提高CRISPR/Cas9介导的动物基因组精确插入效率研究进展
2020-07-22李国玲杨善欣吴珍芳张献伟
李国玲,杨善欣,吴珍芳,,张献伟
综 述
提高CRISPR/Cas9介导的动物基因组精确插入效率研究进展
李国玲1,杨善欣1,吴珍芳1,2,张献伟2
1. 华南农业大学动物科学学院,国家生猪种业工程技术研究中心,广州 510642 2. 温氏食品集团股份有限公司,新兴 527439
基因编辑技术是指通过人为方式在基因组插入、缺失或替换特定碱基,对遗传物质进行精确修饰和定向编辑的一种技术。近年来,锌指核酸内切酶(zinc-finger endonuclease, ZFN)、类转录激活因子效应物核酸酶(transcription activator-like effector nuclease, TALEN)、成簇规律间隔短回文重复序列及其相关系统(clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9, CRISPR/Cas9)等基因编辑技术的出现,使特异性靶向修饰动物基因组序列成为可能。虽然利用CRISPR/Cas9等基因编辑工具可以在细胞基因组高效产生双链断裂(double-strand breaks, DSB),但利用同源定向修复(homology directed repair, HDR)介导的精确插入(knock in, KI)效率却十分低下。本文结合当前基因编辑技术的发展现状,对目前提高CRISPR/Cas9介导的动物基因组KI策略进行了综述,以期为人类疾病模型制备、基因治疗和家畜遗传改良等提供借鉴。
基因编辑;CRISPR/Cas9;精确插入;同源定向修复;非同源末端连接
基因编辑技术是指通过人为方式在基因组插入、缺失或替换特定碱基,对遗传物质进行精确修饰和定向编辑的一种技术。传统的基因编辑方法利用随机整合或同源重组(homologous repair, HR)等方式将DNA片段插入基因组,这种方式存在精确插入(knock in, KI)效率低和外源基因表达不稳定等问题,严重制约了其在农业和医学领域的应用。近年来,随着锌指核酸内切酶(zinc-finger endonuclease, ZFN)、类转录激活因子效应物核酸酶(transcription activator- like effector nuclease, TALEN)和成簇规律间隔短回文重复序列及其相关系统(clustered regularly interspaced short palidromic repeats/CRISPR-associated protein 9, CRISPR/Cas9)等基因编辑技术的出现,以其高效率、特异性靶向等特征在农业和医学领域得到广泛应用,先后在大肠杆菌()、酵母()、果蝇()、斑马鱼()、小鼠()、大鼠()、猪()和恒河猴()等物种中显示了强大的基因编辑能力,展示出广阔的应用前景[1~7]。动物细胞基因组产生双链断裂(double-strand breaks, DSB)后,主要激活体内非同源末端连接(non-homologous end joining, NHEJ)或同源定向修复(homology directed repair, HDR)两种不同的修复机制,其中HDR介导的KI在人类疾病模型制备、基因治疗和农业遗传改良等方面具有重要作用,但是其效率十分低下[7],因此在CRISPR/Cas9高效产生DSB的前提下,如何提高动物基因组KI效率仍然充满挑战。本文结合当前基因编辑技术的发展,对目前提高CRISPR/Cas9介导的动物基因组KI策略进行了综述,以期为人类疾病模型制备、基因治疗和家畜遗传改良等提供借鉴。
1 基因编辑技术
ZFN是由一系列锌指蛋白(zinc finger protein, ZFP)与I限制性核酸内切酶活性区融合而成,其中ZFP负责特异性识别3个连续碱基,I在两个ZFP识别位点相隔5~8 bp时发生二聚体化,发挥核酸酶活性[8,9]。TALEN原理与ZFN相似,激活因子样效应物(transcription activator-like effector, TALE)负责特异性识别靶位点[10~12],I随后切割DNA序列,产生DSB[13]。相对ZFN和TALEN,CRISPR/ Cas9具有切割效率高、构建简单等特征,是目前研究和应用最广泛的基因编辑系统[14,15]。来自化脓性链球菌()的II型CRISPR/Cas9系统主要由sgRNA(single guide RNA)和SpCas9蛋白组成,其中sgRNA通过碱基互补配对原则识别DNA序列,SpCas9蛋白在sgRNA引导下,切割含有NGG或NAG的靶点位[16,17]。为了提高CRISPR/ Cas9系统特异性或靶向的DNA覆盖范围等,科研人员构建不同的SpCas9突变体,如eSpCas9[18]、SpCas9-HF1[19]、EvoCas9[20]、Sniper Cas9[21]、VRER SpCas9[22]及xCas9[23]等。一些结构更简单、容量更小和切割效率相当或更高的基因编辑工具被逐渐开发,如SGN[24]、AsCpf1[25]、StCas9[26]、NmCas9[27]、SaCas9[28]、CjCas9[29]和CasX[30]等。此外在CRISPR/ Cas9系统基础上升级开发的碱基编辑器,如胞嘧啶碱基编辑器(cytosine base editor, CBE)利用胞嘧啶脱氨酶实现靶位点一定范围内C•G到T•A的替换[31],腺嘌呤碱基编辑器(adenine base editor, ABE)利用腺嘌呤脱氨酶实现靶位点一定范围内A•T到G•C的替换[32],有效克服了CRISPR/Cas9介导的单碱基编辑效率低的弊端。目前升级改造的碱基编辑器ABE4max[33]和AncBE4max[34]已经可以高效实现哺乳动物细胞特定位置A•T与G•C之间碱基的置换。尽管SpCas9突变体或其他基因编辑技术一定程度上克服了CRISPR/Cas9系统转染效率低、脱靶效率高、安全性低和靶向位点有限等缺点,但也存在切割效率低、甲基化位点敏感和不适合高通量编辑等弊端[35,36];此外,虽然ABE和CBE不需要供体模板和引入DSB即可实现单碱基编辑,但不断增大的系统结构也导致病毒难以包装和传送;同时ABE和CBE存在编辑窗口有限、不能实现长片段KI或存在明显的DNA/RNA脱靶等问题[35,36],因此如何升级和完善基因编辑技术仍然充满挑战。
2 提高CRISPR/Cas9介导的动物基因组KI策略
自然或人为在基因组中引入DSB是KI发生的前提,传统的基因编辑技术利用供体模板和自然产生的DSB,HDR效率仅为10–7~10–5,对KI技术的应用与研究带来极大困难[7]。近年来,ZFN、TALEN和CRISPR/Cas9等基因编辑工具的发现和应用,可以在动物基因组高效产生DSB,为实现KI的广泛应用提供了契机。细胞基因组产生DSB后,主要激活体内NHEJ或HDR两种不同的修复机制,其中HDR利用同源序列作为供体模板可以实现碱基对的准确插入、缺失或突变,而NHEJ介导的DNA修复容易出错,常常会引入碱基对的随机插入或缺失[37]。但是HDR是细胞KI最为依赖的修复机制,在人类疾病模型制备、基因治疗和家畜遗传改良等方面具有重要的研究价值[37]。在家畜遗传改良中,KI转基因动物具有目的基因表达稳定和有效提高产肉量、改善肉质、抗病能力等特点;同时不引入目的基因外的外源片段,可以减少公众对转基因食品的担忧。Gao等[38]将结核抗性基因精确插入到奶牛基因组,培育了抗牛结核病的新型奶牛品种;Zheng等[39]将小鼠基因插入猪基因组,培育了抵抗寒冷应激的新型猪品种;Hu等[40]将干扰口蹄疫病毒(foot-and-mouth disease virus, FMDV)的shRNA插入猪基因位点,培育了抗FMDV的KI猪。同时KI技术可以快速获得具有特定功能的人类疾病模型,对疾病治疗方法的研究起到推动作用。Yan等[41]将人源突变的基因替换猪基因,成功制备亨廷顿病(huntington’s disease, HD)模型,可以用于研究大型哺乳动物神经性疾病的发病机制及其治疗方案。此外,利用KI技术纠正由定点突变导致的人类疾病,是细胞和体内基因治疗所必需的关键[42,43]。KI技术将免疫细胞或骨髓造血干细胞等分离出来,进行体外培养和扩增后进行精确修复,随后将KI细胞回输入体内达到治疗效果。这种方式目前已经被用于肿瘤[44]、视网膜色素变性[45]、重度联合免疫缺陷病[46]等体外基因治疗,而体内基因治疗利用供体模板直接修复机体致病突变,目前在I型遗传性酪氨酸血症[47]、B型血友病[48]、致命性高血氨症[49]、镰刀型红细胞贫血[50]和白内障[51]等均有应用。但是细胞利用HDR实现KI效率十分低下,仅有0.5%~20%,而与之竞争的NHEJ效率高达60%[7]。仅仅依靠机体的HDR机制实现KI相对比较困难,因此利用CRISPR/Cas9高效产生DSB的前提下,KI效率仍然需要进一步改进。
2.1 靶位点选择
基因组高效产生DSB是实现外源DNA片段KI的前提。目前种类繁多的sgRNA设计软件为科研人员提供了快速、便捷和高效的sgRNA选择。Hsu等[52]开发的CRISPR Design (http://crispr.mit.edu/)可以针对23~500 nt的DNA序列,设计20 nt的sgRNA,同时对人、小鼠和大鼠等物种基因组进行脱靶评估,最终按靶向效率高低进行排序,并用红、绿、黄3种颜色标记脱靶的特异性高低。Stemmer等[53]开发的CCTop (https://crispr.cos.uni-heidelberg.de/index. html)可以针对不大于500 nt的DNA序列设计PAM可变、核心区域可选择的sgRNA序列,同时可对拟南芥、人和斑马鱼等物种的基因组进行脱靶评估。Heigwer等[54]开发的E-CRISP (http://www.e-crisp. org/E-CRISP/index.html)可设计考虑PAM类型、内含子、CpG岛等,并评估人、小鼠和猪等物种基因组脱靶效应的sgRNA序列。由于不同软件所基于的数据和算法不同,导致参考靶向效率评分存在差异。同时最佳效率的sgRNA序列还需要结合体外T7E1酶切法、SSA (single-strand annealing)报告载体检测或Sanger测序法等进一步筛选。此外,选择不同靶基因和同一靶基因不同靶位点,KI效率也存在一定差异。Li等[55]利用CRISPR/Cas9将长片段DNA插入基因和基因位点,发现其效率存在显著性差异。同时靶位点的组蛋白乙酰化水平会影响染色质的致密程度,直接抑制细胞发生HDR。Bin等[56]发现,随着HDAC1、HDAC2蛋白质乙酰化水平的增加,染色质开放程度逐渐增加,KI效率显著提高,因此靶位点的染色质致密程度可能是阻碍KI发生的潜在因素。
2.2 SpCas9蛋白改造
SpCas9蛋白是CRISPR/Cas9系统发挥核酸酶活性的关键,主要包括Ruvc和HNH两个切割DNA正反链的结构域[17]。基于SpCas9蛋白切割结构域特点,Jinek等[57]将SpCas9改造为一个单切口酶D10A Cas9 (Cas9n),该酶在DNA特定位置制造单链切口;只被一个Cas9n切割产生的单链缺口只会进行高保真性的HR途径,这样基本不会引起NHEJ,更有利于实现外源基因KI。同时有研究表明,Cas9蛋白切割域构象也会影响HDR和NHEJ的比例。例如,Kato-Inui等[58]比较了WT-SpCas9、eSpCas9、SpCas9- HF1和HypaCas9共4种SpCas9蛋白,发现不同细胞HDR/NHEJ比值存在明显差异,其中HypaCas9显著提高HEK293T和HeLa的KI效率,分别为6.9倍和7.7倍。因此,可以推断不同的CRISPR/Cas9系统对KI效率存在差异,高效发生KI的SpCas9突变体还有待探索。
DSB附近DNA供体模板的可使用性可能是限制KI效率的重要因素,通过改造SpCas9蛋白富集供体模板在靶位点的局部浓度可以提高KI效率。Carlson-Stevermer等[59]将生物素–链霉素亲和素特异性识别的RNA序列加入sgRNA茎环构建了S1mplex策略,sgRNA引导SpCas9蛋白结合靶位点的同时,可以将生物素–链霉素亲和素修饰的DNA模板富集在DSB附近。研究结果表明,S1mplex策略可以提高HEK293T细胞等位基因KI效率18倍;基于类似的策略,Ma等[60]将SpCas9蛋白与亲和素融合表达构建Cas9-Avidin/Biotin(CAB)系统,通过Avidin富集生物素标记的供体模板,有效将1 kb片段高效KI小鼠基因组;Gu等[61]在小鼠胚胎2-细胞期注射SpCas9-链霉素亲和素融合蛋白和生物素修饰的供体模板,发现KI效率提高了10倍。此外,Savic等[62]将SpCas9蛋白与SNAP蛋白融合表达构建的Cas9-SNAP系统,通过SNAP结合O6-benzylguanine (BG)标记的DNA模板,将HEK293T细胞的KI效率提高了24倍;Aird等[63]将圆环病毒2 (porcine circovirus type 2, PCV2) DNA识别域与SpCas9蛋白融合表达构建Cas9-PCV2系统,发现当DNA模板存在PCV2识别靶序列时,KI效率显著提高15~30倍。
DNA连接酶IV(DNA-ligase IV, LIG4)是NHEJ途经的关键因子,其与Xrcc4类似因子(Xrcc4-like fators, XLF)结合形成Xrcc4-XLF-LIG4复合体,强行将2个DNA断端连接起来,修复DSB[64]。Chu等[65]将SpCas9蛋白与具有降解LIG4蛋白作用的腺病毒蛋白4E1B55K和E4orf6融合表达,显著提高人和小鼠细胞系KI效率3.5~5倍;同时通过siRNA和shRNA干扰表达也可以提高KI效率2~3倍;Zhang等[66]也发现融合表达4E1B55K和E4orf6的CRISPR/Cas9系统可以显著提高诱导多能干细胞(induced pluripotent stem cells, iPSCs)的KI效率2.5~4倍。此外,Rad51、CtIP、Rad50和Rad52等蛋白是DSB修复的直接参与者,在HDR途径中起到关键作用,因此能否通过融合SpCas9蛋白为DSB修复创造有利环境也是值得尝试的。2017年,Shao等[67]利用酵母来源的yRad52与SpCas9融合构建yRad52-Cas9,将KI效率显著提高40%左右;Charpentier等[68]将CtIP与SpCas9融合表达构建Cas9-HE系统显著提高人类细胞系、iPSCs和大鼠受精卵KI效率;Jayavaradhan等[69]将SpCas9蛋白与53BP1的显性失活突变体DN1S融合构建Cas9- DN1S,显著减少了NHEJ发生频率,同时显著提高了不同人类细胞的KI效率;Tran等[70]发现SpCas9蛋白与CtIP、Rad52和Mre11蛋白,而非Rad51C蛋白融合表达,可以提高HEK293T细胞KI效率2倍。同时将包含噬菌体MS2被壳蛋白结合环的sgRNA与Cas9-CtIP融合蛋白组装,通过MS2招募CtIP蛋白完成DNA修复,发现MS2-CtIP系统进一步提高报告系统KI效率。
NHEJ和HDR分别在不同的细胞周期阶段(G1和G2/S)占主导地位,因此SpCas9蛋白时间特异性表达在S期和G2期,可以一定程度提高HDR介导的KI效率。Gutschner等[71]将SpCas9蛋白与人Geminin N(hGem)末端区域的氨基酸序列融合表达。Cas9-hGem融合蛋白可以作为E3泛素连接酶复合物APC/Cdh1的底物,促进其在细胞周期G1期降解,而在S/G2期维持高水平表达。由于G1期不存在HDR,Cas9-hGem策略可以提高KI效率1.42倍。但Howden等[72]利用Cas9-hGem系统处理iPSCs,发现Cas9-hGem并没有增加HDR频率,但其在靶位点诱导NHEJ介导的插入缺失的能力显著降低。尽管SpCas9突变体和融合表达蛋白一定程度上提高了KI效率,但不断增大的系统结构也为后续的应用提出了新的挑战。
2.3 dsDNA供体模板优化
双链DNA (double strand DNA, dsDNA)模板的拓扑结构、同源臂长度等是影响基因组KI效率的关键因素,其中线性化的供体质粒可以进一步提高KI效率。1989年,Bollag等[73]首次发现在供体模板和基因组靶位点附近引入DSB,可提高HR约100倍;随后Shin等[74]结合TALEN技术对斑马鱼和基因进行打靶,发现体外线性化供体模板可以将KI效率提高10%;Auer等[75]发现线性化的供体模板KI效率显著高于超螺旋的质粒;Yao等[76]利用CRISPR/Cas9技术进一步证明了体外线性化的供体模板比环状供体具有更高的KI效率,同时体外线性化供体模板在不同细胞系都获得极高的KI效率;Cristea等[77]发现当供体模板加入ZFN识别位点时,模板在细胞内被线性化可以显著提高外源基因的KI效率,而Zhang等[66]同样发现供体同源臂加入CRISPR/Cas9识别的靶位点,细胞内切割供体长同源臂产生短同源臂时更有利于实现KI。上述研究均表明供体质粒体内或体外线性化对提高KI效率具有一定提高作用。供体同源臂长度对KI效率的提高具有重要的作用。前期研究表明,同源臂长度在200 bp以下时,HDR修复效率有明显下降趋势;当同源臂长度达到约14 kb以上时,这种线性关系才逐渐不明显[78]。但在KI细胞筛选过程中,由于同源臂过长会增加细胞鉴定的难度,权衡KI效率和鉴定难度,传统的供体模板同源臂长度一般都在6~7 kb。随着ZFN、TALEN和CRISPR/Cas9等基因编辑技术的出现,对这一结论提出了新的挑战。Orlando等[79]结合ZFN技术设计同源臂长度在500~1500 bp的供体模板,发现500 bp的同源臂模板也能保持较高的KI效率;Byrne等[80]利用CRISPR/Cas9系统设计同源臂长度100 bp~5 kb的供体模板,发现在iPSCs细胞中同源臂长度在2 kb时,基因置换为小鼠基因的效率最高,并随着同源臂长度变短,效率逐渐降低,同时长于2 kb的同源臂并没有明显提高KI效率;随后Shin等[74]和Chu等[65]也证实KI长片段的供体模板同源臂需要在1~2 kb。当同源臂长度小于350 bp时,HDR效率降低2.5倍;但Shy等[81]在小鼠胚胎干细胞(mouse embryonic stem cell, mESCs)中发现短同源臂(<200 bp)和长同源臂(>1 kb)搭配,KI效率更高。2014年,Nakade等[82]提出一种全新的KI策略——微同源介导的末端连接(micro-homology mediated end joining, MMEJ),即利用5~40 bp短同源臂高效介导外源基因(<1000 bp)精确插入;随后该团队继续对这种策略进行优化,发现利用5~25 bp可以将长片段(5.7~9.6 kb)外源DNA片段高效插入中国仓鼠卵巢细胞(Chinese hamster ovary cells, CHO)基因组,其效率为10%~17%[83,84];Paix等[85]也发现36 bp短同源臂可以将739 bpDNA片段高效插入小鼠受精卵基因组,而在HEK293T细胞中,同源臂长度是33 bp或518 bp 时KI效率是相同的。因此,同源臂的长度可能不是一成不变的,它可能需要根据所使用的基因编辑工具类型、细胞来源、插入片段大小和插入方式等来确定其最优长度。
2.4 ssODN供体模板设计与优化
相比dsDNA模板,单链寡核苷酸(single-strand oligodeoxynucleotide, ssODN)作为供体模板具有更高的KI效率。Liang等[86]对ssODN模板侧翼序列进行优化,发现侧翼为30~40 nt的ssODN模板在HEK293T细胞可以获得最佳的KI效率;Rivera- Torres等[87]发现侧翼为35~50 nt的ssODN模板在HCT116细胞KI效率最佳,而Wang等[1]研究表明侧翼分别是45 nt和71 nt的ssODN在猪胎儿成纤维细胞(porcine fetal fbroblasts, PFFs)具有最高的KI效率(>10%),而过长的ssODN模板对KI效率并没有显著的促进作用;Richardson等[88]通过优化ssODN供体模板的长度和方向,发现利用不对称的ssODN模板(侧翼分别为91 nt和36 nt)在HEK293T获得高达60% KI效率,而Yumlu等[89]和Yang等[90]进一步研究表明采用不对称的ssODN模板在iPSCs可以获得最佳KI效率,但是Moreno-Mateos等[91]发现对称和不对称ssODN模板在斑马鱼的效率是一致的,因此不同来源的细胞系对ssODN侧翼长度和方向要求可能是不一致的,在特定细胞系采用何种方式还有待优化。此外化学修饰的ssODN模板可以减慢其在细胞与胚胎内的降解速度,阻碍NHEJ发生,进一步提高KI效率。Prykhozhij等[92]发现硫代磷酸化标记的ssODN模板可显著提高斑马鱼KI效率;同时有研究表明通过化学修饰的sgRNA[93]或采用其他修饰方式的ssODN模板[94]也可以显著提高KI效率。为了解决ssODN插入片段较短等问题,Miura等[95]和Quadros等[96]开发了Easi-CRISPR (efficient additions with ssDNA inserts-CRISPR)策略:通过先体外转录再逆转录的方法制备长约4~5 kb的ssODN模板;Yoshimi等[97]开发了2H2OP (Two-hit by sgRNA and two oligos with a targeting plasmid)策略:sgRNA同时切割基因组和不具有同源序列的供体质粒,随后两个提供同源碱基的ssODN以“创可贴”形式将供体质粒整合至基因组,为插入更长的DNA片段提供了可能。但是相对dsDNA,长片段的ssODN难以制备,且成本相对较高,因此ssODN常应用于插入片段小于100 bp的基因精确修复。
2.5 小分子化合物调控DNA修复
小分子化合物是一个相对于高分子化合物的概念,通常指相对分子质量小于10,000的一类化合物,如营养素、代谢产物、天然产物和合成的药理学因子等。由于小分子化合物具有结构和功能多样性的特点,给予其无限潜力去控制分子间、蛋白质间的识别及相互作用。近年来,研究人员利用小分子化合物调控DNA修复通路关键蛋白,在提高基因组KI效率方面取得了一定进展[2,7,98~101]。Maruyama等[7]使用Scr7(LIG4抑制剂)处理MelJuSo细胞和A549细胞,将ssODN介导的KI效率提高3~19倍,同时直接注射小鼠受精卵将KI效率提高了2倍;Li等[98]利用小分子化合物Scr7提高猪成纤维细胞(porcine fetal fbroblasts, PFFs)基因组KI效率2~3倍;Ma等[100]使用VE-822(Rad3相关激酶抑制剂)和AZD-7762(检查点激酶CHEK1特异性抑制剂)将人ESCs CRISPR- Cpf1介导的KI效率提高3~6倍;Yu等[101]通过高通量筛选,发现Brefeldin A (蛋白转动抑制剂)或L755507 (β3肾上腺素受体激动剂)显著提高小鼠ESCs、人源HeLa、K562和iPSCs KI效率1.3~2倍;Song等[2]结合CRISPR/Cas9和TALEN技术,利用Rad51蛋白的激活剂RS-1将基因插入大鼠基因效率提高2~5倍,随后Pinder等[102]在HEK293A细胞也进一步证实了该结果;Lin等[103]通过PD0325901和CHIR99021分别抑制mESCs MEK和GSK3b信号通路,提高KI效率1~5倍;Robert等[104]使用DNA-PK抑制剂NU7441和KU-57788显著降低NHEJ效率,同时显著提高CRISPR/Cas9介导的KI效率。DSB修复表面上是通过NHEJ/HDR途径实现,但细胞所在的细胞周期会显著影响DNA修复途径的选择。在DNA修复过程中,NHEJ可以发生在细胞分裂的任一时期,而HDR主要发生在G2和S期[15]。利用这个规律,通过化合物阻滞细胞周期可以为研究者提供一条提高基因组KI效率的思路。Urnov等[105]通过添加长春花碱(vinblastine)将细胞同步化至G2期显著提高HR发生频率约7倍;随后Rahman等[106]利用indirubin-3ʹ-monoxime抑制剂将HeLa、HT-1080和U-2 OS细胞周期停滞在G2期,有效提高了I-SceI和ZFN的KI效率2~5倍,但目前并没有CRISPR/Cas9相关研究报道。Lin等[99]在多种人源细胞中添加能使细胞停滞在G2/S期的Aphidicolin和Nocodazole,显著提高了ssODN介导的KI效率;Yang等[107]利用化合物ABT-751诱导人iPSCs细胞周期停滞在G2期,随后将2~5 kb的序列分别整合至人类基因组5个区域,KI效率提高了3~6倍。尽管一些化合物可将细胞周期显著停滞在G2期,如LiCl[108],但这些化合物并不能提高CRISPR/Cas9介导的KI效率。一些被证实可以将细胞周期显著阻滞在S期的化合物,如硫酸羟脲(hydroxyurea)[109]和2ʹ,3ʹ- 双脱氧胞苷(dideoxycytidine, ddC)[110]等,是否能有效提高哺乳动物细胞KI效率还有待进一步探索。
虽然已经有较多小分子化合物被应用于提高KI效率,但一些小分子化合物在不同细胞系,甚至是同一细胞系的结果都是不一致的。其原因可能是:(1)不同物种来源细胞NHEJ/HDR活性存在差异,导致实验结果有偏差[111],如SCR7可以显著提高大部分人源细胞如HEK293T、A549和MelJuSo等的KI效率,但对兔子胚胎和CHO的KI效率提高并不理想[2,7,108]。(2)采用CRISRR/Cas9系统方式不一致,导致SpCas9蛋白在体内表达时间存在明显差异影响结果[112]。如Lin等[99]通过转染Cas9 RNP复合物显著提高HEK293T ssODN介导的KI效率,而Yan等[113]通过转染SpCas9质粒却不能提高。(3)供体模板形式不同导致细胞KI效率和修复方式有差异影响结果。如前文所述,ssODN介导的KI效率明显高于dsDNA[88];同时也有研究表明,ssODN修复基因组DSB并不是通过HDR途径实现的,而是通过Fanconi anemia(FA)途径[114],这些可能是导致HDR激活剂RS-1不能显著提高斑马鱼胚胎ssODN介导的KI效率的原因。(4)化合物工作浓度不同导致差异。Li等[98]利用100 µmol/L Scr7显著提高PFFs细胞HDR效率1.9倍,而10 µmol/L时效果不明显(从26.2%提高至28.1%);而Xie等[115]使用5 µmol/L Scr7处理PFFs细胞,提高KI效果不理想。此外提高KI效率固然很重要,但是小分子化合物对细胞DNA损伤仍然需要考虑。有研究表明Scr7可显著提高KI效率,但其存在许多未知的风险,如剂量过大具有明显了细胞毒性,而直接注射胚胎会导致胚胎停滞在桑椹胚时期等[116]。Nocodazole、vinblastine和ABT-751等通过与细胞微管蛋白结合竞争性抑制微管蛋白的聚合,导致分裂的细胞不能形成纺锤体微管而使细胞分裂停止,随后引起细胞内谷胱甘肽/活性氧失衡,导致细胞凋亡和DNA损伤[117~119],因此细胞毒性更小、效率更高的小分子化合物还有待发现(见表1)。
2.6 利用NHEJ途径实现KI
相对低频率的HDR途径,NHEJ在不同类型的细胞都高度活跃。He等[127]将供体模板侧翼同源臂替换为sgRNA靶位点开发HITI (homology-independent targeted integration)策略,利用NHEJ途径将4.6 kb的ires-片段插入LO2细胞和人胚胎干细胞(human embryonic stem cells, hESCs)的位点;同样,Auer等[128]利用HITI方式高效地将>5.7 kb的DNA片段插入斑马鱼基因组;Lackner等[129]利用该方法成功对内源基因实现了NanoLuc luciferase和Turbo GFP标记。基于相同的策略,Suzuki等[130]成功将DNA片段精确插入到非分裂细胞基因组位点上;随后该团队对HITI策略进行优化:保留一侧sgRNA序列,另一侧sgRNA序列替换或增加同源臂,开发了SATI (single homology arm donor mediated intron-targeting integration)策略[131]。SATI策略显著提高细胞发生KI效率,同时一定程度克服了HITI策略靶位点插入片段前后颠换等问题。SpCas9蛋白产生的平末端切口不适合被DNA修复蛋白捕获,而ZFN和TALEN产生的粘性末端更适合切口捕获。Maresca等[132]开发了ObLiGaRe (obligate ligation- gated recombination)策略,通过两对ZFP蛋白分别识别供体模板和基因组靶位点,只有I核酸酶发生异源二聚化时才能切割DNA。线性化的供体模板插入基因组靶位点时,I核酸酶同源二聚化无法切割KI的DNA片段,因此有利于提高KI效率;随后Tsai等[133]和Guilinger等[134]采用类似的策略,将dCas9与I核酸酶融合表达,发现dCas9-I显著提高了KI效率。尽管HITI策略、SATI策略和ObLiGaRe策略成功克服了HDR介导KI低的障碍,但由NHEJ介导的KI方式,可能会导致外源基因随机插入到基因组的其他位置,同时靶位点容易插入或缺失少量碱基,对研究应用依然充满技术挑战。
3 结语与展望
ZFN、TALEN、CRISPR/Cas9和基于CRISPR/ Cas9改造的CBE和ABE系统极大地丰富了基因组编辑的应用范围,但不同的基因编辑系统有着其独特的优点,因此针对不同的物种以及不同的细胞系、转染效率和基因位点序列信息选择合适的基因编辑系统尤为重要。总体而言,ZFN编码的序列更小,更容易实现病毒包装与传送。相对ZFN,TALEN特异性更高,但其设计同样繁琐和不适合高通量筛选;同时TALEN蛋白结构更大,只能通过腺病毒或电转染等方式传送至细胞。CRISPR/Cas9系统摆脱了合成和组装具有特异性DNA识别能力蛋白模块的繁琐操作,以其高效率、易设计构建等特征在生物、农业和医学领域得到广泛应用。ABE和CBE是CRISPR/Cas9系统的进一步提升,其不需要供体模板和引入DSB即可实现单碱基编辑,但不断增大的系统结构也导致病毒难以包装和传送;同时ABE和CBE仍然存在编辑窗口有限、不能实现长片段KI或存在明显的DNA/RNA脱靶等问题[35~37]。2019年,美国哈佛大学David Liu实验室开发的全新碱基基因编辑器PE (prime editors),其无需额外的DNA模板即可实现所有12种单碱基的自由转换,且能有效实现多碱基的KI与基因敲除(knock out, KO)[135]。PE基因编辑器的出现极大地丰富了单碱基与小片段增删的基因编辑系统,但目前没有来自其他实验室重复数据的报道,其基因编辑效率还需谨慎对待。
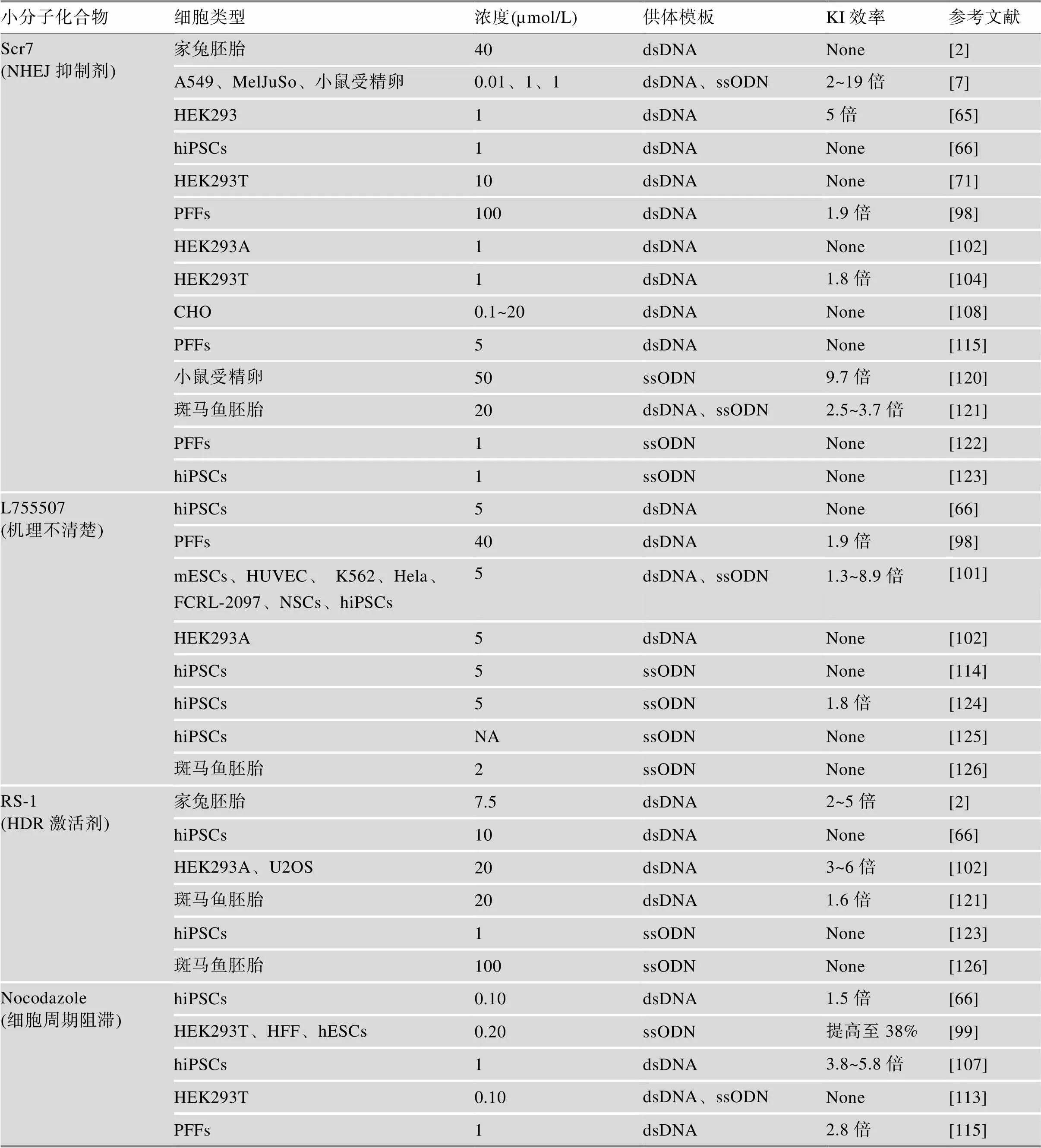
表1 小分子化合物对CRISRR/Cas9介导的KI效率的影响
dsDNA:双链DNA;ssODN:单链寡核苷酸;NA表示小分子化合浓度不详,None表示KI效率没有提高。
细胞基因组产生DSB后,主要激活体内NHEJ或HDR两种不同的修复机制,其中HDR介导的KI效率十分低下,而与之竞争的NHEJ效率却非常高。HDR是细胞KI最为依赖的修复机制,其在人类疾病模型制备,基因治疗和家畜遗传改良等具有重要的研究价值,如KI功能基因达到提高经济动物产肉量、改善肉质、抗病能力等;同时利用KI技术可以制备特定功能的人类疾病模型,和纠正定点突变导致的人类疾病,为研究疾病的发病机制和基因治疗提供方案。但是KI效率的低下极大地限制了其广泛应用,目前已经有多种策略用于提高KI效率,如靶位点选择、SpCas9蛋白改造、dsDNA供体模板优化、ssODN供体模板设计与优化、小分子化合物调控DNA修复和NHEJ途径实现KI等。其中研究人员将SpCas9与其他功能性蛋白融合,通过融合蛋白招募DNA修复因子、调控Cas9蛋白周期特异性降解或富集供体模板等形式一定程度上提高基因组的KI效率,但不断增大的系统容量也为后续病毒包装和传送增加了困难。同时研究也发现不同细胞采用不同类型的修复方式对dsDNA/ssODN供体模板的拓扑结构、同源臂的长度等也有要求,其中HDR介导的dsDNA供体模板同源臂需要在500 bp~1 kb;MMEJ介导的同源臂只需要5~40 bp;而NHEJ介导的KI需要根据HITI、SATI或ObLiGaRe等策略制定其独特的供体模板;尽管NHEJ介导的KI效率非常高,但其DNA片段容易随机插入到基因组的其他位置,增加潜在的安全风险;相比dsDNA供体模板,ssODN插入或替换少量碱基具有更高的编辑效率,而通过化学修饰的ssODN将进一步提高KI效率,但是ssODN模板难以合成,只适用于少量碱基的编辑;此外,使用小分子化合物激活HDR途径关键蛋白、抑制NHEJ途径关键蛋白或阻滞细胞周期至S/G2期也可以提高HDR介导的KI效率,但一些小分子化合物在不同细胞系,甚至同一细胞系的研究结果都有所差异。同时小分子化合物对细胞DNA损伤仍然需要考虑,细胞毒性更小、效率更高的小分子化合物还有待发现。总之,不管采用哪种策略都有其优势和劣势,因此继续完善和提高KI效率仍然需要科研人员努力,这将对家畜遗传改良、人类疾病模型制备和基因治疗等具有重要的意义和价值。
[1] Wang KK, Tang XC, Liu Y, Xie ZC, Zou XD, Li MJ, Yuan HM, Ouyang HS, Jiao HP, Pang DX. Efficient generation of orthologous point mutations in pigs via CRISPR-assisted ssODN-mediated homology-directed repair., 2016, 5(11): e396.
[2] Song J, Yang DS, Xu J, Zhu TQ, Chen YE, Zhang JF. RS-1 enhances CRISPR/Cas9- and TALEN-mediated knock-in efficiency., 2016, 7: 10548.
[3] Niu D, Wei HJ, Lin L, George H, Wang T, Lee IH, Zhao HY, Wang Y, Kan YA, Shrock E, Lesha E, Wang G, Luo YL, Qing YB, Jiao DL, Zhao H, Zhou XY, Wang SQ, Wei H, Güell M, Church GM, Yang LH. Inactivation of porcine endogenous retrovirus in pigs using CRISPR- Cas9., 2017, 357(6357): 1303–1307.
[4] Komor AC, Badran AH, Liu DR. CRISPR-based technologies for the manipulation of eukaryotic genomes., 2017, 168(1–2): 20–36.
[5] Gao YP, Wu HB, Wang YS, Liu X, Chen LL, Li Q, Cui CC, Liu X, Zhang JC, Zhang Y. Single Cas9 nickase induced generation of NRAMP1 knockin cattle with reduced off-target effects., 2017, 18(1): 13.
[6] Chen YC, Zheng YH, Kang Y, Yang WL, Niu YY, Guo XY, Tu ZC, Si CY, Wang H, Xing RX, Pu XQ, Yang SH, Li SH, Ji WZ, Li XJ. Functional disruption of the dystrophin gene in rhesus monkey using CRISPR/Cas9., 2015, 24(13): 3764–3774.
[7] Maruyama T, Dougan SK, Truttmann MC, Bilate AM, Ingram JR, Ploegh HL. Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining., 2015, 33(5): 538–542.
[8] Wah DA, Bitinaite J, Schildkraut I, Aggarwal AK. Structure ofI has implications for DNA cleavage., 1998, 95(18): 10564–10569.
[9] Kim YG, Cha J, Chandrasegaran S. Hybrid restriction enzymes: zinc finger fusions toI cleavage domain., 1996, 93(3): 1156–1160.
[10] Moscou MJ, Bogdanove AJ. A simple cipher governs DNA recognition by TAL effectors., 2009, 326(5959): 1501.
[11] Romer P, Recht S, Lahaye T. A single plant resistance gene promoter engineered to recognize multiple TAL effectors from disparate pathogens., 2009, 106(48): 20526–20531.
[12] Osborn MJ, Starker CG, Mcelroy AN, Webber BR, Riddle MJ, Xia L, Defeo AP, Gabriel R, Schmidt M, von Kalle C, Carlson DF, Maeder ML, Joung JK, Wagner JE, Voytas DF, Blazar BR, Tolar J. TALEN-based gene correction for epidermolysis bullosa., 2013, 21(6): 1151–1159.
[13] Li T, Huang S, Jiang WZ, Wright D, Spalding MH, Weeks DP, Yang B. TAL nucleases (TALNs): hybrid proteins composed of TAL effectors andI DNA-cleavage domain., 2011, 39(1): 359–372.
[14] Pickar-Oliver A, Gersbach CA. The next generation of CRISPR-Cas technologies and applications., 2019, 20(8): 490–507.
[15] Komor AC, Badran AH, Liu DR. CRISPR-based technologies for the manipulation of eukaryotic genomes., 2017, 168(1–2): 20–36.
[16] Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9., 2014, 346(6213): 1258096.
[17] Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering., 2014, 157(6): 1262–1278.
[18] Slaymaker IM, Gao L, Zetsche B, Scott DA, Yan WX, Zhang F. Rationally engineered Cas9 nucleases with improved specificity., 2016, 351(6268): 84–88.
[19] Chen JS, Dagdas YS, Kleinstiver BP, Welch MM, Sousa AA, Harrington LB, Sternberg SH, Joung JK, Yildiz A, Doudna JA. Enhanced proofreading governs CRISPR- Cas9 targeting accuracy., 2017, 550(7676): 407– 410.
[20] Casini A, Olivieri M, Petris G, Montagna C, Reginato G, Maule G, Lorenzin F, Prandi D, Romanel A, Demichelis F, Inga A, Cereseto A. A highly specific SpCas9 variant is identified byscreening in yeast., 2018, 36(3): 265–271.
[21] Lee JK, Jeong E, Lee J, Jung M, Shin E, Kim YH, Lee K, Jung I, Kim D, Kim S, Kim JS. Directed evolution of CRISPR-Cas9 to increase its specificity., 2018, 9(1): 3048.
[22] Kleinstiver BP, Prew MS, Tsai SQ, Topkar VV, Nguyen NT, Zheng ZL, Gonzales AP, Li ZY, Peterson RT, Yeh JR, Aryee MJ, Joung JK. Engineered CRISPR-Cas9 nucleases with altered PAM specificities., 2015, 523(7561): 481–485.
[23] Hu JH, Miller SM, Geurts MH, Tang WX, Chen LW, Sun N, Zeina CM, Gao X, Rees HA, Lin Z, Liu DR. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity., 2018, 556(7699): 57–63.
[24] Xu S, Cao SS, Zou BJ, Yue YY, Gu C, Chen X, Wang P, Dong XH, Xiang Z, Li K, Zhu MS, Zhao QS, Zhou GH. An alternative novel tool for DNA editing without target sequence limitation: the structure-guided nuclease., 2016, 17(1): 186.
[25] Bin MS, Lee JM, Kang JG, Lee NE, Ha DI, Kim DY, Kim SH, Yoo K, Kim D, Ko JH, Kim YS. Highly efficient genome editing by CRISPR-Cpf1 using CRISPR RNA with a uridinylate-rich 3'-overhang., 2018, 9(1): 3651.
[26] Mali P, Yang LH, Esvelt KM, Aach J, Guell M, Dicarlo JE, Norville JE, Church GM. RNA-guided human genome engineeringCas9., 2013, 339(6121): 823–826.
[27] Esvelt KM, Mali P, Braff JL, Moosburner M, Yaung SJ, Church GM. Orthogonal Cas9 proteins for RNA-guided gene regulation and editing., 2013, 10(11): 1116–1121.
[28] Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, Zetsche B, Shalem O, Wu XB, Makarova KS, Koonin EV, Sharp PA, Zhang F.genome editing usingCas9., 2015, 520(7546): 186–191.
[29] Yamada M, Watanabe Y, Gootenberg JS, Hirano H, Ran FA, Nakane T, Ishitani R, Zhang F, Nishimasu H, Nureki O. Crystal structure of the minimal Cas9 fromreveals the molecular diversity in the CRISPR-Cas9 systems., 2017, 65(6): 1109–1121.
[30] Burstein D, Harrington LB, Strutt SC, Probst AJ, Anantharaman K, Thomas BC, Doudna JA, Banfield JF. New CRISPR-Cas systems from uncultivated microbes., 2017, 542(7640): 237–241.
[31] Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage., 2016, 533(7603): 420–424.
[32] Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, Liu DR. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage., 2017, 551(7681): 464–471.
[33] Koblan LW, Doman JL, Wilson C, Levy JM, Tay T, Newby GA, Maianti JP, Raguram A, Liu DR. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction., 2018, 36(9): 843–846.
[34] Rees HA, Liu DR. Base editing: precision chemistry on the genome and transcriptome of living cells., 2018, 19(12): 770–788.
[35] Ren YX, Xiao RD, Lou XM, Fang XD. Research advance and application in the gene therapy of gene editing technologies., 2019, 41(1): 18–27.任云晓, 肖茹丹, 娄晓敏, 方向东. 基因编辑技术及其在基因治疗中的应用. 遗传, 2019, 41(1): 18–28.
[36] Zong Y, Gao CX. Progress on base editing systems., 2019, 41(9): 777–800.宗媛, 高彩霞. 碱基编辑系统研究进展. 遗传, 2019, 41(9): 777–800.
[37] Li S, Yang YY, Qiu Y, Chen Y, Xu LW, Ding QR. Applications of genome editing tools in precision medicine research., 2017, 39(3): 177–188.李爽, 杨圆圆, 邱艳, 陈彦好, 徐璐薇, 丁秋蓉. 基因组编辑技术在精准医学中的应用. 遗传, 2017, 39(03): 177–188.
[38] Gao YP, Wu HB, Wang YS, Liu X, Chen LL, Li Q, Cui CC, Liu X, Zhang JC, Zhang Y. Single Cas9 nickase induced generation ofknockin cattle with reduced off-target effects., 2017, 18(1): 13.
[39] Zheng QT, Lin J, Huang JJ, Zhang HY, Zhang R, Zhang XY, Cao CW, Hambly C, Qin GS, Yao J, Song RG, Jia QT, Wang X, Li YS, Zhang N, Piao ZY, Ye RC, Speakman JR, Wang HM, Zhou Q, Wang YF, Jin WZ, Zhao JG. Reconstitution ofusing CRISPR/Cas9 in the white adipose tissue of pigs decreases fat deposition and improves thermogenic capacity., 2017, 114(45): E9474–E9482.
[40] Hu SW, Qiao J, Fu Q, Chen CF, Ni W, Wujiafu S, Ma SW, Zhang H, Sheng JL, Wang PY, Wang DW, Huang J, Cao LJ, Ouyang HS. Transgenic shRNA pigs reduce susceptibility to foot and mouth disease virus infection., 2015, 4: e06951.
[41] Yan S, Tu ZC, Liu ZM, Fan NN, Yang HM, Yang S, Yang WL, Zhao Y, Ouyang Z, Lai DC, Yang HQ, Li L, Liu QS, Shi H, Xu GQ, Zhao H, Wei HJ, Pei Z, Li SH, Lai LX, Li XJ. A Huntingtin knockin pig model recapitulates features of selective neurodegeneration in Huntington's disease., 2018, 173(4): 989–1002.
[42] Niu XR, Yin SM, Chen X, Shao TT, Li DL. Gene editing technology and its recent progress in disease therapy., 2019, 41(7): 582–598.牛煦然, 尹树明, 陈曦, 邵婷婷, 李大力. 基因编辑技术及其在疾病治疗中的研究进展. 遗传, 2019, 41(7): 582–598.
[43] Qu L, Li HS, Jiang YH, Dong CS. The molecular mechanism of CRISPR/Cas9 system and its application in gene therapy of human diseases., 2015, 37(10): 974–982.璩良, 李华善, 姜运涵, 董春升. CRISPR/Cas9系统的分子机制及其在人类疾病基因治疗中的应用. 遗传, 2015, 37(10): 974–982.
[44] Firth AL, Menon T, Parker GS, Qualls SJ, Lewis BM, Ke E, Dargitz CT, Wright R, Khanna A, Gage FH, Verma IM. Functional gene correction for cystic fibrosis in lung epithelial cells generated from patient iPSCs., 2015, 12(9): 1385–1390.
[45] Howden SE, Maufort JP, Duffin BM, Elefanty AG, Stanley EG, Thomson JA. Simultaneous reprogramming and gene correction of patient fibroblasts., 2015, 5(6): 1109–1118.
[46] Chang CW, Lai YS, Westin E, Khodadadi-Jamayran A, Pawlik KM, Lamb LJ, Goldman FD, Townes TM. Modeling human severe combined immunodeficiency and correction by CRISPR/Cas9-enhanced gene targeting., 2015, 12(10): 1668–1677.
[47] Yin H, Xue W, Chen SD, Bogorad RL, Benedetti E, Grompe M, Koteliansky V, Sharp PA, Jacks T, Anderson DG. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype., 2014, 32(6): 551–553.
[48] Guan YT, Ma YL, Li Q, Sun ZL, Ma L, Wu LJ, Wang LR, Zeng L, Shao YJ, Chen YT, Ma N, Lu WQ, Hu KW, Han HH, Yu YH, Huang YH, Liu MY, Li DL. CRISPR/ Cas9-mediated somatic correction of a novel coagulator factor IX gene mutation ameliorates hemophilia in mouse., 2016, 8(5): 477–488.
[49] Yang Y, Wang LL, Bell P, Mcmenamin D, He ZN, White J, Yu HW, Xu CY, Morizono H, Musunuru K, Batshaw ML, Wilson JM. A dual AAV system enables the Cas9-mediated correction of a metabolic liver disease in newborn mice., 2016, 34(3): 334–338.
[50] Dewitt MA, Magis W, Bray NL, Wang TJ, Berman JR, Urbinati F, Heo SJ, Mitros T, Munoz DP, Boffelli D, Kohn DB, Walters MC, Carroll D, Martin DI, Corn JE. Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells., 2016, 8(360): 134r–360r.
[51] Wu YX, Liang D, Wang YH, Bai MZ, Tang W, Bao SM, Yan ZQ, Li DS, Li JS. Correction of a genetic disease in mouse via use of CRISPR-Cas9., 2013, 13(6): 659–662.
[52] Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li YQ, Fine EJ, Wu XB, Shalem O, Cradick TJ, Marraffini LA, Bao G, Zhang F. DNA targeting specificity of RNA-guided Cas9 nucleases., 2013, 31(9): 827–832.
[53] Stemmer M, Thumberger T, Del SKM, Wittbrodt J, Mateo JL. CCTop: an intuitive, flexible and reliable CRISPR/Cas9 target prediction tool., 2015, 10(4): e0124633.
[54] Heigwer F, Kerr G, Boutros M. E-CRISP: fast CRISPR target site identification., 2014, 11(2): 122–123.
[55] Li GL, Zhang XW, Wang HQ, Mo JX, Zhong CL, Shi JS, Zhou R, Li ZC, Yang HQ, Wu ZF, Liu DW. CRISPR/ Cas9-mediated integration of large transgene into piglocus., 2020, 10(2): 467–473.
[56] Liu B, Chen SW, Rose A, Chen D, Cao FY, Zwinderman M, Kiemel D, Aissi M, Dekker FJ, Haisma HJ. Inhibitionof histone deacetylase 1 (HDAC1) and HDAC2 enhances CRISPR/Cas9 genome editing., 2020, 48(2): 517–532.
[57] Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A, Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors., 2009, 326(5959): 1509–1512.
[58] Kato-Inui T, Takahashi G, Hsu S, Miyaoka Y. Clustered regularly interspaced short palindromic repeats (CRISPR)/ CRISPR-associated protein 9 with improved proof- reading enhances homology-directed repair., 2018, 46(9): 4677–4688.
[59] Carlson-Stevermer J, Abdeen AA, Kohlenberg L, Goedland M, Molugu K, Lou M, Saha K. Assembly of CRISPR ribonucleoproteins with biotinylated oligonucleotidesan RNA aptamer for precise gene editing., 2017, 8(1): 1711.
[60] Ma M, Zhuang FF, Hu XB, Wang BL, Wen XZ, Ji JF, Xi JJ. Efficient generation of mice carrying homozygous double-floxp alleles using the Cas9-Avidin/Biotin-donor DNA system., 2017, 27(4): 578–581.
[61] Gu B, Posfai E, Rossant J. Efficient generation of targeted large insertions by microinjection into two-cell- stage mouse embryos., 2018, 36(7): 632–637.
[62] Savic N, Ringnalda FC, Lindsay H, Berk C, Bargsten K, Li YZ, Neri D, Robinson MD, Ciaudo C, Hall J, Jinek M, Schwank G. Covalent linkage of the DNA repair template to the CRISPR-Cas9 nuclease enhances homology-directed repair., 2018, 7: e33761.
[63] Aird EJ, Lovendahl KN, St MA, Harris RS, Gordon WR. Increasing Cas9-mediated homology-directed repair efficiency through covalent tethering of DNA repair template., 2018, 1: 54.
[64] Aceytuno RD, Piett CG, Havali-Shahriari Z, Edwards RA, Rey M, Ye RQ, Javed F, Fang SJ, Mani R, Weinfeld M, Hammel M, Tainer JA, Schriemer DC, Lees-Miller S P, Glover J. Structural and functional characterization of the PNKP-XRCC4-LigIV DNA repair complex., 2017, 45(10): 6238–6251.
[65] Chu VT, Weber T, Wefers B, Wurst W, Sander S, Rajewsky K, Kuhn R. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells., 2015, 33(5): 543–548.
[66] Zhang JP, Li XL, Li GH, Chen WQ, Arakaki C, Botimer GD, Baylink D, Zhang L, Wen W, Fu YW, Xu J, Chun N, Yuan WP, Cheng T, Zhang XB. Efficient precise knockin with a double cut HDR donor after CRISPR/ Cas9-mediated double-stranded DNA cleavage., 2017, 18(1): 35.
[67] Shao SM, Ren CH, Liu ZT, Bai YC, Chen ZL, Wei ZH, Wang X, Zhang ZY, Xu K. Enhancing CRISPR/Cas9- mediated homology-directed repair in mammalian cells by expressingRad52., 2017, 92: 43–52.
[68] Charpentier M, Khedher A, Menoret S, Brion A, Lamribet K, Dardillac E, Boix C, Perrouault L, Tesson L, Geny S, De Cian A, Itier JM, Anegon I, Lopez B, Giovannangeli C, Concordet JP. CtIP fusion to Cas9 enhances transgene integration by homology-dependent repair., 2018, 9(1): 1133.
[69] Jayavaradhan R, Pillis DM, Goodman M, Zhang F, Zhang Y, Andreassen PR, Malik P. CRISPR-Cas9 fusion to dominant-negative 53BP1 enhances HDR and inhibits NHEJ specifically at Cas9 target sites., 2019, 10(1): 2866.
[70] Tran NT, Bashir S, Li X, Rossius J, Chu VT, Rajewsky K, Kühn R. Enhancement of precise gene editing by the association of Cas9 with homologous recombination factors., 2019, 10: 365.
[71] Gutschner T, Haemmerle M, Genovese G, Draetta GF, Chin L. Post-translational regulation of Cas9 during G1 enhances homology-directed repair., 2016, 14(6): 1555–1566.
[72] Howden SE, Mccoll B, Glaser A, Vadolas J, Petrou S, Little MH, Elefanty AG, Stanley EG. A Cas9 variant for efficient generation of indel-free knockin or gene- corrected human pluripotent stem cells., 2016, 7(3): 508–517.
[73] Bollag RJ, Waldman AS, Liskay RM. Homologous recombination in mammalian cells., 1989, 23: 199–225.
[74] Shin J, Chen J, Solnica-Krezel L. Efficient homologous recombination-mediated genome engineering in zebrafish using TALE nucleases., 2014, 141(19): 3807–3818.
[75] Auer TO, Duroure K, De Cian A, Concordet JP, Del BF. Highly efficient CRISPR/Cas9-mediated knock-in in zebrafish by homology-independent DNA repair., 2014, 24(1): 142–153.
[76] Yao X, Wang X, Liu JL, Hu XD, Shi LY, Shen XW, Ying WQ, Sun XY, Wang X, Huang PY, Yang H. CRISPR/Cas9 - mediated precise targeted integrationusing a double cut donor with short homology arms., 2017, 20: 19–26.
[77] Cristea S, Freyvert Y, Santiago Y, Holmes MC, Urnov FD, Gregory PD, Cost GJ.cleavage of transgene donors promotes nuclease-mediated targeted integration., 2013, 110(3): 871–880.
[78] Fujitani Y, Yamamoto K, Kobayashi I. Dependence of frequency of homologous recombination on the homology length., 1995, 140(2): 797–809.
[79] Orlando SJ, Santiago Y, Dekelver RC, Freyvert Y, Boydston EA, Moehle EA, Choi VM, Gopalan SM, Lou JF, Li J, Miller JC, Holmes MC, Gregory PD, Urnov FD, Cost GJ. Zinc-finger nuclease-driven targeted integration into mammalian genomes using donors with limited chromosomal homology., 2010, 38(15): e152.
[80] Byrne SM, Ortiz L, Mali P, Aach J, Church GM. Multi-kilobase homozygous targeted gene replacement in human induced pluripotent stem cells., 2015, 43(3): e21.
[81] Shy BR, Macdougall MS, Clarke R, Merrill BJ. Co-incident insertion enables high efficiency genome engineering in mouse embryonic stem cells., 2016, 44(16): 7997–8010.
[82] Nakade S, Tsubota T, Sakane Y, Kume S, Sakamoto N, Obara M, Daimon T, Sezutsu H, Yamamoto T, Sakuma T, Suzuki KT. Microhomology-mediated end-joining- dependent integration of donor DNA in cells and animals using TALENs and CRISPR/Cas9., 2014, 5: 5560.
[83] Aida T, Nakade S, Sakuma T, Izu Y, Oishi A, Mochida K, Ishikubo H, Usami T, Aizawa H, Yamamoto T, Tanaka K. Gene cassette knock-in in mammalian cells and zygotes by enhanced MMEJ., 2016, 17(1): 979.
[84] Sakuma T, Nakade S, Sakane Y, Suzuki KT, Yamamoto T. MMEJ-assisted gene knock-in using TALENs and CRISPR-Cas9 with the PITCh systems., 2016, 11(1): 118–133.
[85] Paix A, Folkmann A, Goldman DH, Kulaga H, Grzelak MJ, Rasoloson D, Paidemarry S, Green R, Reed RR, Seydoux G. Precision genome editing using synthesis- dependent repair of Cas9-induced DNA breaks., 2017, 114(50): E10745–E10754.
[86] Liang XQ, Potter J, Kumar S, Ravinder N, Chesnut JD. Enhanced CRISPR/Cas9-mediated precise genome editing by improved design and delivery of gRNA, Cas9 nuclease, and donor DNA., 2017, 241: 136–146.
[87] Rivera-Torres N, Strouse B, Bialk P, Niamat RA, Kmiec EB. The position of DNA cleavage by TALENs and cell synchronization influences the frequency of gene editing directed by single-stranded oligonucleotides., 2014, 9(5): e96483.
[88] Richardson CD, Ray GJ, Dewitt MA, Curie GL, Corn JE. Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA., 2016, 34(3): 339–344.
[89] Yumlu S, Stumm J, Bashir S, Dreyer AK, Lisowski P, Danner E, Kühn R. Gene editing and clonal isolation of human induced pluripotent stem cells using CRISPR/ Cas9., 2017, 121–122: 29–44.
[90] Yang LH, Guell M, Byrne S, Yang JL, De Los AA, Mali P, Aach J, Kim-Kiselak C, Briggs AW, Rios X, Huang PY, Daley G, Church G. Optimization of scarless human stem cell genome editing., 2013, 41(19): 9049–9061.
[91] Moreno-Mateos MA, Fernandez JP, Rouet R, Vejnar CE, Lane MA, Mis E, Khokha MK, Doudna JA, Giraldez AJ. CRISPR-Cpf1 mediates efficient homology-directed repair and temperature-controlled genome editing., 2017, 8(1): 2024.
[92] Prykhozhij SV, Fuller C, Steele SL, Veinotte CJ, Razaghi B, Robitaille JM, Mcmaster CR, Shlien A, Malkin D, Berman JN. Optimized knock-in of point mutations in zebrafish using CRISPR/Cas9., 2018, 46(17): e102.
[93] Hendel A, Bak RO, Clark JT, Kennedy AB, Ryan DE, Roy S, Steinfeld I, Lunstad BD, Kaiser RJ, Wilkens AB, Bacchetta R, Tsalenko A, Dellinger D, Bruhn L, Porteus MH. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells., 2015, 33(9): 985–989.
[94] Gutierrez-Triana JA, Tavhelidse T, Thumberger T, Thomas I, Wittbrodt B, Kellner T, Anlas K, Tsingos E, Wittbrodt J. Efficient single-copy HDR by 5' modified long dsDNA donors., 2018, 7: e39468.
[95] Miura H, Quadros RM, Gurumurthy CB, Ohtsuka M. Easi-CRISPR for creating knock-in and conditional knockout mouse models using long ssDNA donors., 2018, 13(1): 195–215.
[96] Quadros RM, Miura H, Harms DW, Akatsuka H, Sato T, Aida T, Redder R, Richardson GP, Inagaki Y, Sakai D, Buckley SM, Seshacharyulu P, Batra SK, Behlke MA, Zeiner SA, Jacobi AM, Izu Y, Thoreson WB, Urness LD, Mansour SL, Ohtsuka M, Gurumurthy CB. Easi-CRISPR: a robust method for one-step generation of mice carrying conditional and insertion alleles using long ssDNA donors and CRISPR ribonucleoproteins., 2017, 18(1): 92.
[97] Yoshimi K, Kunihiro Y, Kaneko T, Nagahora H, Voigt B, Mashimo T. ssODN-mediated knock-in with CRISPR- Cas for large genomic regions in zygotes., 2016, 7: 10431.
[98] Li GL, Zhang XW, Zhong CL, Mo JX, Quan R, Yang J, Liu DW, Li ZC, Yang HQ, Wu ZF. Small molecules enhance CRISPR/Cas9-mediated homology-directed genome editing in primary cells., 2017, 7(1): 8943.
[99] Lin S, Staahl BT, Alla RK, Doudna JA. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery., 2014, 3: e04766.
[100] Ma XJ, Chen X, Jin Y, Ge WY, Wang WY, Kong LH, Ji JF, Guo X, Huang J, Feng XH, Fu JF, Zhu SY. Small molecules promote CRISPR-Cpf1-mediated genome editing in human pluripotent stem cells., 2018, 9(1): 1303.
[101] Yu C, Liu YX, Ma TH, Liu K, Xu SH, Zhang Y, Liu HL, La Russa M, Xie M, Ding S, Qi LS. Small molecules enhance CRISPR genome editing in pluripotent stem cells., 2015, 16(2): 142–147.
[102] Pinder J, Salsman J, Dellaire G. Nuclear domain 'knock-in' screen for the evaluation and identification of small molecule enhancers of CRISPR-based genome editing., 2015, 43(19): 9379–9392.
[103] Lin ZY, Zhang YL, Gao TY, Wang LD, Zhang Q, Zhou J, Zhao J. Homologous recombination efficiency enhanced by inhibition of MEK and GSK3β., 2014, 52(11): 889–896.
[104] Robert F, Barbeau M, Éthier S, Dostie J, Pelletier J. Pharmacological inhibition of DNA-PK stimulates Cas9-mediated genome editing., 2015, 7: 93.
[105] Urnov FD, Miller JC, Lee YL, Beausejour CM, Rock JM, Augustus S, Jamieson AC, Porteus MH, Gregory PD, Holmes MC. Highly efficient endogenous human gene correction using designed zinc-finger nucleases., 2005, 435(7042): 646–651.
[106] Rahman SH, Bobis-Wozowicz S, Chatterjee D, Gellhaus K, Pars K, Heilbronn R, Jacobs R, Cathomen T. The nontoxic cell cycle modulator indirubin augments transduction of adeno-associated viral vectors and zinc- finger nuclease-mediated gene targeting., 2013, 24(1): 67–77.
[107] Yang D, Scavuzzo MA, Chmielowiec J, Sharp R, Bajic A, Borowiak M. Enrichment of G2/M cell cycle phase in human pluripotent stem cells enhances HDR-mediated gene repair with customizable endonucleases., 2016, 6(1): 21264.
[108] Lee JS, Grav LM, Pedersen LE, Lee GM, Kildegaard HF. Accelerated homology-directed targeted integration of transgenes in Chinese hamster ovary cellsCRISPR/ Cas9 and fluorescent enrichment., 2016, 113(11): 2518–2523.
[109] Tsakraklides V, Brevnova E, Stephanopoulos G, Shaw AJ. Improved gene targeting through cell cycle synchronization., 2015, 10(7): e133434.
[110] Borel F, Lacroix FB, Margolis RL. Prolonged arrest of mammalian cells at the G1/S boundary results in permanent S phase stasis., 2002, 115(Pt 14): 2829–2838.
[111] Miyaoka Y, Berman JR, Cooper SB, Mayerl SJ, Chan AH, Zhang B, Karlin-Neumann GA, Conklin BR. Systematic quantification of HDR and NHEJ reveals effects of locus, nuclease, and cell type on genome- editing., 2016, 6: 23549.
[112] Kouranova E, Forbes K, Zhao GJ, Warren J, Bartels A, Wu YM, Cui XX. CRISPRs for optimal targeting: delivery of CRISPR components as DNA, RNA, and protein into cultured cells and single-cell embryos., 2016, 27(6): 464–475.
[113] Yan NN, Sun YS, Fang YY, Deng JR, Mu L, Xu K, Mymryk JS, Zhang ZY. A universal surrogate reporter for efficient enrichment of CRISPR/Cas9-mediated homology-directed repair in mammalian cells., 2019, 19: 775–789.
[114] Richardson CD, Kazane KR, Feng SJ, Zelin E, Bray NL, Schafer AJ, Floor SN, Corn JE. CRISPR-Cas9 genome editing in human cells occursthe Fanconi anemia pathway., 2018, 50(8): 1132–1139.
[115] Xie ZC, Pang DX, Wang KK, Li MJ, Guo NN, Yuan HM, Li JN, Zou XD, Jiao HP, Ouyang HS, Li ZJ, Tang XC. Optimization of a CRISPR/Cas9-mediated knock-in strategy at the porcinelocus in porcine foetal fibroblasts., 2017, 7(1): 3036.
[116] Li GL, Zhong CL, Mo JX, Quan R, Wu ZF, Li ZC, Yang HQ, Zhang XW. Advances in site-specific integration of transgene in animal genome., 2017, 39(2): 98–109.李国玲, 钟翠丽, 莫健新, 全绒, 吴珍芳, 李紫聪, 杨化强, 张献伟. 动物基因组定点整合转基因技术研究进展. 遗传, 2017, 39(2): 98–109.
[117] Wang NZ, Liu JR, Wang C, Bai LY, Jiang XF. Asymmetric total syntheses of (–)-jerantinines A, C, and E, (–)-16-methoxytabersonine, (–)-vindoline, and (+)- vinblastine., 2018, 20(1): 292–295.
[118] Chiu WH, Luo SJ, Chen CL, Cheng JH, Hsieh CY, Wang CY, Huang WC, Su WC, Lin CF. Vinca alkaloids cause aberrant ROS-mediated JNK activation, Mcl-1 downregulation, DNA damage, mitochondrial dysfunction, and apoptosis in lung adenocarcinoma cells., 2012, 83(9): 1159–1171.
[119] Wei RJ, Wu WR, Pan CT, Yu CY, Li CF, Chen LR, Liang SS, Shiue YL. Inhibition of the formation of autophagosome but not autolysosome augments ABT-751-induced apoptosis in TP53-deficient Hep-3B cells., 2019, 234(6): 9551–9563.
[120] Singh P, Schimenti JC, Bolcun-Filas E. A mouse geneticist's practical guide to CRISPR applications., 2015, 199(1): 1–15.
[121] Zhang YB, Zhang ZW, Ge W. An efficient platform for generating somatic point mutations with germline transmission in the zebrafish by CRISPR/Cas9-mediated gene editing., 2018, 293(17): 6611–6622.
[122] Gerlach M, Kraft T, Brenner B, Petersen B, Niemann H, Montag J. Efficient knock-in of a point mutation in porcine fibroblasts using the CRISPR/Cas9-GMNN fusion gene., 2018, 9(6): 296.
[123] Riesenberg S, Maricic T. Targeting repair pathways with small molecules increases precise genome editing in pluripotent stem cells., 2018, 9(1): 2164.
[124] Liu YL, Yang Y, Kang XJ, Lin B, Yu Q, Song B, Gao G, Chen YY, Sun XF, Li XP, Bu L, Fan Y. One-step biallelic and scarless correction of a β-thalassemia mutation in patient-specific iPSCs without drug selection., 2017, 6: 57–67.
[125] Steyer B, Bu Q, Cory E, Jiang K, Duong S, Sinha D, Steltzer S, Gamm D, Chang Q, Saha K. Scarless genome editing of human pluripotent stem cellstransient puromycin selection., 2018, 10(2): 642–654.
[126] Boel A, De Saffel H, Steyaert W, Callewaert B, De Paepe A, Coucke PJ, Willaert A. CRISPR/Cas9-mediated homology-directed repair by ssODNs in zebrafish induces complex mutational patterns resulting from genomic integration of repair-template fragments., 2018, 11(10): dmm035352.
[127] He XJ, Tan CL, Wang F, Wang YF, Zhou R, Cui DX, You WX, Zhao H, Ren JW, Feng B. Knock-in of large reporter genes in human cellsCRISPR/Cas9- induced homology-dependent and independent DNA repair., 2016, 44(9): e85.
[128] Auer TO, Del BF. Homology-independent integration of plasmid DNA into the zebrafish genome., 2016, 1451: 31–51.
[129] Lackner DH, Carre A, Guzzardo PM, Banning C, Mangena R, Henley T, Oberndorfer S, Gapp BV, Nijman S, Brummelkamp TR, Bürckstümmer T. A generic strategy for CRISPR-Cas9-mediated gene tagging., 2015, 6: 10237.
[130] Suzuki K, Tsunekawa Y, Hernandez-Benitez R, Wu J, Zhu J, Kim EJ, Hatanaka F, Yamamoto M, Araoka T, Li Z, Kurita M, Hishida T, Li M, Aizawa E, Guo SC, Chen S, Goebl A, Soligalla RD, Qu J, Jiang TS, Fu X, Jafari M, Esteban CR, Berggren WT, Lajara J, Nunez- Delicado E, Guillen P, Campistol JM, Matsuzaki F, Liu GH, Magistretti P, Zhang K, Callaway EM, Zhang K, Belmonte JC.genome editingCRISPR/Cas9 mediated homology-independent targeted integration., 2016, 540(7631): 144–149.
[131] Suzuki K, Yamamoto M, Hernandez-Benitez R, Li Z, Wei C, Soligalla RD, Aizawa E, Hatanaka F, Kurita M, Reddy P, Ocampo A, Hishida T, Sakurai M, Nemeth AN, Nunez DE, Campistol JM, Magistretti P, Guillen P, Rodriguez EC, Gong JH, Yuan YL, Gu Y, Liu GH, Lopez-Otin C, Wu J, Zhang K, Izpisua BJ. Precisegenome editingsingle homology arm donor mediated intron-targeting gene integration for genetic disease correction., 2019, 29(10): 804–819.
[132] Maresca M, Lin VG, Guo N, Yang Y. Obligate ligation-gated recombination (ObLiGaRe): custom- designed nuclease-mediated targeted integration through nonhomologous end joining., 2013, 23(3): 539–546.
[133] Tsai SQ, Wyvekens N, Khayter C, Foden JA, Thapar V, Reyon D, Goodwin MJ, Aryee MJ, Joung JK. Dimeric CRISPR RNA-guidedI nucleases for highly specific genome editing., 2014, 32(6): 569–576.
[134] Guilinger JP, Thompson DB, Liu DR. Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification., 2014, 32(6): 577–582.
[135] Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM, Chen PJ, Wilson C, Newby GA, Raguram A, Liu DR. Search-and-replace genome editing without double-strand breaks or donor DNA., 2019, 576(7785): 149.
Recent developments in enhancing the efficiency of CRISPR/Cas9- mediated knock-in in animals
Guoling Li1, Shanxin Yang1, Zhenfang Wu1,2, Xianwei Zhang2
Gene-editing technology can artificially modify genetic material of targeted loci by precise insertion, deletion, or replacement in the genomic DNA. In recent years, with the developments of zinc-finger endonuclease (ZFN), transcription activator-like effector nuclease (TALEN), clustered regularly interspaced short palindromic repeats/CRISPR- associated protein 9 (CRISPR/Cas9) technologies, such precise modifications of the animal genomes have become possible. Although gene-editing tools, such as CRISPR/Cas9, can efficiently generate double-strand breaks (DSBs) in mammalian cells, the homology-directed repair (HDR) mediated knock-in (KI) efficiency is extremely low. In this review, we briefly describe the current development of gene-editing tools and summarize the recent strategies to enhance the CRISPR/Cas9- mediated KI efficiency, which will provide a reference for the generation of human disease models, research on gene therapy and livestock genetic improvement.
gene editing; CRISPR/Cas9; knock in; homology directed repair; non-homologous end joining
2020-03-04;
2020-04-24
国家转基因重大专项(编号:2016ZX08006002)资助[Supported by the National Transgenic Major Projects (No. 2016ZX08006002)]
李国玲,在读博士研究生,专业方向:基因编辑。E-mail: 792268184@qq.com
张献伟,博士,硕士生导师,研究方向:遗传育种。E-mail: zxianw@163.com
10.16288/j.yczz.20-056
2020/6/2 11:50:24
URI: http://kns.cnki.net/kcms/detail/11.1913.R.20200601.1621.003.html
(责任编委: 谷峰)
