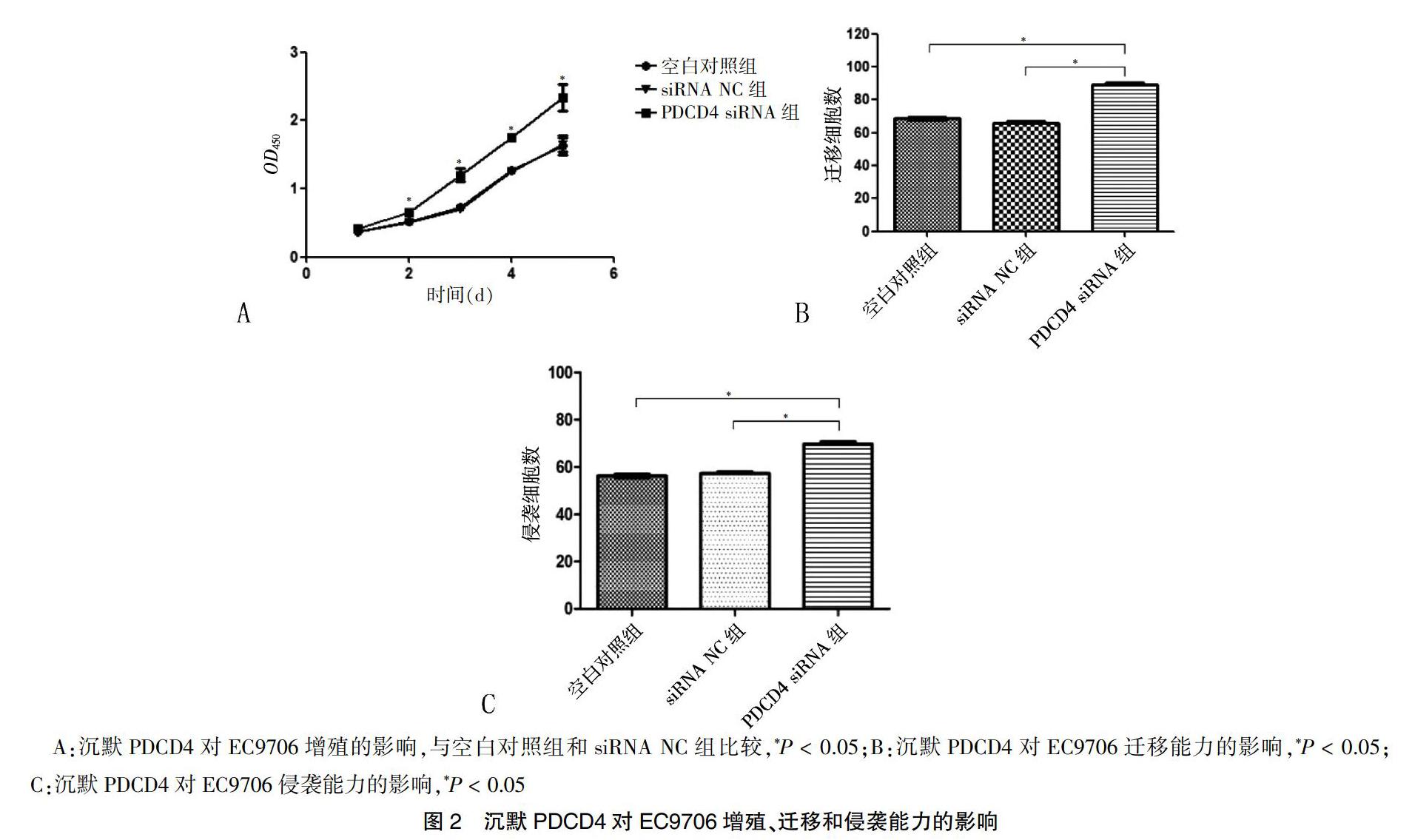
CCK-8检测结果显示,转染后2 d起,PDCD4 siRNA组EC9706细胞的增殖能力高于空白对照组和siRNA NC组,差异有统计学意义(P < 0.05)(图2A)。Transwell迁移实验结果显示,PDCD4 siRNA组穿过小室的细胞计数多于空白对照组和siRNA NC组,差异有统计学意义(P < 0.05)(图2B)。Transwell侵袭实验结果显示,PDCD4 siRNA组穿透Matrigel胶的侵袭细胞数多于空白对照组和siRNA NC组,差异有统计学意义(P < 0.05)(图2C)。Transwell迁移和侵袭实验表明下调PDCD4可促进食管癌EC9706细胞的增殖、迁移和侵袭能力。
2.3 PDCD4参与miR-21调节食管癌EC9706细胞增殖、迁移和侵袭的作用
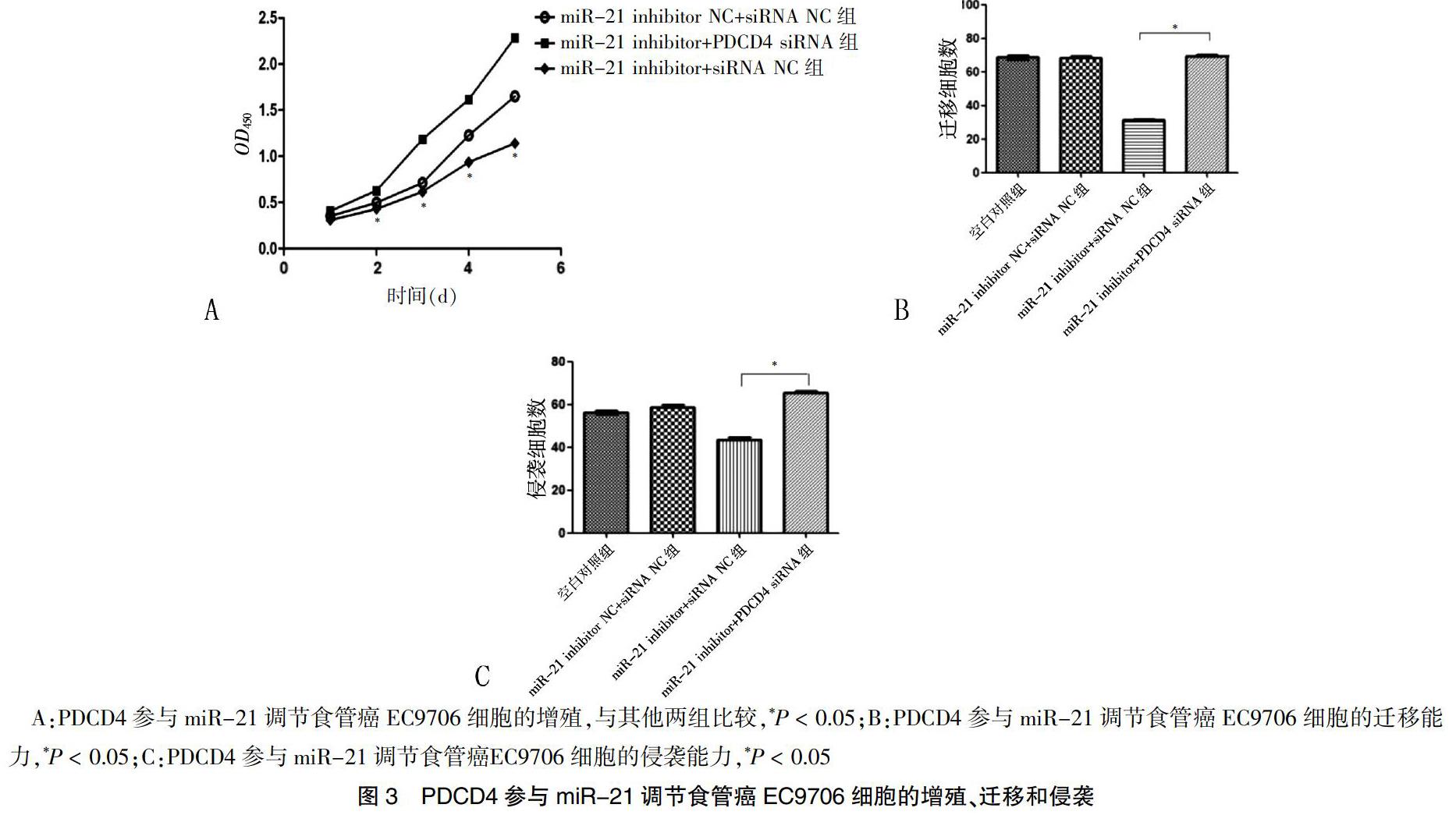
转染后2 d起,miR-21 inhibitor+siRNA NC组EC9706细胞的增殖能力低于miR-21 inhibitor+PDCD4 siRNA组和miR-21 inhibitor NC+siRNA NC组,差异有统计学意义(P < 0.05)(图3A)。Transwell迁移实验结果显示,miR-21 inhibitor+PDCD4 siRNA组穿过小室的细胞计数多于miR-21 inhibitor+siRNA NC组,差异有统计学意义(P < 0.05)(图3B)。Transwell侵袭实验结果显示,miR-21 inhibitor+PDCD4 siRNA组穿透Matrigel胶的侵袭细胞数多于miR-21 inhibitor+siRNA NC组,差异有统计学意义(P < 0.05)(图3C)。Transwell迁移和侵袭实验证实,虽然miR-21 inhibitor抑制了食管癌细胞的增殖、迁移、侵袭能力,但在下调miR-21的同时下调PDCD4,可以逆转miR-21 inhibitor引起的食管癌EC9706细胞增殖、迁移、侵袭能力的下降,提示miR-21参与调控食管癌细胞恶性表型可能是通过调控PDCD4的表达实现的。
2.4 AP-1促进食管癌EC9706细胞的增殖、迁移和侵袭能力
转染后4 d起,c-Jun siRNA组EC9706细胞的增殖能力低于空白对照组和siRNA NC组,差异有统计学意义(P < 0.05)(图4A)。Transwell迁移侵袭实验显示,c-Jun siRNA组穿过小室的细胞计数、穿透Matrigel胶的侵袭细胞数均少于siRNA NC组,差异有统计学意义(P < 0.05)(图4B~C)。结果提示,沉默c-Jun可以抑制食管癌EC9706细胞的生物恶性表型。
3 讨论
miR-21是一种常见的致癌基因,在包括肺癌、宫颈癌、肾癌、肝癌等多种恶性肿瘤中均呈现异常表达。miR-21的异常表达参与肿瘤细胞的增殖、凋亡、侵袭、转移等多种生物学行为,且与肿瘤患者的预后密切相关,对判断肿瘤的恶性程度及患者预后具有一定的临床参考价值[6-8]。miR-21可作用于多个与凋亡相关的靶基因,如PDCD4、Sprouty1、Sprouty2、PTEN、FasL、bcl-2、TIMP3、REC等[9-11]。其中,PDCD4是一种肿瘤抑制基因,PDCD4通过多个信号通路参与调节肿瘤细胞的生物学行为[12]。
目前,關于miR-21/PDCD4与食管癌生物学行为、机制及临床相关性研究也有一些报道。miR-21在食管癌中高表达,可以负向调控PDCD4基因[13]。研究显示,miR-21是通过与PDCD4基因3′UTR序列结合而下调蛋白翻译[14]。张建东等[15]和Zhang等[16]研究表明,在Ⅱ期食管鳞癌术后患者中,肿瘤浸润深度、淋巴结转移、病变长度与癌组织中miR-21的表达呈正相关。COX回归模型发现,Ⅱ期食管鳞癌患者miR-21和PDCD4表达量均为影响患者PFS的因素,高表达miR-21提示预后较差,而高表达PDCD4提示预后较好。
PDCD4可通过一系列过程作用于AP-1,抑制其转录活性,阻断其介导的信号转导通路,抑制肿瘤增殖。而miR-21又存在着AP-1的直接靶点,是AP-1的靶目标,调节器活化的AP-1可以上调miR-21转录。因此miR-21与AP-1构成一个自我调节的环路,对肿瘤的增殖、侵袭进一步产生影响。而miR-21/PDCD4/AP-1反馈环路对食管癌细胞的报道罕见。虞勇等[17]报道,在柯萨奇病毒B3感染大鼠心脏微血管内皮细胞后,miR-21表达上调,miR-21与PDCD4、转录因子AP-1之间分别具有负向调控作用。Zhu等[18]报道,miR-21可通过miR-21-PDCD4-AP-1反馈环路调控其在肝癌细胞的生物学功能。Chang等[19]研究表明,牙龈卟啉单胞菌可能通过miR-21/PDCD4/AP-1负反馈信号通路调节细胞周期蛋白D1的表达来促进口腔鳞状细胞癌的增殖。目前miR-21/PDCD4/AP-1反馈环路对食管癌细胞的作用,国内外尚鲜见报道。
本研究分别应用CCK-8、Transwell迁移和Transwell侵袭检测miR-21/PDCD4/AP-1反馈环路对食管癌EC9706细胞增殖、迁移和侵袭能力的影响。与空白对照组和NC组比较,外源性高表达miR-21可以促进食管癌EC9706细胞的增殖、迁移和侵袭能力;相反,下调miR-21表达则可抑制食管癌恶性表型。通过沉默PDCD4使PDCD4低表达,可以促进食管癌细胞的增殖、迁移和侵袭能力。迁移实验中,与NC组比较,miR-21 inhibitor+siRNA细胞迁移至膜背面的细胞数明显减少,但在下调miR-21的同时沉默PDCD4,可以逆转miR-21 inhibitor引起的食管癌EC9706迁移的下降。同样在侵袭实验中,也得到类似的结果。说明通过沉默PDCD4可以逆转外源性miR-21下调引起的食管癌EC9706细胞增殖、迁移和侵袭能力的下降,提示miR-21调控食管癌细胞恶性表型可能是通过调控PDCD4的表达来实现。外源性低表达c-Jun可以抑制食管癌EC9706细胞的迁移、侵袭能力。结果表明,miR-21在食管癌细胞中发挥“抑癌基因样”作用,其可能通过miR-21/PDCD4/AP-1调控生物学作用。但其具体作用机制有待进一步研究。有研究表明,miR-21对PDCD4有直接调控作用,PDCD4通过干扰c-Jun和c-Fos的反式激活可以抑制AP-1的活性[20]。而PDCD4对c-Jun的抑制是通过抑制MAP4K1经MAP2K1→TAK1→MKK4→JNK信号通路激活JNK,发挥作用。miR-21/PDCD4/AP-1调控食管癌细胞增殖、迁移和侵袭的机制需进一步探索。
综上所述,在食管癌中,miR-21、PDCD4与AP-1呈负相关,miR-21可能通过miR-21/PDCD4/AP-1反馈环路调控食管癌细胞的增殖、迁移和侵袭。本研究为进一步了解食管癌发生、发展的分子机制和为阻止食管癌生长、侵袭和转移提供新的治疗靶点奠定了一定的基础。
[参考文献]
[1] Liu M,Yu J,Wang D,et al. Epigenetically upregulated microRNA-602 is involved in a negative feedback loop with FOXK2 in esophageal squamous cell carcinoma [J]. Mol Ther,2019,27(10):1796-1809.
[2] Kiuchi J,Komatsu S,Imamura T,et al. Low levels of tumour suppressor miR-655 in plasma contribute to lymphatic progression and poor outcomes in oesophageal squamous cell carcinoma [J]. Mol Cancer,2019,18(1):2.
[3] Junker F,Chabloz A,Koch U,et al. Dicer1 imparts essential survival cues in Notch-driven T-ALL via miR-21-mediated tumor suppressor Pdcd4 repression [J]. Blood,2015,126(8):993-1004.
[4] Wang P,Guan Q,Zhou D,et al. miR-21 inhibitors modulate biological functions of gastric cancer cells via PTEN/PI3K/mTOR pathway [J]. DNA Cell Biol,2018,37(1):38-45.
[5] Zhang Z,Zha Y,Hu W,et al. The autoregulatory feedback loop of microRNA-21/programmed cell death protein 4/activation protein-1 (MiR-21/PDCD4/AP-1) as a driving force for hepatic fibrosis development [J]. J Biol Chem,2013,288(52):37082-37093.
[6] 杨鲸蓉,吴波.microRNA-21在食管癌中的研究进展[J].中国医药导报,2017,14(16):40-43.
[7] Irimie-Aghiorghiesei AI,Pop-Bica C,Pintea S,et al. Prognostic value of miR-21:an updated meta-analysis in head and neck squamous cell carcinoma (HNSCC) [J]. J Clin Med,2019,8(12):E2041.
[8] Zhang N,Hu Z,Qiang Y,et al. Circulating miR-130b- and miR-21-based diagnostic markers and therapeutic targets for hepatocellular carcinoma [J]. Mol Genet Genomic Med,2019,7(12):e1012.
[9] Hu Y,Rao SS,Wang ZX,et al. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function [J]. Theranostics,2018,8(1):169-184.
[10] Liu Y,Ren L,Liu W,et al. MiR-21 regulates the apoptosis of keloid fibroblasts by caspase-8 and the mitochondria-mediated apoptotic signaling pathway via targeting FasL [J]. Biochem Cell Biol,2018,96(5):548-555.
[11] Wang F,Gao X,Zhang R,et al. LncRNA TUG1 ameliorates diabetic nephropathy by inhibiting miR-21 to promote TIMP3-expression [J]. Int J Clin Exp Pathol,2019, 12(3):717-729.
[12] 姜艷,杨大群,王聪洋,等.抑癌基因PDCD4与肿瘤关系的研究进展[J].现代肿瘤医学,2015,23(22):3363-3366.
[13] Liu T,Liu Q,Zheng S,et al. MicroRNA-21 promotes cell growth and migration by targeting programmed cell death 4 gene in Kazakh′s esophageal squamous cell carcinoma [J]. Dis Markers,2014,2014:232837.
[14] Fassan M,Pizzi M,Battaglia G,et al. Programmed cell death 4 (PDCD4) expression during multistep Barrett′s carcinogenesis [J]. J Clin Pathol,2010,63(8):692-696.
[15] 張建东,贺利民,马磊,等.miR-21、PDCD4表达对Ⅱ期食管鳞癌术后患者生存期的影响[J].肿瘤学杂志,2017, 183(12):5-12.
[16] Zhang J,Ma L,Shi D,et al. Prognostic significance of miR-21 and PDCD4 in patients with stage Ⅱ esophageal carcinoma after surgical resection [J]. J Cell Biochem,2018,119(6):4783-4791.
[17] 虞勇,虞莹,刘桂剑,等.miR21对心脏微血管内皮细胞PDCD4/AP1通路的调节作用[J].中国分子心脏病学杂志,2015,15(6):1534-1538.
[18] Zhu Q,Wang Z,Hu Y,et al. miR-21 promotes migration and invasion by the miR-21-PDCD4-AP-1 feedback loop in human hepatocellular carcinoma [J]. Oncol Rep,2012,27(5):11660-11668.
[19] Chang CR,Wang HY,Liu JC,et al. Porphyromonas gingivalis infection promoted the proliferation of oral squamous cell carcinoma cells through the miR-21/PDCD4/AP-1 negative signaling pathway [J]. ACS Infect Dis,2019,5(8):1336-1347.
[20] Talotta F,Cimmino A,Matarazzo MR,et al. An autoregulatory loop mediated by miR-21 and PDCD4 controls the AP-1 activity in RAS transformation [J]. Oncogene,2009,28(1):73-84.
(收稿日期:2020-02-28)
