L-半胱氨酸处理对采后青脆李果实苯丙烷代谢的影响
2020-08-12陈力维邓丽莉曾凯芳
陈力维,令 阳,邓丽莉,,曾凯芳,
L-半胱氨酸处理对采后青脆李果实苯丙烷代谢的影响
陈力维1,令 阳1,邓丽莉1,2,曾凯芳1,2※
(1. 西南大学食品科学学院,重庆 400715;2.西南大学食品贮藏与物流研究中心,重庆 400715)
L-半胱氨酸(L-cysteine)作为生物体中的常见氨基酸,已有研究发现其能有效延缓青脆李果实的衰老和品质的下降。然而,L-半胱氨酸处理对李果实中苯丙烷代谢途径合成酚类物质的影响尚不清楚。因此,该研究以青脆李果实为试材,采用1 g/L L-半胱氨酸浸泡处理后于(20±1)℃贮藏,研究贮藏期间苯丙烷代谢途径中关键酶活性以及总酚、总黄酮等相关代谢产物的变化规律,同时测定果实中糖酸含量和抗氧化活性的变化。结果表明,李果实在贮藏过程中可溶性固形物(Total soluble solids,TSS)含量和可滴定酸(Titratable acid,TA)呈逐渐降低的趋势,L-半胱氨酸处理显著延缓了李果实TSS和TA的下降(<0.05)。在贮藏期间,苯丙烷代谢途径中关键酶活性均呈逐渐上升的趋势,与对照组相比,处理组中苯丙氨酸解氨酶、4-香豆酸辅酶A连接酶活性更高。果实中总酚、总黄酮含量在贮藏期间先降低后上升,在贮藏前三天,处理组中总酚、总黄酮含量显著高于对照组(<0.05)。对酚类物质单体含量的测定发现,处理后的果实中绿原酸、咖啡酸、丁香酸、芦丁等酚类物质单体含量显著高于对照组(<0.05)。抗氧化活性与总酚、总黄酮的变化趋势一致,经L-半胱氨酸处理后的果实保持了较高的抗氧化活性。相关性分析结果表明,果实中苯丙氨酸解氨酶、肉桂酸-4-羟基化酶和4-香豆酸辅酶A连接酶与果实酚类物质含量及抗氧化活性显著相关(<0.05)。总体来说,1 g/L L-半胱氨酸浸泡处理能够延缓青脆李果实贮藏品质下降,同时能够激活苯丙烷代谢途径关键酶,促进果实中酚类物质的积累。
农产品;贮藏;L-半胱氨酸;李果实;苯丙烷代谢;酚类物质;抗氧化活性
0 引 言
青脆李果实营养丰富,因含有糖、酸、蛋白质、脂肪、维生素、花青素、酚类化合物、类黄酮、矿物质等多种营养元素[1-2],具有较高的抗氧化活性,且能够促进胃肠道消化,深受消费者喜爱。但李果实采收期高温多雨,且果实皮薄肉厚,容易腐烂变质,采收后旺盛的呼吸作用会促进果实衰老[3]。依据现有文献报道,UV-B照射[4]、热空气[5]、水杨酸[6]、-氨基丁酸[7]等多种采后处理方式可通过诱导果实次生代谢途径改变,促进抗逆物质的合成,从而延缓果实采后衰老,维持果实品质。近年来,天然氨基酸作为安全的外源处理物在农业生产上具有广阔的应用前景。外源氨基酸处理能够调节果蔬的生长发育,改善果蔬品质[8],提高果实的抗逆能力[9]。
L-半胱氨酸是具有抗褐变和抗氧化的巯基化合物[10],在植物初生代谢和次生代谢中都具有重要作用[11]。目前,L-半胱氨酸广泛应用于食品工业以控制鲜切果蔬的褐变过程[12]。在酚类物质氧化过程中,半胱氨酸通过竞争酶促褐变结合位点减缓褐变过程,将其应用于荔枝果实采后保鲜可以有效减缓果皮褐变[12-13]。另外,有研究发现L-半胱氨酸能诱导果实提高对环境胁迫的耐受力,将其用于龙眼、黄瓜等果蔬中可以提高果实的抗氧化活性,延缓果实的衰老[14-15]。苯丙烷代谢途径是果蔬酚类物质合成以及产生诱导抗性的关键途径,其产物具有抗氧化活性且具有抗菌活性,如对-香豆酸[16]、咖啡酸[17]、绿原酸[18]等。同时,苯丙烷代谢途径相关产物能够提高果实的食用价值和经济价值,对果蔬采后保鲜具有重要意义[19]。已有文献报道,L-半胱氨酸处理能在一定程度上延缓青脆李果实采后衰老和品质下降[20],其中1 g/L L-半胱氨酸处理的效果最好。但L-半胱氨酸处理对青脆李果实苯丙烷代谢途径的影响尚不清楚。因此,本文探讨了1 g/L L-半胱氨酸处理对采后青脆李果实苯丙烷代谢途径的影响,以期为采后青脆李果实贮藏保鲜提供理论依据。
1 材料与方法
1.1 材料与试剂
供试材料“青脆李”(. ‘Qingcui’),品种为巫山脆李,产自重庆市梁平区果园,于2018年9月1日采收,果实成熟度为八成熟,采摘后当天运回实验室。挑选无病害、无机械伤且大小均匀,成熟度一致的果实。摊平散去田间热后,室温条件下(20 ℃,相对湿度为80%~90%)平铺于试验台待用。
纯度为99%的L-半胱氨酸,美国Adamas-Bata公司;-巯基乙醇、三羟甲基氨基甲烷(Tris)、荧光素钠盐、水溶性维生素E(Trolox)、2,2′-偶氮二异丁基脒盐酸盐(2,2′-azobis[2-methylpropionamidine] dihydrochloride,ABAP)(均为分析纯),Sigma-Aldrich西格玛奥德里奇(上海)贸易有限公司;甲醇、原儿茶酸、绿原酸、丁香酸、咖啡酸、对香豆酸、芦丁(均为色谱纯),成都普瑞法科技有限公司。
1.2 仪器与设备
AvantiTM J-30I高速冷冻离心机,美国Beckman公司;UV1000紫外分光光度计,北京莱伯泰科科技有限公司;LC-20A高效液相色谱仪(配有光电二极管阵列紫外可见光检测器和LabSolutions 工作站),日本岛津公司;SYNERGYH1MG全自动酶标仪,美国Bio Tek仪器有限公司。
1.3 研究方法
1.3.1 青脆李果实处理及取样
参考令阳等[20]的方法。果实用2%(体积分数)次氯酸钠浸泡消毒1 min后,用清水冲洗,于室温条件下自然晾干。试验分为2个处理组(每组包含3次重复),清水(对照组)、1 g/L L-半胱氨酸(处理组)浸泡10 min。待完全晾干后,所有果实用聚乙烯薄膜袋(170 mm× 140 mm)单果包装,贮藏在(20±1)℃、相对湿度为80%~90%的环境中。
定期取样,样品用液氮快速冷冻后保存在-40℃冰箱,用于后续指标测定,每次测定重复3次。
1.3.2 可溶性固形物含量和可滴定酸的测定
可溶性固形物:参考曹建康的方法[21],使用数显手持式折光仪测定样品中可溶性固形物的含量。结果用质量分数(%)表示。
可滴定酸:参考曹建康的方法[21],酸碱滴定法,加入酚酞作为指示剂,用已标定的氢氧化钠溶液进行滴定。用蒸馏水代替样品,作为空白对照。可滴定酸用质量分数(%)表示。
1.3.3 酚类物质代谢相关酶活性测定
苯丙氨酸解氨酶(Phenylalanine Ammonia Lyase,PAL):根据Yao和Tian的方法[22],反应液于37 ℃水浴保温1 h后立即加入0.1 mL 6 mol/L HCl终止反应。在波长290 nm下分别测定反应管和对照管的吸光度值(OD1和OD0),重复3次。以每小时吸光度值变化0.01为一个酶活力单位(U)。
肉桂酸-4-羟基化酶(Cinnamate-4-Hydroxylase,C4H):参考Lamb和Rubery的方法[23],反应液于37 ℃水浴保温1 h后立即加入0.2 mL 6 mol/L HCl终止反应。在波长340 nm下分别测定反应管和对照管的吸光度值(OD1和OD0),重复3次。以每小时吸光度值变化0.01为一个酶活力单位(U)。
4-香豆酸辅酶A连接酶(4-Coumaric Coenzyme A Ligase,4CL):参考Li等的方法[24],反应液于25℃水浴保温10 min后,在波长340 nm下分别测定反应管和对照管的吸光度值(OD1和OD0),重复3次。以每分钟吸光度值变化0.001为一个酶活力单位(U)。
1.3.4 总酚含量测定
总酚含量测定参考Chu等的方法[25],采用福林-酚法,以没食子酸为标准品,于760 nm波长处测其吸光度值,结果以每克样品中所含的没食子酸当量表示。
1.3.5 总黄酮含量测定
总黄酮含量测定参照吴瑛等的方法[26],采用硝酸铝-亚硝酸钠比色法测定总黄酮含量,以芦丁为标准品,于510 nm波长处测定吸光度值,结果以每克样品中所含的芦丁当量表示。
1.3.6 酚类物质的定性定量分析
酚类物质的定性定量分析采用高效液相色谱法[27]。高效液相色谱(High-Performance Liquid Chromatography, HPLC)条件:流动相A(1% 甲酸),流动相B(乙腈),洗脱梯度:0~5 min 3%~9% B;5~15 min,9%~16% B;15~45 min 16%~50% B;45~55 min 50% B;55~60 min 50%~3% B;60~62 min 3% B;流速1 mL/min,柱温25 ℃,进样量20L。检测器:光电二极管阵列紫外可见光检测器。色谱柱:SHIMADZU Shim-pack GIST C18(4.6 mm×250 mm,5m)。根据保留时间和吸收光谱与标准品对照定性,外标法定量。基于鲜质量,酚类物质单体含量表示为g/g。
1.3.7 氧化自由基吸收能力(Oxygen Radical Absorbance Capacity,ORAC)测定
参考Wolfe等的方法[28],根据测定值分别按照以下公式计算荧光衰减曲线下的面积(Area Under Fluorescence Decay Curve,AUC)和ORAC值:
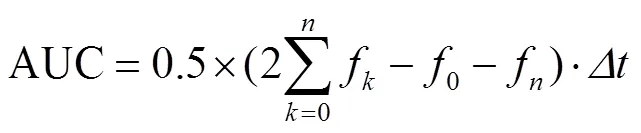

式中f为第个测定点时的相对荧光强度;0为初始测定时的相对荧光强度;f为第个测定点时的相对荧光强度;Δ为相邻两个测定点之间的时间间隔,min;Trolox为标准品水溶性维生素E的浓度,mol/L;样品为样品中酚类物质浓度,mol/L。ORAC值以每克物质相当于微摩尔Trolox的量表示(mol/g)。
1.4 数据分析
以上指标均取3个平行样品,重复测定3次。采用Excel 2016软件统计分析数据,运用OriginPro 9.0.0(Northampton, MA 01060 USA)软件绘制图表,应用SPSS 23.0 (SPSS Inc., Chicago, IL, USA)软件对数据进行差异显著性分析和相关性分析。
2 结果与分析
2.1 L-半胱氨酸处理对青脆李果实可溶性固形物含量和可滴定酸的影响
如图1所示,随着贮藏时间的延长,果实中可溶性固形物含量呈先上升后下降的趋势,可滴定酸呈下降趋势。贮藏第6天,对照组和L-半胱氨酸处理组的果实中TSS含量均达到峰值,分别为12.87%和13.23%。在贮藏期内,L-半胱氨酸处理后,果实中的TSS含量和TA均显著高于对照组(<0.05)。
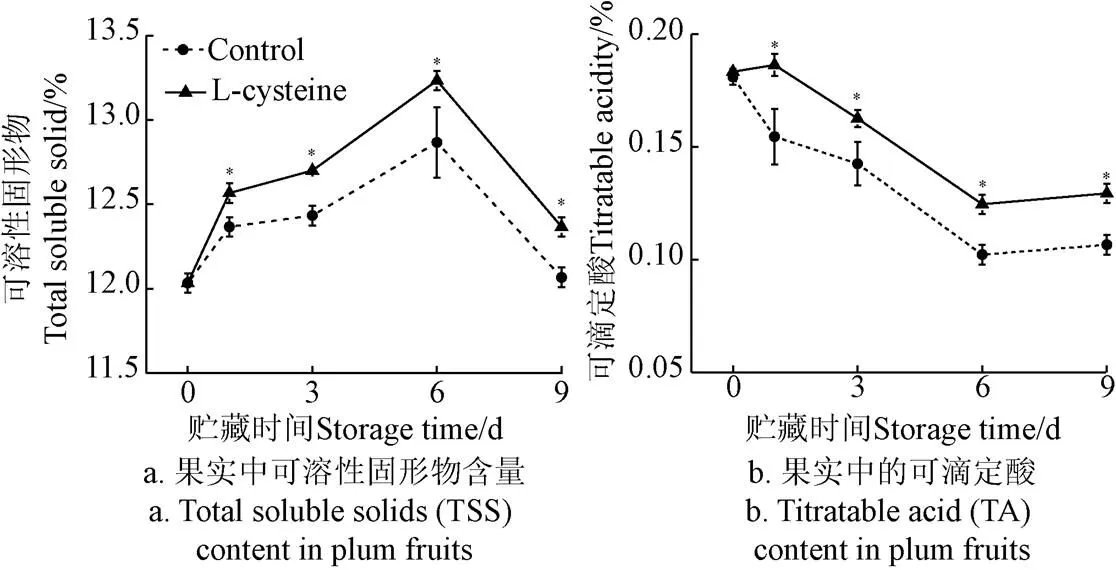
注:数据为3次试验的平均值。“*”代表同一贮藏时间的差异显著(P<0.05),下同。
2.2 L-半胱氨酸处理对青脆李果实苯丙烷代谢相关酶活性的影响
在植物体内,PAL、4CL、C4H是植物苯丙烷类代谢途径中的关键酶类,其活力大小与酚类、黄酮类等物质的合成密切相关。如图2所示,青脆李果实中PAL、4CL酶活性随贮藏时间的延长呈上升趋势,C4H酶活性呈先下降后上升的趋势。在果实贮藏第1天和第6天,L-半胱氨酸处理后PAL酶活性均显著高于对照组(<0.05),其中,第6天时处理后PAL酶活性达到峰值,比对照组高30.66%。在果实贮藏第3天,L-半胱氨酸处理后4CL酶活性显著高于对照组(<0.05),比对照组高11.03%。在贮藏期间,L-半胱氨酸处理后对C4H酶活性无显著影响(>0.05)。

图2 L-半胱氨酸处理对李果实苯丙烷类代谢相关酶活性的影响
2.3 L-半胱氨酸处理对青脆李果实总酚和总黄酮含量的影响
如图3所示,在贮藏过程中,青脆李果实中总酚类含量呈先下降后上升的趋势,L-半胱氨酸处理能延缓果实总酚含量的下降,在贮藏第1天和第3天显著高于对照组(<0.05),分别比对照组高4.86%和19.01%。黄酮类物质作为酚类物质的其中一类,总黄酮含量在贮藏过程中的变化趋势与总酚含量变化一致,L-半胱氨酸处理后,果实总黄酮含量在贮藏第1天和第3天分别比对照组高52.91%、46.33%。说明1 g/L L-半胱氨酸处理后在贮藏前期能够诱导果实中酚类物质的积累。
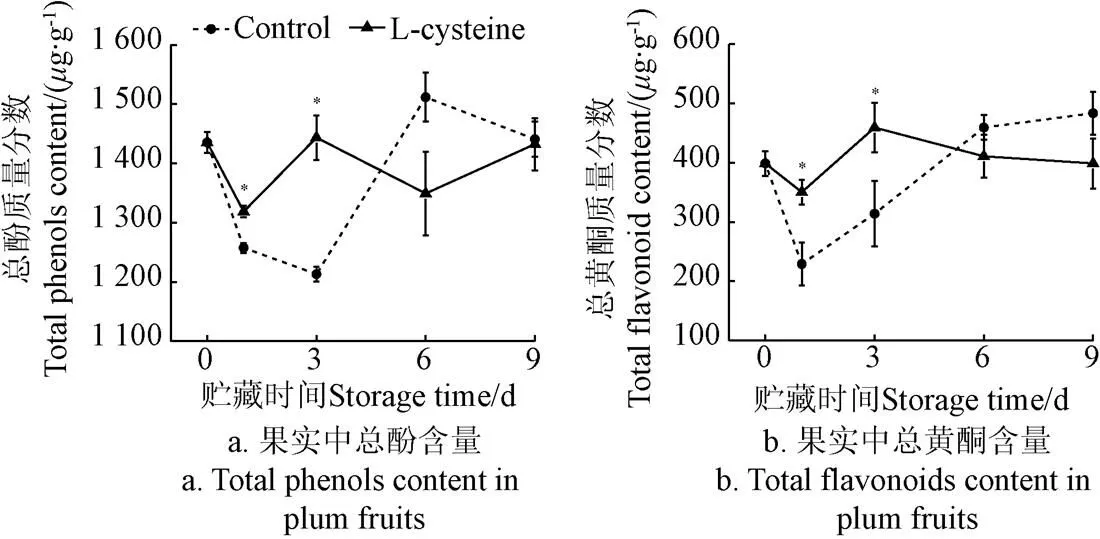
图3 L-半胱氨酸处理对李果实总酚和总黄酮含量的影响
2.4 L-半胱氨酸处理对青脆李果实多酚组分含量的影响
如图4所示,共检测到果实中6种含量较明显的酚类物质单体,包括原儿茶酸(图4a)、对香豆酸(图4b)、绿原酸(图4c)、咖啡酸(图4d)、丁香酸(图4e)和芦丁(图4f)。在贮藏过程中,原儿茶酸、绿原酸、咖啡酸、对香豆酸含量呈先下降后上升的趋势,丁香酸、芦丁含量呈上升趋势。L-半胱氨酸处理后,咖啡酸含量在贮藏6 d后显著高于对照组(<0.05),绿原酸含量在贮藏前3 d显著高于对照组(<0.05),芦丁含量在贮藏前6 d均显著高于对照组(<0.05)。
2.5 L-半胱氨酸处理对青脆李果实氧化自由基吸收能力(ORAC)的影响
氧化自由基吸收能力是衡量果实抗氧化活性的重要指标。如图5所示,在贮藏过程中,青脆李果实的氧化自由基能力变化趋势与总酚含量变化趋势基本一致,其中,在贮藏第1天和第3天,L-半胱氨酸处理后均显著高于对照组(<0.05),分别比对照组高9.85%、21.23%。
2.6 青脆李果实中酚类物质与酶、抗氧化活性的相关性分析
在青脆李果实贮藏过程中,果实中酚类物质与苯丙烷代谢途径关键酶活性、果实抗氧化活性的相关性分析结果如表1所示,其中PAL、4CL、C4H 3种酶活性均与果实中总黄酮、酚类物质单体、抗氧化活性显著正相关(<0.05),说明苯丙烷途径中酶活性的升高促进了青脆李果实酚类物质的合成和积累,从而提高了果实的抗氧化活性。因此,L-半胱氨酸处理后提高了苯丙烷代谢途径中3种酶活性,促进了酚类物质的合成,提高了果实的抗氧化活性。此外,果实的氧化自由基吸收能力与酚类物质均呈极显著相关(<0.01)。
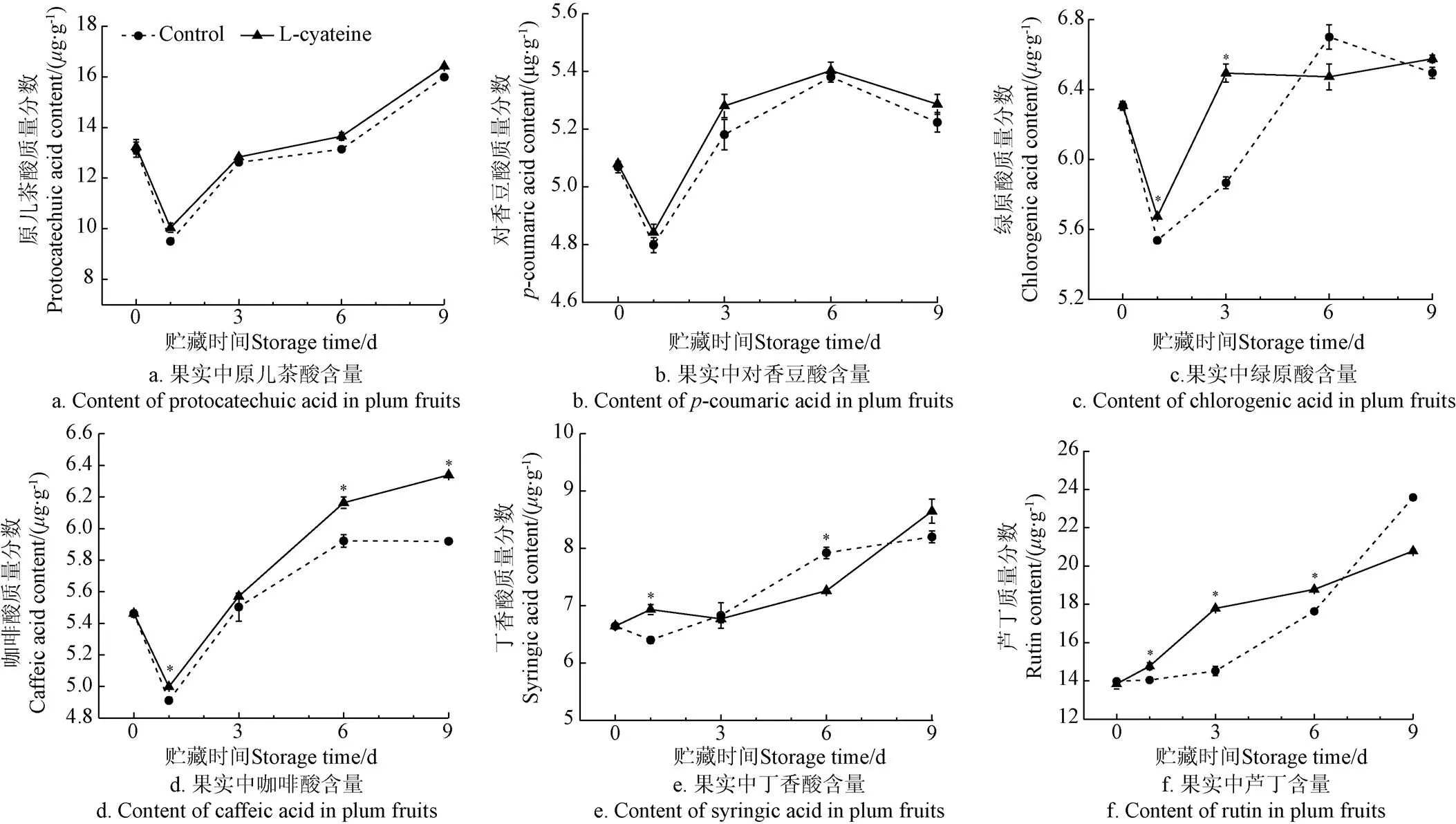
图4 L-半胱氨酸处理对李果实多酚组分含量的影响

图5 L-半胱氨酸处理对李果实氧化自由基吸收能力的影响
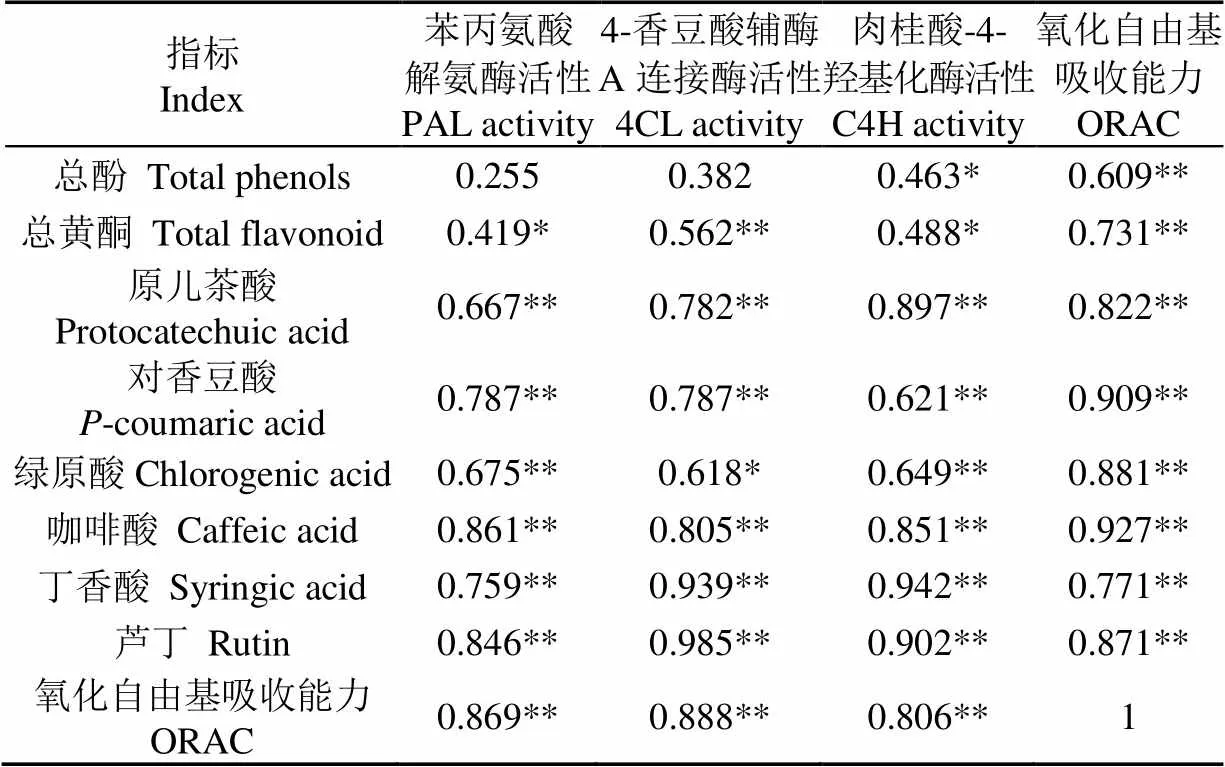
表1 青脆李果实中酚类物质与酶、抗氧化活性的相关性分析
注:“**”表示极显著(<0.01),“*”表示显著(<0.05)。
Note: ** indicate extremely significant effect at<0.01, * indicate significant effect at<0.05.
3 讨 论
青脆李成熟于高温高湿的夏季,且是一种典型的呼吸跃变型果实,在采后常温贮藏过程极易后熟软化[29],大大降低其经济价值。为缓解此类问题,已有物理、化学、生物等手段应用于李果实的采后保鲜[3]。L-半胱氨酸作为一种具有活性硫醇基且强还原性的氨基酸,广泛应用于医药业、食品工业及农业等,已有研究表明将其作为果蔬采后处理措施可有效提高果实抗氧化活性,延缓果实的衰老[14]。本研究主要探讨了L-半胱氨酸处理对青脆李果实品质的影响及其对苯丙烷代谢途径的诱导作用。
可溶性固形物、可滴定酸是评价果实品质的重要参数,本试验结果表明,1 g/L L-半胱氨酸处理维持了果实中可溶性固形物含量和可滴定酸,说明L-半胱氨酸对果实无不利影响且能够延缓果实品质下降。
果蔬在采后贮藏过程中受到环境胁迫时,会诱导苯丙烷代谢途径中关键酶活性的提高[30]。苯丙氨酸解氨酶(PAL)作为苯丙烷途径中的第一个关键酶,它是莽草酸途径与黄酮类化合物等产物之间的桥梁。本文中,经L-半胱氨酸处理后的李果实中PAL活性先增加后降低且始终高于对照组,这可能与PAL活性调节机制有关,该酶活性具有产物抑制特性,受到肉桂酸及其衍生物的反馈调节[31]。4-香豆酸辅酶A连接酶(4CL)处于苯丙烷代谢途径中合成不同类型产物的转折点,它能催化肉桂酸、香豆酸等辅酶A酯的合成。L-半胱氨酸处理后李果实中4CL活性增加。以上结果可能是因为L-半胱氨酸可作为诱导因子,激发果实中PAL、4CL活性的升高,以促进李果实中酚类物质的合成[32]。这与经温度[5]、光照[33]、精油[34]等处理对果实的影响结果相似。
此外,酚类化合物是植物体中主要的次级代谢产物,这些物质不仅在果蔬采后对病原菌的防御反应中起着关键作用[35-37],同时具有较强抗氧化活性,能够提高果实营养价值[38]。本研究中,贮藏前期L-半胱氨酸处理后显著促进了李果实中总酚、总黄酮的积累,该结果说明L-半胱氨酸能够引起李果实的应激反应,诱导果实中抗性物质的合成。这与Gao等[39]用褪黑素处理桃果实后提高PAL活性、促进果实中酚类物质积累的结论相似。此外,本研究结果中,L-半胱氨酸处理李果实后,显著促进了绿原酸、咖啡酸、芦丁的积累,这与采后处理会影响果实内部多酚组分变化的研究报道相似,例如,水杨酸处理柑桔果实后引起苯丙烷途径基因差异表达,造成绿原酸、咖啡酸、对香豆酸等物质高于对照组[6]。百里香油熏蒸通过增加黄桃果实中儿茶素、绿原酸和咖啡酸的含量,降低了采后褐腐病的发生[40]。有研究报道发现,对香豆酸、绿原酸、咖啡酸、芦丁等物质具有抗真菌活性[18,41]和抗氧化活性[36]。本研究中青脆李果实的抗氧化活性(ORAC)在贮藏过程中的变化趋势与总酚、总黄酮含量变化(图3)相同,根据相关性分析结果,苯丙烷代谢途径中关键酶活性的高低与果实中酚类物质含量和抗氧化活性显著相关,综上结果说明L-半胱氨酸能够诱导果实苯丙烷代谢途径中关键酶活性改变,促进酚类物质的积累,提高果实抗氧化活性,从而提高李果实的营养品质。
4 结 论
与清水对照处理相比,1 g/L L-半胱氨酸处理能显著减缓青脆李果实中可溶性固形物含量和可滴定酸的下降(<0.05);1 g/L L-半胱氨酸处理诱导了李果实中苯丙烷代谢途径关键酶苯丙氨酸解氨酶和4-香豆酸辅酶A连接酶活性的提高,促进了绿原酸、咖啡酸以及芦丁等物质的积累,提高了李果实在贮藏过程中的抗氧化活性。相关性分析结果也表明酶活性与酚类物质和抗氧化活性显著相关(<0.05)。由此说明,1 g/L L-半胱氨酸处理能够激活青脆李果实中苯丙烷代谢途径同时延缓果实贮藏品质的下降。
[1] Nowicka P, Wojdylo A, Samoticha J, et al. Evaluation of phytochemicals, antioxidant capacity, and antidiabetic activity of novel smoothies from selected Prunus fruits[J]. Journal of Functional Foods, 2016, 25: 397-407.
[2] Glew R H, Ayaz F A, Millson M, et al. Changes in sugars, acids and fatty acids in naturally parthenocarpic date plum persimmon (L.) fruit during maturation and ripening[J]. European Food Research and Technology, 2005, 221(1/2): 113-118.
[3] 郭丹,郝义,韩英群.李子采后特性及贮藏保鲜技术研究进展[J]. 食品工业,2015,36(9):237-240.
Guo Dan, Hao Yi, Han Yingqun. Research advancement in postharvest characteristic and storage technology of plum[J]. Food Industry, 2015, 36(9): 237-240. (in Chinese with English abstract)
[4] Liu C, Zheng H, Sheng K, et al. Effects of postharvest UV-C irradiation on phenolic acids, flavonoids, and key phenylpropanoid pathway genes in tomato fruit[J]. Scientia Horticulturae, 2018, 241: 107-114.
[5] Wei Y, Zhou D, Peng J, et al. Hot air treatment induces disease resistance through activating the phenylpropanoid metabolism in cherry tomato fruit[J]. Journal of Agricultural and Food Chemistry, 2017, 65(36): 8003-8010.
[6] Zhou Y, Ma J, Xie J, et al. Transcriptomic and biochemical analysis of highlighted induction of phenylpropanoid pathway metabolism of citrus fruit in response to salicylic acid,and oligochitosan[J]. Postharvest Biology and Technology, 2018, 142: 81-92.
[7] Aghdam M S, Kakavand F, Rabiei V, et al. Gamma-Aminobutyric acid and nitric oxide treatments preserve sensory and nutritional quality of cornelian cherry fruits during postharvest cold storage by delaying softening and enhancing phenols accumulation[J]. Scientia Horticulturae, 2019, 246: 812-817.
[8] 高晶晶,冯新新,段春慧,等. ALA提高苹果叶片光合性能与果实品质的效应[J]. 果树学报,2013,30(6):944-951.
Gao Jingjing, Feng Xinxin, Duan Chunhui, et al. Effects of 5-aminolevulinic acid (ALA) on leaf photosynthesis and fruit quality of apples[J]. Journal of Fruit Science, 2013, 30(6): 944-951. (in Chinese with English abstract)
[9] 许猛. 复合氨基酸制剂对小白菜和棉花抗逆性的影响[D].北京:中国农业科学院,2018.
Xu Meng. Effects of a Compound Amino Acid Preparation on Stress Resistance of Pak Choi and Cotton[D]. Beijing: Chinese Academy of Agricultural Sciences, 2018. (in Chinese with English abstract)
[10] Ainsworth E A, Gillespie K M. Estimation of total phenolic contents and other oxidation substrates in plant tissue using Folin-Ciocalteu reagent[J]. Nature Protocols, 2007, 2: 875-877.
[11] 王小芳,杨玲娟,董晓宁,等. 植物半胱氨酸合成及调控研究进展[J]. 植物生理学报,2011,47(1):37-48.
Wang Xiaofang, Yang Lingjuan, Dong Xiaoning, et al. Advancement in research on synthesis and regulation of cysteine in plants[J]. Plant Physiology Journal, 2011, 47(1): 37-48. (in Chinese with English abstract)
[12] Ali S, Khan A S, Malik A U. Postharvest L-cysteine application delayed pericarp browning, suppressed lipid peroxidation and maintained antioxidative activities of litchi fruit[J]. Postharvest Biology and Technology, 2016, 121: 135-142.
[13] Ali S, Khan A S, Malik A U, et al. Postharvest application of antibrowning chemicals modulates oxidative stress and delays pericarp browning of controlled atmosphere stored litchi fruit[J]. Journal of Food Biochemistry, 2019, 43(3): e12746.
[14] Li T, Wu Q, Zhou Y, et al. L-Cysteine hydrochloride delays senescence of harvested longan fruit in relation to modification of redox status[J]. Postharvest Biology and Technology, 2018, 143: 35-42.
[15] 高荣侠. 外源半胱氨酸和一氧化氮对黄瓜镉胁迫的缓解效应[D]. 泰安:山东农业大学,2013.
Gao Rongxia. The Physiological Effects of Exogenous Cysteine and Nitric Oxide on Alleviating cd Toxicity in Cucumber[D]. Taian: Shandong Agricultural Universty, 2013. (in Chinese with English abstract)
[16] Yuan S, Ding X, Zhang Y, et al. Characterization of defense responses in the ‘green ring’and ‘red ring’on jujube fruit upon postharvest infection byand the activation by the elicitor treatment[J]. Postharvest Biology and Technology, 2019, 149: 166-176.
[17] Garcia-Jimenez A, Teruel-Puche J A, Garcia-Ruiz P A, et al. Action of tyrosinase on caffeic acid and its n-nonyl ester. Catalysis and suicide inactivation[J]. International Journal of Biological Macromolecules, 2018, 107: 2650-2659.
[18] Jiao W, Li X, Wang X, et al. Chlorogenic acid induces resistance againstin peach fruit by activating the salicylic acid signaling pathway[J]. Food Chemistry, 2018, 260: 274-282.
[19] 陈存坤,张慧杰,纪海鹏,等. 臭氧精准处理提高采后草莓抗氧化酶活性和酚类物质含量[J]. 农业工程学报,2019,35(10):274-280.
Chen Cunkun, Zhang Huijie, Ji Haipeng, et al. Ozone treatment improving antioxidant enzyme activity and phenolic content of postharvest strawberry[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2019, 35(10): 274-280. (in Chinese with English abstract)
[20] 令阳,邓丽莉,姚世响,等. L-半胱氨酸处理对青脆李果实常温贮藏品质的影响[J]. 食品科学,2019,40(21):222-228.
Ling Yang, Deng Lili, Yao Shixiang, et al. Effect of L-cysteine treatment on the quality of ‘qingcui’ plum fruit during storage at room temperature[J]. Food Science, 2019, 40(21): 222-228. (in Chinese with English abstract)
[21] 曹健康. 果蔬采后生理生化实验指导[M]. 北京:中国轻工业出版社,2007.
[22] Yao H, Tian S. Effects of pre-and post-harvest application of salicylic acid or methyl jasmonate on inducing disease resistance of sweet cherry fruit in storage[J]. Postharvest Biology and Technology, 2005, 35(3): 253-262.
[23] Lamb C J, Rubery P H. A spectrophotometric assay for transcinnamic acid 4-hydroxylase activity [J]. Analytical Biochemistry, 1975, 68(2): 554-561.
[24] Li G, Zhu S, Wu W, et al. Exogenous nitric oxide induces disease resistance againstthrough activating the phenylpropanoid pathway in peach fruit[J]. Journal of the Science of Food and Agriculture, 2017, 97(9): 3030-3038.
[25] Chu Y F, Sun J I E, Wu X, et al. Antioxidant and anti proliferative activities of common vegetables[J]. Journal of Agricultural and Food Chemistry, 2002, 50(23): 6910-6916.
[26] 吴瑛,王秀芳,袁守亮. 响应面分析昆仑雪菊水溶性黄酮类化合物的提取工艺[J]. 食品科学,2013,34(6):129-133.
Wu Ying, Wang Xiufang, Yuan Shouliang. Process optimization by response surface methodology for the extraction of water soluble flavonoids from coreopsis tinctoria flowers[J]. Food Science, 2013, 34(6):129-133. (in Chinese with English abstract)
[27] Usenik V, Stampar F, Kastelec D. Phytochemicals in fruits of twoL. plum cultivars during ripening[J]. Journal of the Science of Food and Agriculture, 2013, 93(3): 681-692.
[28] Wolfe K L, Kang X, He X, et al. Cellular antioxidant activity of common fruits[J]. Journal of Agricultural and Food Chemistry, 2008, 56(18): 8418-8426.
[29] 罗冬兰,林明俊,尤勇刚,等. 李子贮藏保鲜技术研究进展[J]. 南方农业,2018,12(34):56-58.
Luo Donglan, Lin Mingjun, You Yonggang, et al. Research progress for plum storage and fresh-keeping technology[J]. South China Agriculture, 2018, 12(34):56-58. (in Chinese with English abstract)
[30] Tosetti R, Tardelli F, Tadiello A, et al. Molecular and biochemical responses to wounding in mesocarp of ripe peach (L. Batsch) fruit[J]. Postharvest Biology & Technology, 2014, 90: 40-51.
[31] Zhang X, Liu C J. Multifaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids[J]. Molecular Plant, 2015, 8(1): 17-27.
[32] 葛铭佳,张丽媛,艾佳音,等. 热激和山梨酸钾处理对猕猴桃果实灰霉病的抑制效应[J]. 农业工程学报,2020,36(7):316-323.
Ge Mingjia, Zhang Liyuan, Ai Jiayin, et al. Inhibitory effects of heat water and potassium sorbate on gray mold in postharvest kiwifruit[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2020, 36(7): 316-323. (in Chinese with English abstract)
[33] Kokalj D, Zlatić E, Cigić B, et al. Postharvest light-emitting diode irradiation of sweet cherries (L.) promotes accumulation of anthocyanins[J]. Postharvest Biology and Technology, 2019, 148: 192-199.
[34] Wei Y, Shao X, Wei Y, et al. Effect of preharvest application of tea tree oil on strawberry fruit quality parameters and possible disease resistance mechanisms[J]. Scientia Horticulturae, 2018, 241: 18-28.
[35] Kim H G, Kim G S, Lee J H, et al. Determination of the change of flavonoid components as the defence materials ofMarc. fruit peel againstby liquid chromatography coupled with tandem mass spectrometry[J]. Food Chemistry, 2011, 128(1): 49-54.
[36] Ballester A R, Lafuente M T, de Vos R C H, et al. Citrus phenylpropanoids and defence against pathogens. Part I: metabolic profiling in elicited fruits[J]. Food Chemistry, 2013, 136(1): 178-185.
[37] Telles A C, Kupski L, Furlong E B. Phenolic compound in beans as protection against mycotoxins[J]. Food Chemistry, 2017, 214: 293-299.
[38] Skrovankova S, Sumczynski D, Mlcek J, et al. Bioactive compounds and antioxidant activity in different types of berries[J]. International Journal of Molecular Sciences, 2015, 16(10): 24673-24706.
[39] Gao H, Lu Z M, Yang Y, et al. Melatonin treatment reduces chilling injury in peach fruit through its regulation of membrane fatty acid contents and phenolic metabolism[J]. Food Chemistry, 2018, 245: 659-666.
[40] Khumalo K N, Tinyane P, Soundy P, et al. Effect of thyme oil vapour exposure on the brown rot infection, phenylalanine ammonia-lyase (PAL) activity, phenolic content and antioxidant activity in red and yellow skin peach cultivars[J]. Scientia Horticulturae, 2017, 214: 195-199.
[41] Ma L, He J, Liu H, et al. The phenylpropanoid pathway affects apple fruit resistance to[J]. Journal of Phytopathology, 2018, 166(3): 206-215.
Effects of L-cysteine treatment on phenylpropanoid metabolism of postharvest “Qingcui” plum fruit
Chen Liwei1, Ling Yang1, Deng Lili1,2, Zeng Kaifang1,2※
(1.,400715,;2.,,400715,)
The phenylpropanoid pathway, one of the important secondary metabolic pathways in fruits and vegetables, can produce a wide range of phenolic substances, which have many biological activities, such as antioxidant, antibacterial and immunity enhancing. The type and content of produced phenols determine the flavor and quality of fruits, particularly on the nutrition and health. Since L-cysteine is a typical amino acid in living organism, previous studies have found that exogenous L-cysteine treatment can effectively delay the senescence and quality loss of plum fruit during storage after harvest. However, there are few reports on the effect of L-cysteine treatment on the synthesis of phenolic compounds in fruit. Taking ‘Qingcui’ plum fruit as the test material, this study aims to investigate the effect of L-cysteine treatment on the phenylpropanoid metabolism pathway, in order to provide theoretical support for the shelf life of fruit and preservation during postharvest storage. Specifically, the plum fruit was soaked with L-cysteine solution at 1 g/L for 10 min, and then stored at (20±1)℃ with 85%-90% relative humidity. The effect of L-cysteine treatment on key enzymes activities in phenylpropanoid pathway was investigated, including phenylalanine ammonia lyase (PAL), 4-coumaric coenzyme A ligase (4CL), and cinnamate-4-hydroxylase (C4H), as well as the change rule of total phenols, flavonoids and other metabolites. The antioxidant activity of plum fruit was also evaluated. The results showed that L-cysteine treatment significantly(<0.05) delayed the decrease of total soluble solid and titratable acidity content of plum fruit during postharvest storage, indicating that can maintain an excellent quality of fruit. Moreover, the activities of key enzymes increased gradually in the phenylpropane metabolic pathway during storage. The activities of PAL and 4CL of plum fruit in the treatment group were higher than that in the control group. Compared with control group, L-cysteine treatment can increase the content of total phenols and total flavonoids significantly in the first three days of storage, where the content decreased first, and then increased. In the determination of phenolic monomers, protocatechuic acid,-coumaric acid, chlorogenic acid, and caffeic acid decreased first and then increased during storage, while syringic acid and rutin increased gradually. The contents of phenolic monomers in the treated fruits, such as chlorogenic acid, caffeic acid, syringic acid, and rutin, were significantly higher than that in the control group(<0.05). The trend of antioxidant activity was consistent with that of total phenols and flavonoids, while the fruits maintained high antioxidant activity during storage after L-cysteine treatment. The correlation analysis revealed that the activities of PAL, 4CL and C4H enzyme in fruit were significantly correlated to the content of phenolic substances and antioxidant capacity (<0.05), whereas, the antioxidant activity in the fruit was extremely significantly correlated with total phenols, total flavonoids and other metabolic substances (<0.01). These findings demonstrated that 1 g/L L-cysteine treatment can efficiently activate the phenylpropanoid pathway of fruit, thereby to promote the accumulation of phenolic substances. Therefore, the L-cysteine treatment can effectively enhance the storage quality of ‘Qingcui’ plum fruit.
agricultural products; storage; L-cysteine; plum fruit; phenylpropanoid metabolism; phenolics; antioxidant capacity
陈力维,令阳,邓丽莉,等. L-半胱氨酸处理对采后青脆李果实苯丙烷代谢的影响[J]. 农业工程学报,2020,36(13):257-263.doi:10.11975/j.issn.1002-6819.2020.13.030 http://www.tcsae.org
Chen Liwei, Ling Yang, Deng Lili, et al. Effects of L-cysteine treatment on phenylpropanoid metabolism of postharvest “Qingcui” plum fruit[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2020, 36(13): 257-263. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2020.13.030 http://www.tcsae.org
2020-03-24
2020-06-03
重庆市硕士研究生科研创新项目(CYS19114);重庆市技术创新与应用发展专项重点项目(cstc2019jscx-dxwtBX0027)
陈力维,主要从事农产品加工及贮藏工程方面研究。Email:chenliwei013211@163.com
曾凯芳,博士,教授,博士生导师,主要从事果蔬贮藏与保鲜的教学与研究工作。Email:zengkaifang@163.com
10.11975/j.issn.1002-6819.2020.13.030
TS255.3
A
1002-6819(2020)-13-0257-07
