甜椒内质网小分子热激蛋白基因(CaHSP22.5)克隆及其在转基因烟草中的表达分析(英文)
2020-07-07苏振华张泽鑫李妹芳郭尚敬冀芦沙
苏振华 张泽鑫 李妹芳 郭尚敬 冀芦沙


摘要:【目的】克隆甜椒內质网小分子热激蛋白基因(CaHSP22.5),并检测其表达特性,为探究其在植物抗逆中的调控机制提供理论依据。【方法】克隆CaHSP22.5基因并构建其表达载体pBI121-CaHSP22.5,转化农杆菌LBA4404后通过叶盘法转化野生型烟草,即获得CaHSP22.5转基因株系;以转pBI121空载体的野生型烟草为对照组。对转基因株系和野生型烟草进行4 ℃ 6 h低温处理,根据幼苗成活率测定CaHSP22.5基因表达对植株耐寒性的影响。采用脉冲振幅调制叶室荧光仪Li-6400测量4 ℃处理10 h后野生型植株与CaHSP22.5转基因株系叶绿素荧光,根据非光化学淬灭系数(NPQ)值测定CaHSP22.5基因表达对在低温下植株光合作用的影响;使用过氧化氢(H2O2)钛络合物法对烟草叶片中H2O2进行定量分析,通过测定活性氧(ROS)积累程度分析CaHSP22.5对植物光合系统的影响;采用检测柠檬酸合成酶(CS)活性的方法,在体外通过对变性CS复性的影响测定CaHSP22.5的分子伴侣活性。【结果】经低温处理后发现CaHSP22.5转基因株系13号、24号和32号幼苗的平均存活率分别为84%、75%和52%,而野生型烟草的平均存活率为30%,CaHSP22.5转基因株系烟草幼苗成活率菌高于野生型幼苗。低温处理10 h后发现CaHSP22.5转基因植株NPQ较野生型植株显著升高(P<0.05),说明转基因烟草中CaHSP22.5的积累有利于保护光合系统,在低温胁迫下清除ROS。同时发现低温胁迫导致植株ROS产量增加,CaHSP22.5转基因植系与野生型相比ROS积累水平较低。不同浓度的CaHSP22.5均可使变性的CS复性,且其CS活性均高于未添加CaHSP22.5的CS活性。【结论】CaHSP22.5蛋白可增强植株的耐寒性及光合作用,在植物体内可降低ROS积累,减轻脂质过氧化,同时具有分子伴侣活性。
关键词: 甜椒;内质网小分子热激蛋白;低温胁迫;转基因烟草;耐受性
【Research significance】Chilling stress is one of the major environmental stresses. Many plants have developed the resistance mechanisms to chilling stress at the molecule and protein levels. Chilling treatments have been shown to induce the expression of cold acclimation-related genes. It has been repo-rted that small heat shock proteins(sHSPs) can protect plant cells against environmental stresses inclu-ding oxidative stress,drought,cold,and heavy metals(Ezemaduka et al.,2017; Kumar et al.,2018). The effects of sHSP on heat tolerance in plants in recent studies have focused on the in?ux of molecular cha-perone. sHSP is involved in nearly all aspects of the high temperature stress in plant development and participates in many regulatory processes. However,the interaction mechanism between the sHSP and cold stress is still unknown,such as whether the low temperature directly potentiates the expression of HSP genes or not. Cloning the endoplasmic reticulum small heat shock proteins(CaHSPs) of Capsicum annuum L. to study their effects on plants provides a theoretical basis for exploring the regulatory mechanism of CaHSP22.5 in plant resistance. 【Research progress】A class of HSPs that accumulated in response to low temperature has been reported for spinach(Lambert et al.,2011) and soybean(Lopes-Caitar et al.,2013; Shukla et al.,2017). There were six classes of sHSPs in plants,with molecular weights ranging from 20 to 44 kD(Zhong et al.,2013). In plants,sHSPs were encoded by nuclear multigene families and localized in different cellular compartments(Fu et al.,2016). Class CI,CII,and CIII sHSPs localized in the cytosol or nucleus,while other classes localized in the chloroplasts,mitochondria,endoplasmic reticulum(ER),and peroxisomes(Li et al.,2015). Extensive studies have been ca-rried out on the biological functions of these sHSPs.Researches have shown that sHSPs accumulated after chilling stress in diverse plant species such as mulberry(Ukaji et al.,2010),potato(Savic et al.,2012),maize(Wu et al.,2015) and Arabidopsis(Wang et al.,2017). The sHSPs played dual roles in tomato(Lycopersicon esculentum); they protected the photosystem II complex(PSII) from oxidative stress,and they promoted color change during fruit maturation(Huther et al.,2013). The ER localization of sHSPs(ER-sHSP) during heat stress has been reported in pea and soybean(Helm et al.,1993). ER-sHSPs were shown to protect the endomembrane of plants from heat damage(Fu et al.,2016). Previous studies have proposed mechanisms by which ER-sHSPs attenuate chilling stress,including the molecular chaperone activity of ER-sHSPs(McHaourab et al.,2009). 【Research breakthrough point】Although many studies have revealed the functions of various sHSPs,few studies have focused on ER-localized sHSPs. More studies are necessary to understand the general roles of ER-HSPs in the cold and heat acclimation of plants. 【Solving problems】The purpose of this study was to explore the protective effects of an ER-sHSP against chilling stress. CaHSP22.5 is a sHSP from C. annuum L. that shows a high sequence similarity to most reported ER-localized sHSPs. Several parameters were evaluated in transgenic and wild-type tobacco plants under chilling stress,including chlorophyll fluorescence,the accumulation of hydrogen peroxide(H2O2) and superoxide radicals([O] ),and the extent of lipid peroxidation. In addition,the molecular chaperone function of CaHSP22.5 was detected in vitro to elucidate the cellular function of this ER-sHSP to provide a theoretical basis for exploring the regulatory mechanism of CaHSP22.5 in plant stress resistance.
1 Materials and methods
1. 1 Plant materials and growth conditions
Seeds of sweet pepper(C. annuum L.,line 156) were germinated at 25 ℃ for 3 d on moistened filter paper. Sprouted seeds were planted in plastic pots filled with sterilized soil(20 cm in diameter and 15 cm in height,two plants per pot). The seedlings were grown at 25-30℃/15-20 ℃(day/night temperature regime) under a 14-h photoperiod[300-400 μmol/(m2·s) PFD] in a greenhouse.
The chilling treatments were applied to plants at the 4-leaf stage. After the chilling treatment,samples of roots,shoots,leaves,flowers and fruit were collected and immediately frozen by liquid nitrogen. All samples were stored at -80 ℃ until analysis. Details of the experiments have been described by Guo et al.(2007).
1. 2 Isolation and sequencing of CaHSP22.5 gene from sweet pepper
Total RNA was isolated from sweet pepper leaves using an RNeasy Plant Mini Kit(Qiagen). The total RNA was amplified by reverse transcription polymerase chain reaction(RT-PCR) and analyzed by RNA gel blot. A total of 2 mg RNA was denatured at 70 ℃ for 5 min and then 2 mL Avian Mye-loblastosis Virus reverse transcriptase(AMV-RT) was added in the reaction system. The transcription reaction mixture was mixed briefly and incubated at 42 ℃ for 1 h. The reaction was terminated by hea-ting to 85 ℃ for 10 min. Degenerate primers were designed based on conserved regions of ER-sHSP sequences from other plants including soybean(Glycine max L.),pea(Pisum sativum L.),potato(Solanum tuberosum),tomato(Lycopersicon esculentum),and Arabidopsis thaliana(Fig.1). The primers for RT-PCR were as follows: forward primer(5'-AAAGA GATC GTATATATCCT-3') and reverse primer(5'-CA AGA ACAAGAATAGCTTG-3').
A 328-bp fragment was amplified from cDNA prepared from sweet pepper leaves. The cDNA amplification product was cloned into the pGEM-T-easy vector(TaKaRa) and sequenced. After sequencing the above specific PCR fragment, 5'- and 3'-RACE(rapid amplification of cDNA ends) were performed to obtain the full-length cDNA with the Gibco-BRL kit(Gibco-BRL).
The putative full-length CaHSP22.5 sequence was amplified from cDNA with the specific primers CaHSP22.5F and CaHSP22.5R. The amplification conditions for PCR were as follows:initial denatura-tion for 5 min at 94 ℃, followed by 35 cycles of 94 ℃ for 50 s, 53 ℃ for 45 s, and 72 ℃ for 80 s. The final extension cycle was 10 min at 72 ℃ and the reaction was terminated by cooling to 4 ℃. All primers were synthesized by the Bioasia Bio-engineering Limited Company. Nucleotide and deduced amino acid sequences were analyzed with DNAMAN version 5.2(Lynnon Biosoft). Sequence data in this article have been deposited in GenBank under the acce-ssion number FJ827067.
1. 3 Vector construction and plant transformation
The full-length CaHSP22.5 cDNA from sweet pepper was sub-cloned into the pBI121 expression vector under the control of the 35S promoter between Sac I and BamH I sites. The recombinant pBI121-CaHSP22.5 expression vector was construc-ted and transformed into Agrobacterium tumefaciens LBA4404. Leaf-disk transformation was performed with wild-type tobacco as previously described(Sun et al., 2013). Thirty-seven individual T1 kanamycin-resistant lines were obtained from tissue culture and confirmed to harbor the transgene by gel blot analysis of genomic DNA and RNA(data not shown). Wild-type plants, the transgenic line with pBI121 vector alone as the control line(CK) and the CaHSP22.5 line(transgenic line over-expressing CaHSP22.5 gene) 13,24,23(random selection) were subjected to RNA and protein gel blot analysis. Four lines(CK,13,24,and 32) were selected for RNA and protein gel blot analyses. and three lines(lines 13,24, and 32) were selected for physiological ana-lysis.
1. 4 Northern and western blotting analysis
In each experiment,20 μg total RNA was analyzed using at least two independent blots. Hybridization was performed as previously described(Guo et al.,2007). The template was the 0.4 kb cDNA fragment at the 5' end of CaHSP22.5. The probe was prepared and labeled with[a-32P] dCTP using the Prime-a-Gene labeling system(Promega). Leaf discs sampled from 10 plants per treatment were ground in liquid nitrogen and the proteins were quantified(Li et al.,2015).
For immuno-blotting,samples with equal amounts of protein were separated by sodium dodecyl sulfate poly-acrylamide gel electrophoresis(SDS-PAGE). For the SDS-PAGE analysis,10% separating gels,4% concentrating gels,and 10% SDS were used. The proteins were transferred to a polyvinylidene difluoride(PVDF) membrane(Millipore) and probed with antibodies against CaHSP22.5(Johnstone,Herbert,Lockhart and Pascoe) and then with a secondary antibody(Johnstone,Herbert,Lockhart and Pascoe). The antibodies against CaHSP and β-actin(Johnstone,Herbert,Lockhart and Pascoe) were obtained from Santa Cruz Biotechnology.
1. 5 Seed germination
Seeds of wild-type and transgenic tobacco(N. tabacum L. cv. NC89) were sterilized in 0.1% mercuric chloride for 10 min,then immersed in full strength Murashige and Skoog(MS) medium and stratified at 4 ℃ for 2 d in the dark prior to germination. Seedlings were grown in a growth chamber at 25 ℃ under a 16 h light/8 h dark[450 μmol/(m2·s)]photoperiod. At least 100 seeds of each genotype were sterilized and sown on MS medium. The germination results were calculated based on at least three independent experiments.
Six well-developed tobacco plants were transplanted into plastic pots filled with sand. The plants were cultured with half-strength Hoagland nutrient solution for the following experiments.
1. 6 Fluorescence measurements
A pulse amplitude modulated leaf chamber fluorometer(Li-6400,Li-Cor,Inc). was used to measure chlorophyll fluorescence. The light-adapted and dark-adapted chlorophyll fluorescence for gas exchange were measured as described elsewhere(He et al.,2009). F0(minimal fluorescence in the dark-adapted state) was measured with a low-intensity red light source(630 nm) and Fm(maximal fluorescence) was measured with a saturating light pulse of 8000 μmol/(m2·s).
The maximum quantum use efficiency of PSII in the dark-adapted state was calculated using the formula: Fv/Fm=(Fm-F0)/Fm. The leaf was dark-adap-ted for at least 15 min prior to measuring Fv/Fm. F'0 was the minimal value of fluorescence in the light-adapted state at 800 μmol(m2·s); F'm was the maximum value of fluorescence in the light-adapted state at 800 μmol/(m2·s); Fs was steady-state fluorescence,and was recorded after continuously illuminating the leaves with actinic light for 8 min. The following ratios were calculated: effective quantum use efficiency of PSII in the light-adapted state,F'v/F'm=(F'm–F'o)/F'm; effective quantum yield,ΦPSII=(F'm–Fs)/F'm; photochemical quenching,qP=(F'm–Fs)/F'm–F'0; and non-photochemical quenching,NPQ=(Fm–F'm)/F'm.
1. 7 Analysis of H2O2 and [O]
H2O2 was quantified using the titanium hydro-peroxide complex method(Mukherjee and Choudharr,1983). Fresh leaves(1 g) were frozen in liquid nitrogen and then immersed in 10 mL cooled acetone at 10 ℃. Then,4 mL titanium reagent and 5 mL ammonium solution were added to the mixture to precipitate the titanium hydro-peroxide complex. The reaction mixture was centrifuged at 10000×g for 10 min,and the precipitate was dissolved in 10 mL 2 mol/L H2SO4 and re-centrifuged. The absorbance of the supernatant was measured at 415 nm against a blank titanium reagent with a UV-visible light spectrophotometer (Model M 36,Beck). The [O]concentration was determined as described previously(Jardine et al.,2013). The [O]concentration was determined by monitoring nitrite formation from hydroxylamine in the presence of [O] . The absorbance of the mixture was determined at 530 nm.
ROS-scavenging enzymes was extracted follo-wing the method of Mukherjee and Choudharr(1983),extraction of ROS-scavenging enzymes was done from 1 g leaf tissue with 10 mL of phosphate buffer(pH 6.5).
1. 8 Molecular chaperone activity assays
The molecular chaperone activity of CaHSP22.5 was monitored by evaluating the refolding of denatured citrate synthetase(CS,Sigma-Aldrich)(Lee et al.,1995). The CS activity assay was carried out at 25 ℃ as reported elsewhere(Ukaji et al.,2010). The CS(1.5 μmol/L) was denatured for 1.5 h at 25 ℃,in a solution of 6 mol/L guanidine hydrochloride,2 mmol/L DTT,and 100 mmol/L Tris-HCl(pH 8.0). During the refolding process,2.5 μL denatured CS was diluted with 247.5 μL refolding solution containing different amounts of CaHSP22.5. CaHSP22.5 was purified by gst-tag protein purification kit(Merck).
For thermal inactivation experiments,150 nmol/L CS was incubated in 50 mmol/L HEPES-KOH(pH 8.0)in the absence or presence of 150 nmol/L CaHSP22.5. The CS was incubated at 22 ℃ and then CS was incubated at 42 ℃ for 60 min in the absence or presence of CaHSP22.5(150 nmol/L or 450 nmol/L),finally it was transferred to 4 ℃. At various time points,25 μL aliquots were taken to assay CS activity.
1. 9 Statistical analysis
The data were subjected to one-way analysis of variance(ANOVA) using SPSS 16.0. Differences among mean values were compared with Duncans test,and were considered significant at P<0.05. Each data point represented mean ± SE(n=3).
2 Results and analysis
2. 1 Isolation and characterization of cDNA of CaHSP22.5 gene from sweet pepper
The full-length sequence of CaHSP22.5 gene was 807 bp,and the complete open reading frame(ORF) sequence was 603 bp(position 50-653). This gene was designated as CaHSP22.5(GenBank accession number:FJ827067).
The CaHSP22.5 sequence encoded a polypeptide of approximately 21 kD. The deduced amino acid sequence of CaHSP22.5 showed high homology to other ER-localized sHSPs,including PsHSP22.7 from pea(M33898),GmHSP22.0 from soybean(X63198),StHSPI19 from potato(S70186),AtHSP22.0 from Arabidopsis(U11501),and LeHSP21.5 from tomato (AB026983)(Fig.1 and Fig.2). Three conserved motifs(blocks I-III) were revealed by analyzing the amino acid sequence(Fig.1). Block I was the putative ER signal sequence in the N-terminal region; block II with PGL and RGP regions showed high homology with sequences in other sHSPs; block III was a putative ER retention signal sequence in the C-terminal region.
A phylogenetic tree was constructed to elucidate the evolutionary relationships among ER-sHSPs. Full-length ER-sHSPs from representative higher plants were analyzed. DNAMAN software was used to draw the phylogenic tree. The results demonstrated the evolutionary relationships among the following six ER-sHSPs:Ca-ERsHSP from sweet pepper,LeHSP21.5 from tomato,StC119 from potato,AtHSP22.0 from Arabidopsis,GmHSP22.0 from soybean,and PsHSP22.7 from pea. Through the phylogenetic tree,it could be found that the deduced amino acid sequence of CaHSP22.5 showed high simila-rity to LeHSP21.5 from tomato and StC119 from potato,and the bootstrap value(76%) showed that the phylogenetic tree was more reliable(Fig.2).
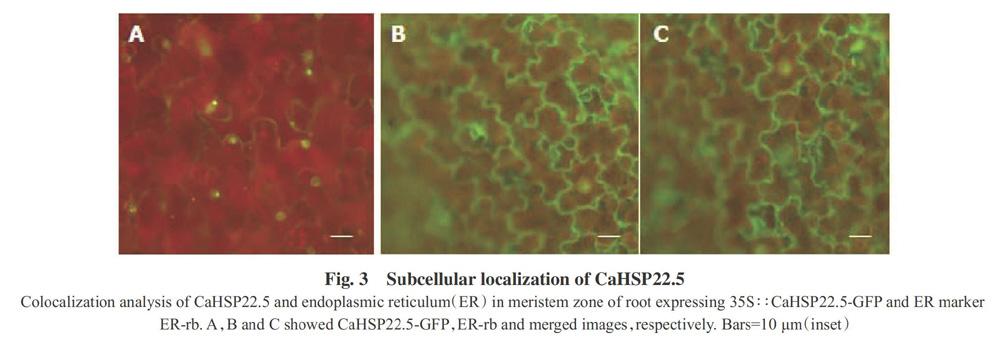
To confirm whether CaHSP22.5 was an ER-localized protein,the subcellular localization of CaHSP22.5 was determined in the root of transgenic sweet pepper expressing 35S∶∶CaHSP22.5-GFP by staining with ER-Tracker Red(Invitrogen,catalog No. E34250)(Fig.3). The 35S∶∶CaHSP22.5-GFP construct was introduced into sweet pepper by Agrobacterium tumefaciens-mediated infiltration,and the transformed tissue was examined by confocal microscopy(Fig.3). It was found that CaHSP22.5 showed ER localization.
2. 2 Expression of CaHSP22.5 gene in sweet pe-pper during heat stress
Sweet pepper samples were subjected to a heat treatment(42 ℃),and calyxes,leaves,stems,roots and green fruits were collected for analysis. These tissues were analyzed by RNA blotting to determine the expression pattern of CaHSP22.5 gene(Fig.4). The transcripts were relatively more abundant in photosynthetic tissues(leaves and fruits) of plants subjected to the heat treatment. These tissues were used in the following northern blot analysis.
Samples of sweet pepper leaves for RNA extraction were exposed to 42 ℃ in the light for 16 h and then in the dark for 8 h(Fig.5). The transcript level of CaHSP22.5 gene peaked at 8 h and gradua-lly decreased thereafter during the light period. Total protein was extracted from the leaves after exposure to 42 °C over the same diurnal cycle. The proteins were analyzed by western blotting with antibodies against CaHSP22.5. The results of the western blotting analysis showed the same trend as that of the nor-thern blotting analysis(Fig.6).
2. 3 Enhanced chilling tolerance of transgenic tobacco over-expressing CaHSP22.5 gene
There were no obvious morphological differen-ces between wild-type and transgenic plants. A strong positive signal was detected in the transgenic line over-expressing CaHSP22.5 gene,while weak signals were detected in wild-type plants and CK(Fig.7). In the protein gel blots,strong protein signals were detected in all transgenic tobacco lines over-expressing CaHSP22.5 gene,and a weak signal was detected in wild-type plants(Fig.8).
The effects of CaHSP22.5 to protect transgenic tobacco against chilling stress was further analyzed. The ratio of seed germination and percentage of plant survival were calculated(Table 1). Seed germination was not significantly different between the transgenic lines(line 13,line 24 and line 32) and wild-type after a chilling treatment(4 ℃ for 24 h). The survival rate of the transgenic and wild-type tobacco plants under chilling stress was also measured. Four-week-old CaHSP22.5 transgenic and wild-type tobacco plants were grown on medium containing 50 mg/L kanamycin,and then the plants were exposed to 4 ℃ for 6 h(Fig.9). The average survival percen-tages of line 13,line 24 and line 32 seedlings was 84%,75% and 52%,while that of the wild-type plants was 30%.
2. 4 Enhanced photosynthesis of transgenic tobacco overexpressing CaHSP22.5 gene under chilling stress
The influence of CaHSP22.5 on photosynthesis was investigated in wild-type and transgenic tobacco lines(line 13,line 24,and line 32) during chilling stress. The measured parameters included Fv/Fm,qP,F'v/F'm,ΦPSII,and ΦNPQ.
All the plants were treated at 4 ℃ for 10 h. The differences in Fv/Fm and qP between wild-type and transgenic lines were not significant(P>0.05,the same below)(Fig.10-A and Fig.10-B). F'v/F'm and ΦPSII significantly decreased in transgenic lines during the 10-h chilling treatment(P<0.05,the same below),especially in line 13. There was a much greater decrease in F'v/F'm and ΦPSII in wild-type plants than in transgenic plants(Fig.10-C and Fig.10-D). During low temperature stress,the significant decrease in ΦPSII of lines 13,24 and 32 compared with wild type may have resulted from the down-regulation of PSII electron transport in vivo(Fig.10-D). The down-regulation of PSII was associated with a decrease in F'v/F'm(Fig.10-C). The parameter of qP did not differ significantly between transgenic lines and wild type under chilling stress (Fig.10-B). The NPQ significantly increased in transgenic lines under chilling stress(Fig.10-E).
2. 5 Reduced reactive oxygen species accumulation and alleviated lipid peroxidation in transgenic tobacco over-expressing CaHSP22.5 gene under chilling stress
Chilling stress led to an increase in reactive oxy-gen species(ROS) production. Compared with wild-type,the transgenic tobacco lines showed lower ROS (H2O2,[O] ) accumulation(Fig.11). These results suggested that the generation of ROS was alleviated in plants over-expressing CaHSP22.5 gene during chil-ling(4 ℃ for 10 h). Measured the ability of exogenous CaHSP22.5 to degrade ROS(H2O2,[O] ). Some ROS were degraded without enzymes(H2O+H2O2), because ROS was unstable(Fig.12-A). In vitro,ROS were not directly cleared by exogenous CaHSP22.5. However,the concentrations of ROS were decreased more by enzymes extracted from transgenic lines than by enzymes extracted from wild-type plants (Fig.12-B). These results suggested that CaHSP22.5 may indirectly clear ROS in vitro by regulating the relevant enzymes.
2. 6 Molecular chaperone activity of CaHSP22.5 in vitro
CaHSP22.5 might serve as a molecular chapero-ne in the resistance response to chilling stress. When 150 nmol/L and 450 nmol/L CaHSP22.5 was added to 150 nmol/L CS monomer,the activity of CS recovered to approximately 15% and 20%,respectively(Fig.13-A). The refolded activity of CS was 9% in the control. These results showed that only a small proportion of CS could be reactivated in the absence of CaHSP22.5(Fig.13-A).
To further analyze the molecular chaperone function of CaHSP22.5,a CS thermal inactivation experiment was performed. When CS was incubated at 45 ℃ alone,CS activity rapidly decreased to below 15% of maximum activity in 20 min(Fig.13-B). However,with the addition of 150 nmol/L or 450 nmol/L CaHSP22.5,CS activity after the heat and cold treatment was approximately 50% and 55% of the maximum in 20 min,respectively(Fig.13-B). After the addition of CaHSP22.5,there was no significant difference in CS activity after subsequent heat inactivation at 45 °C for 60 min. The heat-denatured CS was then treated at 4 ℃. Samples to which 150 nmol/L or 450 nmol/L CaHSP22.5 was added showed CS activity recovered to 70%(150 nmol/L) and 80% (450 nmol/L) of the maximum. In the sample without CaHSP22.5,the activity of heat-denatured CS recovered to only approximately 60% of the maximum activity.
At indicated times,CS activity was assayed as described in Materials and Method section. Data were mean values±SE of three individual experiments. Asterisks indicated significant differences among mean values.
3 Discussion
CaHSP22.5 is an ER-localized sHSP in sweet pepper. The deduced amino acid sequence of CaHSP22.5 showed high sequence similarity with other reported ER-localized sHSPs,especially in the conserved regions. Previous studies on various sHSPs from different species have shown that they were characterized by a core α-crystallin domain of 100 amino acids(Wadhwa et al.,2010),which was flanked by other peptides,including a transit peptide of variable length at the N-terminal arm,a divergent sequence,and a putative ER-retention signal KDEL in the C-terminal region(Fujikawa et al.,2006). The analysis in the paper on the CaHSP22.5 sequence indicated that the SSL region in block I was the putative ER-localization sequence; and that the KDEL region in block III was a putative ER-retention signal sequence. In further studies,the specific localization of CaHSP22.5 in the ER should be further investigated by immuno-electron microscopy.
A phylogenetic tree was constructed to compare CaHSP22.5 with ER-sHSP proteins from other dicots. CaHSP22.5 showed 76% homology to LeHSP21.5(from tomato) and to StCI19(from potato). These results indicated that CaHSP22.5 belonged to the nightshade ER-sHSP protein family.
Some studies have reported that sHSPs were induced by chilling(4 ℃). For example,CP sHSP was expressed in tomato under low temperature(Huther et al.,2013). The research group has been interested in genes related to high temperature and chilling stress in plants,and identified the expression patterns of some specific chilling-resistance genes in previous studies(Guo et al.,2007;Li et al.,2014). Analysis on the spatiotemporal expression pattern of CaHSP22.5 during chilling stress showed that its expression level was higher in leaves and fruit than in stems,flowers,and roots. These results indicated that CaHSP22.5 played a role in green organs. In the leaves of sweet pepper,the mRNA level of CaHSP22.5 was up-regulated under chilling stress. However,the protein expression level of CaHSP22.5 fluctuated: it was abundant during the first 12 h at 4 ℃ and gradually decreased during the last 12 h. There was a difference between transcript abundance and the protein level of CaHSP22.5 in sweet pepper leaves.
To further analyze the functions of CaHSP22.5,the full-length cDNA of CaHSP22.5 was introduced into tobacco plants,and the transgenic lines were subjected to northern and western blot analysis. The results showed that CaHSP22.5 was expressed in transgenic tobacco lines and the level of HSP was higher than that in wild-type plants. The abundance of newly synthesized CaHSP22.5 increased as a result of the over-expression of CaHSP22.5 gene.
The activity of sHSP may have increased accordingly. The results showed that the total protein level in leaves was not significantly different between transgenic tobacco lines and wild-type plants. Both the CaHSP22.5 transcript abundance and CaHSP22.5 protein level increased in transgenic tobacco leaves under chilling stress,suggesting that CaHSP22.5 was involved in the response to chilling stress. The percentage of plant survival was higher in transgenic plants than in wild-type plants under chilling stress. These results further confirmed that CaHSP22.5 could protect transgenic tobacco plants against chilling stress.
CaHSP22.5 might participate in a variety of processes to protect plants against chilling damage. In this study,the mRNA hybridization signal of CaHSP22.5 was found in both shoots and roots of sweet pepper,indicating that CaHSP22.5 may also be expressed in non-photosynthetic tissue. These findings suggested that ER-sHSP might not only protect photosynthesis,but also other process in wild-type sweet pepper.
Previous studies have shown that sHSPs played a vital role in the photo-protection of both PSII and PSI(Huther et al.,2013). In the present study,transgenic and wild-type plants showed differences in the activities of PSII electron transport under chilling stress,as determined by measuring chlorophyll fluorescence in vivo. Chilling stress leads to a decrease in ΦPSII in plants. The increased NPQ of CaHSP22.5 could be higher because of the lower rates of photosynthesis. These results indicated that the photoinhibition of PSII and PSI during chilling stress could be alleviated by the over-expression of CaHSP22.5 in tobacco.
Recent studies have shown that ROS inhibit the repair of photo-inhibited PSII in vivo(Nishiyama et al.,2005). The concentrations of H2O2 and [O]signi-ficantly increased in transgenic and wild-type plants under chilling stress,but to lower levels in transgenic plants than in wild-type plants. The accumulation of ROS in vivo was alleviated by expression of CaHSP22.5 during chilling stress. In addition,the accumulation of CaHSP22.5 in vivo was associated with the repair of PSII after cold-enhanced photo-inhibition. Exogenous CaHSP22.5 could not directly decrease the concentration of ROS in vitro. However,ROS concentrations were greatly decreased by enzymes extracted from transgenic plants,but only slightly reduced by enzymes extracted from wild-type. These results suggested that the accumulation of CaHSP22.5 in transgenic tobaccos was beneficial for protecting the photosynthetic system and for clearing ROS under chilling stress.
It has been speculated that HSPs stabilize proteins to increase resistance to chilling stress. Exportable proteins and membrane proteins were generally produced in association with the ER(Alvim et al.,2001; Koizumi et al.,2001),and ER-localized CaHSP22.5 was proposed to play a vital role in maintaining membrane stability under chilling stress. In future research,the membrane stability in CaHSP22.5-over-expressing transgenic tobacco plants should be further studied.
Only basic information about ER-sHSPs has been reported previously. The ER-sHSPs have not been well studied as ER-localized molecular chaperones specific to plants(Baldwin et al.,2011). The molecular chaperone activity of CaHSP22.5 was tes-ted to facilitate the refolding of CS after heat treatment and chemical treatment. The results were compared those of other studies(Fujikawa et al.,2006;Basha et al.,2012; Zhong et al.,2013). Without CaHSP22.5,little CS reactivation occurred. Howe-ver,the addition of CaHSP22.5 resulted in greater reactivation of CS. It suggested that CaHSP22.5 could act as a molecular chaperone,and this role may be part of the response to chilling stress.
In conclusion,the expression of CaHSP22.5 was correlated with the function of photosystems under chilling stress. Further studies should be conduc-ted to explore the specific protection mechanisms and the sites of interaction between this sHSP and biomolecules in photosystems. Tobacco plants over-expressing CaHSP22.5 showed enhanced tolerance to chilling stress.
4 Conclusion
In this study,CaHSP22.5 gene encoding a sHSP in sweet pepper was introduced into tobacco and the chilling resistance of the transgenic plants was examined. Several parameters indicative of the response to chilling stress were examined in transgenic and wild-type tobacco plants. The results showed that CaHSP22.5 accumulated at the RNA and protein levels in the transgenic plants. The transgenic lines showed alleviation of photo-inhibition of PSII and PSI and reduced ROS accumulation under chilling stress. The molecular chaperone function of CaHSP22.5 was confirmed in vitro.
Acknowledgements:
We thank Jennifer Smith,PhD,from Liwen Bianji,Edanz Group China(www.liwenbianji.cn/ac),for editing the English text of a draft of this manuscript.
References:
Alvim F C,Carolino S M B,Cascardo J C M,Nunes C C,Martinez C A,Otoni W C,Fontes E P B. 2001. Enhanced accumulation of BiP in transgenic plants confers tolerance to water stress[J]. Plant Physiology,126(3):1042-1054.
Baldwin A J,Hilton G R,Lioe H,Bagnéris C,Benesch J L,Kay L E. 2011. Quaternary dynamics of aB-crystallin as a direct consequence of localised tertiary fluctuations in the C-terminus[J]. Journal of Molecular Biology,413(2):310-320.
Basha E,ONeill H,Vierling E. 2012. Small heat shock proteins and α-crystallins: Dynamic proteins with flexible functions[J]. Trends in Biochemical Sciences,37(3):106-117.
Ezemaduka A N,Wang Y B,Li X J. 2017. Expression of CeHSP17 protein in response to heat shock and heavy metal ions[J]. Journal of Nematology,49(3):334-340.
Fu C,Liu X X,Yang W W,Zhao C M,Liu J. 2016. Enhanced salt tolerance in tomato plants constitutively expressing heat-shock protein in the endoplasmic reticulum[J]. Genetics and Molecular Research. doi:10.4238/gmr. 15028301.
Fujikawa S,Ukaji N,Nagao M,Yamane K,Takezawa D,Arakawa K. 2006. Functional role of winter-accumulating proteins from mulberry tree in adaptation to winter-induced stresses[M]//Chen T,Uemura M,Fujikawa S. Cold hardiness in plants. Wallingford: CABI Press:181-202.
Guo S J,Zhou H Y,Zhang X S,Li X G,Meng Q W. 2007. Overexpression of CaHSP26 in transgenic tobacco allevia-tes photoinhibition of PSII and PSI during chilling stress under low irradiance[J]. Journal of Plant Physiology,164(2):126-136.
He Y,Zhu Z J,Yang J,Ni X L,Zhu B. 2009. Grafting increases the salt tolerance of tomato by improvement of photosynthesis and enhancement of antioxidant enzymes activity[J]. Environmental and Experimental Botany,66(2):270-278.
Helm K W,LaFayette P R,Nagao R T,Key J L,Vierling E. 1993. Localization of small heat shock proteins to the higher plant endomembrane system[J]. Molecular and Cellular Biology,13(1):238-247.
Huther C M,Ramm A,Rombaldi C V,Bacarin M A. 2013. Physiological response to heat stress of tomato ‘Micro-Tom plants expression high and low levels of mitochondrial sHSP23.6 protein [J]. Plant Growth Regulation,70(2):175-185.
Jardine K J,Meyers K,Abrell L,Abrell L,Alves E G,Yanez Serrano A M,Kesselmeier J,Karl T,Guenther A,Chambers J Q,Vickers C. 2013. Emissions of putative isoprene oxidation products from mango branches under abio-tic stress[J]. Journal of Experimental Botany,64(12):3697-3708.
Koizumi N,Martinez I M,Kimata Y,Kohno K,Sano H,Chrispeels M J. 2001. Molecular characterization of two Arabidopsis Ire1 homologs,endoplasmic reticulum-loca-ted transmembrane protein kinases[J]. Plant Physiology,127(3): 949-962.
Kumar R R,Goswami S,Singh K,Dubey K,Rai G K,Singh B,Singh S,Grover M,Mishra D,Kumar S,Bakshi S,Rai A,Pathak H,Chinnusamy V,Praveen S. 2018. Characteri-zation of novel heat-responsive transcription factor (TaHSFA6e) gene involved in regulation of heat shock proteins(HSPs)-A key member of heat stress-tolerance network of wheat[J]. Journal of Biotechnology,279: 1-12.
Lambert W,Koeck P J,Ahrman E,Purhonen P,Cheng K,Elmlund D,Hebert H,Emanuelsson C. 2011. Subunit arrangement in the dodecameric chloroplast small heat shock protein Hsp21[J]. Protein Science,20(2):291-301.
Lee G J,Pokala N,Vierling E. 1995. Structure and in vitro molecular chaperone activity of cytosolic small heat shock proteins from pea[J]. Journal of Biological Che-mistry,270(18):10432-10438.
Li Y J,Wang B,Dong R R,Hou B K. 2015. AtUGT76C2,an Arabidopsis cytokinin glycosyltransferase is involed in drought stress adaptation[J]. Plant Science,236:157-167.
Li M F,Guo S J,Xu Y,Meng Q W,Li G,Yang X H. 2014. Glycine betaine-mediated potentiation of HSP gene expression involves calcium signaling pathways in tobacco exposed to NaCl stress[J]. Physiologia Plantarum,150(1):63-75.
Lopes-Caitar V S,de Carvalho M C,Darben L M,Kuwahara M K,Nepomuceno A L,Dias W P,Abdelnoor R V,Marcelino-Guimar?es F C. 2013. Genome-wide analysis of the Hsp20 gene family in soybean: Comprehensive sequence,genomic organization and expression profile ana-lysis under abiotic and biotic stresses[J]. BMC Geno-mics, 14(1):577.
McHaourab H S,Godar J A,Stewart P L. 2009. Structure and mechanism of protein stability sensors: Chaperone activity of small heat shock proteins[J]. Biochemistry,48(18): 3828-3837.
Mukherjee S P,Choudhari M A. 1983. Implications of water stress induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in vigna seedlings[J].Physiologia Plantarum,58(2): 166-170.
Nishiyama Y,Allakhverdiev S I,Murata N. 2005. Inhibition of the repair of photosystem II by oxidative stress in cyanobacteria [J]. Photosynthesis Research,84(1-3):1-7.
Savic J,Dragicevic I,Pantelic D,Oljaca J,Momcilovic I. 2012. Expression of small heat shock proteins and heat tolerance in potato(Solanum tuberosum L)[J]. Archives of Biological Sciences,64(1): 135-144.
Shukla V,Upadhyay R K,Tucker M L,Giovannoni J J,Rudrabhatla S V,Mattoo A K. 2017. Transient regulation of three clustered tomato class-I small heat-shock chaperone genes by ethylene is mediated by SIMADS-RIN transcription factor[J]. Scientific Reports,7(1):6474
Sun Y G,Wang B,Jin S H,Qu X X,Li Y J,Hou B K. 2013. Ectopic expression of Arabidopsis glycosyltransferase UGT85A5 enhances salt stress tolerance in tobacco[J]. PLoS One,8(3):e59924.
Ukaji N,Kuwabara C,Kanno Y,Mitsunori S,Takezawa D,Arakawa K,Fujikawa S. 2010. Endoplasmic reticulum-localized small heat shock protein that accumulates in mulberry tree(Morus bombycis Koidz.) during seasonal cold acclimation is responsive to abscisic acid[J]. Tree Physio-logy,30(4): 502-513.
Wadhwa R,Ryu J,Gao R,Choi I K,Morrow G,Kaur K,Kim I,Kaul S C,Yun C O,Tanguay R M. 2010. Proproliferative functions of drosophila small mitochondrial heat shock protein 22 in human cells[J]. The Journal of Biological Chemistry,285(6): 3833-3839.
Wang M L,Zou Z W,Li Q H,Xin H H,Zhu X J,Chen X,Li X H. 2017. Heterologous expression of three Camellia sinensis small heat shock protein genes confers temperature stress tolerance in yeast and Arabidopsis thaliana [J]. Plant Cell Reports,36(7):1125-1135.
Wu X L,Gong F P,Yang L,Hu X L,Tai F T,Wang W. 2015. Proteomic analysis reveals differential accumulation of small heat shock proteins and late embryogenesis abundant proteins between ABA-deficient mutant Vp5 seeds and wild-type Vp5 seeds in maize[J]. Frontiers in Plant Science,5:801.
Zhong L L,Zhou W,Wang H J,Ding S H,Lu Q T,Wen X G,Peng L W,Zhang L X,Lu C M. 2013. Chloroplast small heat shock protein HSP21 interacts with plastid nucleoid protein pTAC5 and is essential for chloroplast development in Arabidopsis under heat stress[J]. The Plant Cell,25(8):2925-2943.
(責任编辑 陈德元)
