Hydrothermal evolution and source of metallogenic materials of Beiya Au polymetallic deposit,western Yunnan, China
2020-07-07DENGJiafangSUNFengyueXINWeiXUZhihePANZhongcuiWUDongqianandTIANNan
DENG Jiafang, SUN Fengyue, XIN Wei, XU Zhihe, PAN Zhongcui,WU Dongqian and TIAN Nan
College of Earth Sciences, Jilin University, Changchun 130061, China
Abstract: The Beiya porphyry-skarn gold-polymetallic deposit is one of the largest gold deposits in China and it also contains significant amounts of silver and base metals. The ore-bearing monzonitic granite porphyry occurs as a stock, of which the skarn type gold-copper-iron ore bodies are controlled by the contact zone between alkali-rich monzonitic granite porphyry and the limestone, and the gold-silver polymetallic mineralization is controlled by interlayer structure. Alteration and mineralization occur around the intrusion and exterior of monzonitic granite porphyry. Ore mineral formation sequence is as follows: skarn minerals→magnetite→pyrite→chalcopyrite/bornite+pyrite+gold→pyrite+galena+gold (silver). Petrographic studies of fluid inclusions indicate that the following types of inclusions exist in the pre-mineralization quartz-pyrite stage: gas-liquid two-phase inclusions (L-type), three-phase inclusions with daughter minerals (D-type) and gas-rich inclusions (V-type). The colorless transparent quartz in the main gold-chalcopyrite-pyrite stage mainly consists of L-type and V-type inclusions, whereas the inclusions in the late gold-silver-galena stage are mainly L-type. The evolution of ore-forming fluids shows a trend from high temperature, high salinity to medium-low temperature and low salinity. Medium-low density fluids play a dominant role in mineral component migration and transportation. Fluid cooling and boiling are the main mechanisms of gold-copper precipitation, while the involvement of atmospheric water and pH reduction are the main mechanisms of gold-silver polymetallic precipitation. The fluids in the quartz-pyrite stage before mineralization and the main gold-chalcopyrite-pyrite stage are dominated by magmatic water, while in the gold-silver-galena stage the fluids are dominated by atmospheric water. Isotope tracers show that S and Pb are mainly derived from monzonitic granite porphyry, not from limestone of the Beiya Formation.
Keywords: Beiya; Au polymetallic deposit;hydrothermal evolution; porphyry-skarn deposit
0 Introduction
Porphyry-skarn deposits are one of the most important types of deposits with great economic significance (Cookeetal., 2005; Sillitoe, 2010), containing about 80% of the world’s copper resources, 95% of Mo resources, and 30% of Au, and they are also important sources of Ag, Zn, Sn and W. As a typical super-large skarn-type gold polymetallic deposit, Beiya deposit has been mined since 2000, and currently has proven ore reserves of 130 million metric tons (Mt), with grades of 2.42 g/t for Au, 0.48% for Cu, 25.5% for Fe, 38.85 g/t for Ag, 1.24% for Pb, and 0.53% for Zn (Heetal., 2015), therefore, it is of great theoretical significance in scientific research. Although a lot of research results have been published, the evolution of ore-forming fluids is still controversial (Heetal., 2017; Liuetal., 2018), which is mainly due to the insufficient temperature measurement data of fluid inclusions during the whole sulfide stage. Many researchers believe that the sulfur in skarn deposits is magmatic origin (Ault, 2004; Kamvong & Zaw, 2009), but some researchers argue for the derivation of sulfur from the host carbonate rocks (Ishiharaetal., 2000, 2002). Therefore, the source of ore-forming materials still needs more data constraints, and lead isotope study is very important to determine the source of metallogenic metals. Is porphyry the only source of metallogenic metals? Does Beiya Formation limestone contribute to mineralization? These problems are very important for understanding the source of ore-forming materials in Beiya gold polymetallic deposit.
In this study, the fluid inclusion petrography and microthermometry for pyrite-bearing fume-gray quartz veins, chalcopyrite-pyrite veins, lead-zinc mineralized veins in surrounding strata are studied respectively, so that we can delineate the complete fluid evolution process from pre-metallogenic to main metallogenic period, and to provide new constraints on the source of metallogenic materials.
1 Geological setting
The Beiya gold polymetallic deposit is located in the central of the Jinshajiang--Ailaoshan magmatic belt, which extends along the western margin of the Yangtze Craton (Fig.1a). The Pliocene--Oligocene potassium-ultrapotassic magmatic belt extends more than 2 000 kilometers along the Jinshajiang--Ailaoshan tectonic belt and is closely related to a series of porphyry-skarn deposits including Beiya gold deposit in the region (Heetal., 2016). Felsic intrusive rocks in the northern part of Jinshajiang--Ailaoshan tectonic belt are scattered near Yulong in the northern part, Weixi--Dali in the central part (Fig.1b), and Luchun--Jinping in the southern part. Zircon U-Pb dating data show that the intrusion ages of potassic/ultrapotassic igneous rocks in the central and southern segments are between 37--32 Ma (Luetal., 2012, Huangetal., 2010), which is obviously later than the crystallization age of 41--36 Ma for potassic igneous rocks in Yulong belt.(Ruietal., 1984; Houetal., 2003). The tectonic-magmatic-metallogenic mechanism of this metallogenic belt was controlled by the collision between Indian and Asian plates during Cenozoic.
1.1 Ore deposit geology
Beiya gold polymetallic deposit is composed of six ore occurrences, namely Wandongshan, Hongnitang and Jingouba in the west, and Weiganpo, Bijia-shan and Guogaishan in the east (this paper focuses on Wandongshan). The strata of the mining area mainly consist of the Late Permian Emeishan Formation basalt, overlain by sandstone and breccia of Lower Triassic Qintianbao Formation and limestone of Beiya Formation (Fig.2a). Beiya Formation limestone is the main host rock for precious metal mineralization. Metallic skarn usually occurs in the contact area between limestone and monzonitic granite porphyry (Fig.2b). Small interbed structural zones and fault zones are widely developed in carbonate rocks, which are favorable for gold polymetallic precipitation. Extensive Quaternary sediments are distributed around the valleys.
The main structure of Beiya area comprises the Ma’anshan sub-fault (branch of Jinshajiang--Honghe strike-slip fault) and the NS-trending Beiya syncline (branch of Songgui syncline). Several ore occurrences of Beiya deposit are located on the east and west sides of the Beiya syncline axis (Fig.2a).
A large number of feldspathic and mafic rocks are exposed in this area. Magmatism can be divided into two stages: Emeishan continental flood basalt was formed about 260 Ma, and potassic/ultrapotassic igneous rocks intruded during 37--34 Ma including emplacement of large number of monzonitic granite porphyries, quartz syenite porphyries and syenite porphyries and lamprophyre dike swarms (Heetal., 2013, 2014). In Beiya mining area, monzonitic granite porphyry is cut by younger lamprophyre and biotite monzonitic granite porphyries (Fig.2b). The widely occurred monzonitic granite porphyry intrusions are controlled by the NS-trending regional fault system, and intrude into carbonate rocks. The outcrops of these porphyries mostly occur as dikes and stocks of about 36 Ma, which are closely related to the mineralization.

Fig.1 Distribution of potassic igneous rocks in southwestern margin of Yangtze Craton (a) and tectonic location of the studied area in Southwest China (b) (after Deng et al., 2016)
The biotite granite porphyries and lamprophyre dikes cut across monzonitic granite porphyry and orebodies, which have no genetic relationship with mineralization. The monzonitic granite porphyry intrusions are widely distri-buted and controlled by the NS-trending regional fault system. These porphyries intrude into carbonate rocks.
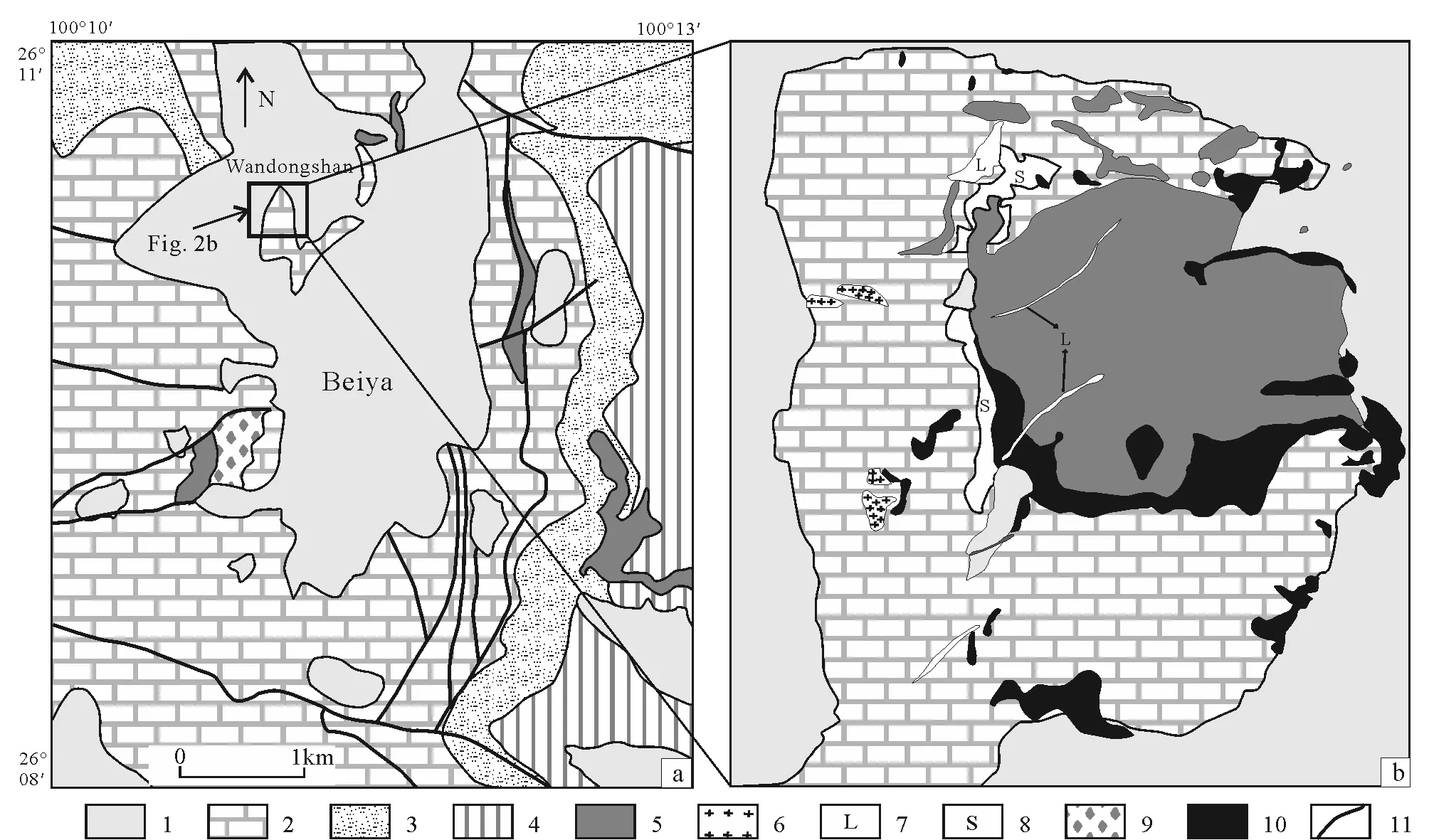
1.Quaternary; 2.limestone of Middle Triassic Beiya Formation; 3.Lower Triassic classic rock series; 4.Upper Permian basalts; 5.monzonitic granitic porphyry; 6.biotite granite porphyry; 7.lamprophyre; 8.skarn; 9.hydrothermal breccia; 10.Au-Cu orebody; 11.faults.Fig.2 Geological maps of Beiya ore field (a) and Wandongshan mining area (b) (after Dong, 2018)
1.2 Mineralization
There are three types of primary orebodies in Beiya deposit, namely porphyry type, skarn type and hydrothermal vein type. The lateritic supergene gold mineralization may be formed by secondary weathering and leaching of primary ore. All mineralization types are closely related to alkali-rich porphyry intrusions spatially. Among them, skarn type ore bodies are the most important (Table 1).
Skarn ore body is located near the contact zone between the porphyry and Upper Triassic limestone (Fig.3). Gangue minerals are composed of garnet, diopside, tremolite, epidote, chlorite, actinolite, tremolite, talc, calcite and sericite. The main ore minerals include magnetite (Fig.4a), chalcopyrite/porphyry (Fig.4b), pyrite, galena, sphalerite and natural gold.
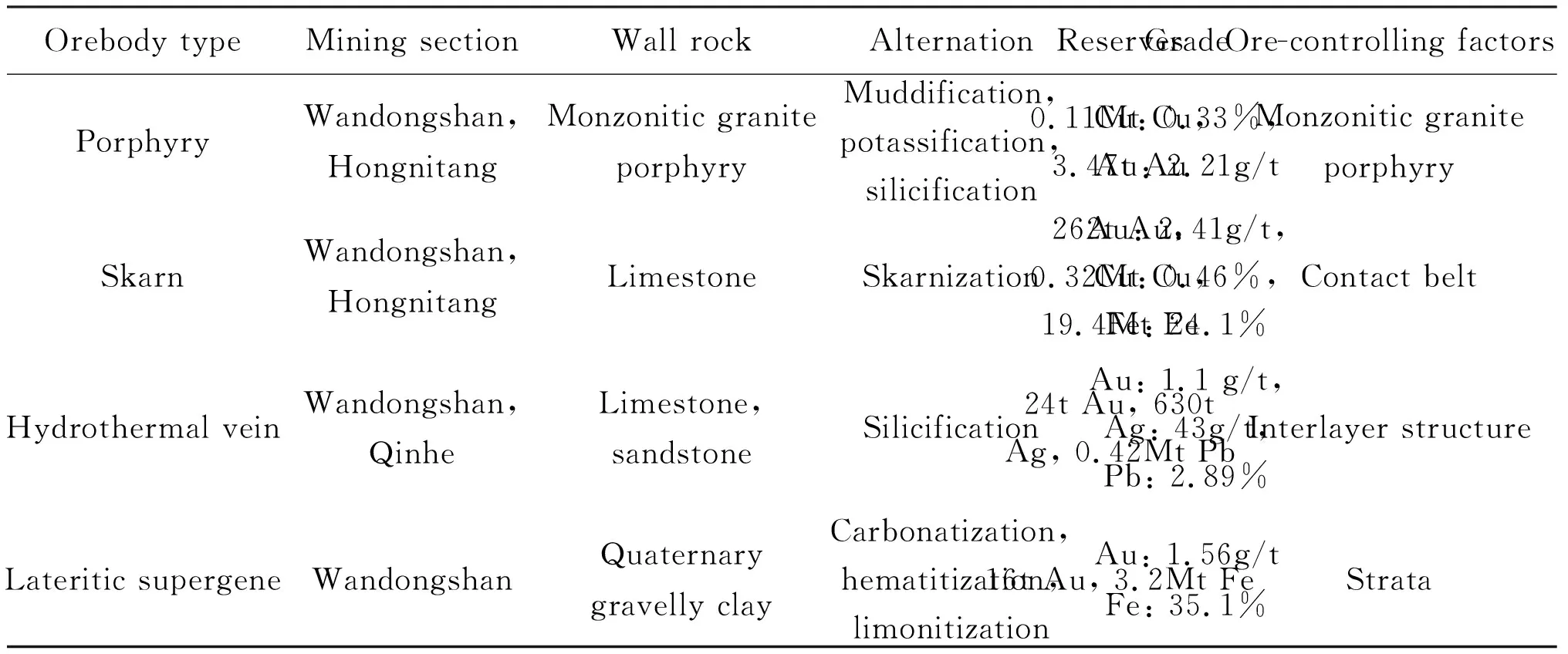
Table 1 Various types of orebodies and characteristics of Beiya gold polymetallic deposit (after Dong, 2018)

Fig.3 Profile of No.72 exploration line in Wandongshan mining area of Beiya deposit(after Dong, 2018)
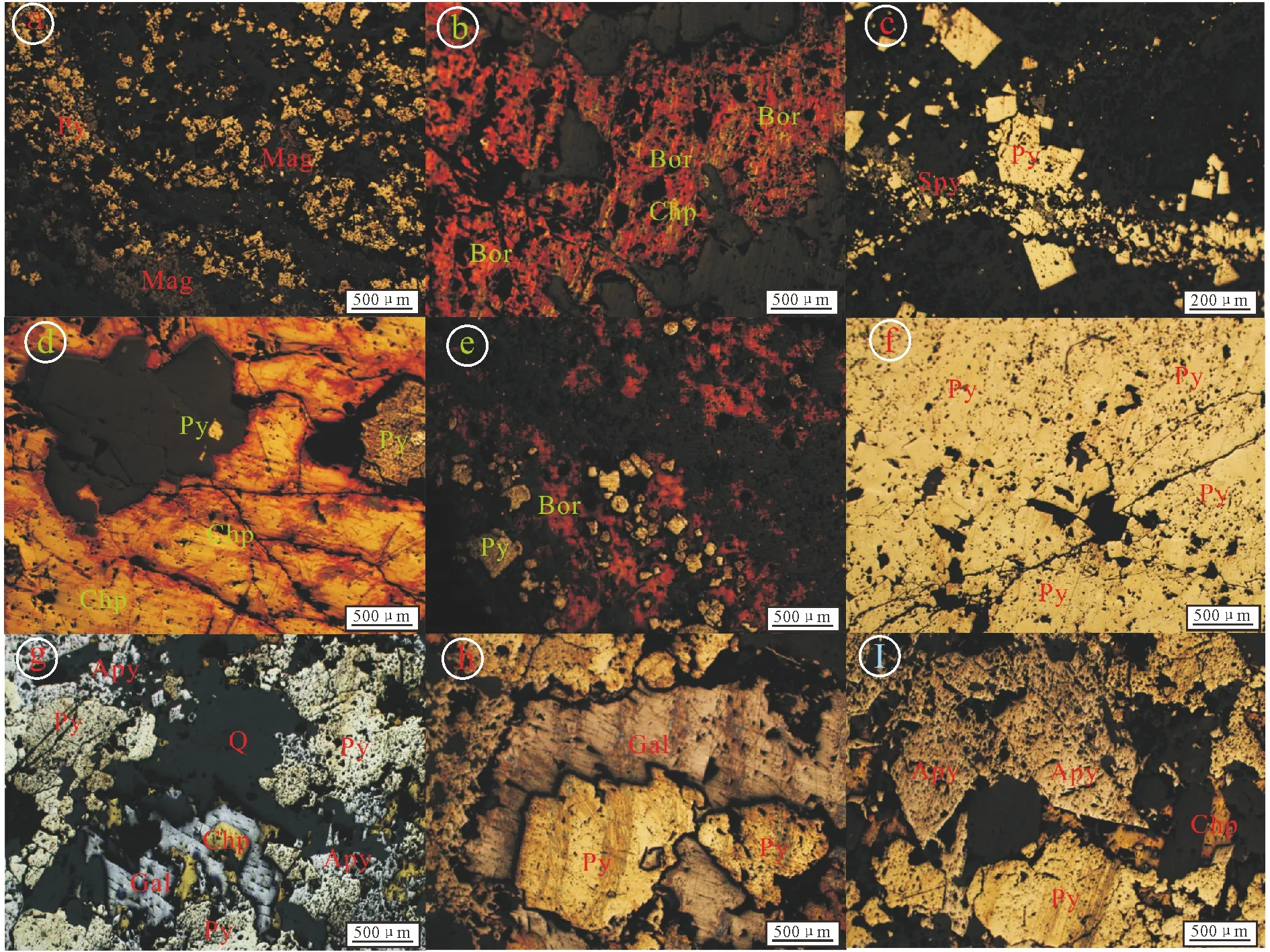
(a) Pyrite and magnetite automorphic-semi-automorphic texture; (b) solid solution exsolution of porphyrite and chalcopyrite; (c) pyrite metasomatized by sphalerite; (d) solid solution exsolution of chalcopyrite and porphyrite; (e) pyrite metasomatized by bornite; (f) massive pyrite; (g) pyrite metasomatized by galena; (h) pyrite metasomatized by galena; (i) automorphic-semi-automorphic texture of arsenopyrite and pyrite; Mag- magnetite; Py- pyrite; Apy- arsenopyrite; Qz- quartz; Spy- sphalerite; Chp- chalcopyrite; Bor- bornite; Gal- galena. Fig.4 Photomicrographs of ore textures in Beiya gold polymetallic deposit
Porphyry gold-copper-iron orebodies occur in primary cooling and shear cracks of intrusive bodies, in the form of disseminated, or veined pyrite (Fig.4c,f) and chalcopyrite/bornite ores (Fig.4d,e). The main ore minerals are chalcopyrite, porphyry and pyrite, and small amount of magnetite and molybdenite. The gangue minerals comprise quartz, potassium feldspar, sericite, chlorite and plagioclase.
Structure-controlled hydrothermal vein-type lead, gold and silver orebodies are distributed in the faults in the Beiya Formation or the interlayer structural fractures near the contact zone between Beiya Formation and Qintianbao Formation. Mineral assemblages include galena (Fig.4g,h), sphalerite, pyrite, chalcopyrite, arsenopyrite (Fig.4i) and limonite. Gangue minerals are quartz, siderite, calcite and dolomite.
Secondary/supergene mineralization (lateritic mi-neralization): secondary mineralization is formed by weathering and erosion of primary minerals, which generally occurs near the top of the primary ore.
2 Analytical method
2.1 Microthermometric analyses of fluid inclusions
The microthermometric analyses of fluid inclusions were carried out using a Linkam THMS 600 heating-freezing stage at the Geologic Laboratory of College of Earth Sciences, Jilin University. After formal testing, the heating-freezing stage was calibrated using artificial fluid inclusions of 25% CO2-H2O and pure H2O. The precision of measurements was ±0.1℃ at temperatures below 31℃±2℃ at temperatures above 30℃. Most measurements were carried out at a heating rate of (0.2--5)℃/min and the rate of (0.1--0.2)℃/min was adopted near phase transformations in testing.
2.2 Staple isotopes
Samples were prepared in Hongxin, Langfang, and then stable isotope analyses were carried out at the Institute of Nuclear Industrial Geology, Beijing.
2.2.1 H-O isotopes
The quartz samples were crushed and treated to 0.25 to 0.5 mm in size. The quartz grains were hand-picked through binoculars; the samples with purity over 99% should be more than 5 g. The hydrogen isotope ratios were measured by method described by Simon (2001). The released water was trapped by decrepitation method, reduced to H2by zinc. The oxygen was liberated from quartz by reaction with BrF5. Then the H-O isotopes were analyzed with the MAT-253 mass spectrometer, following international standard SMOW. The analysis precision for hydrogen and oxygen is ±2×10-3and ±0.2×10-3, respectively. The δ18O value of fluid was calculated from homogenization temperature of fluid inclusion in quartz and oxygen isotope equation. Oxygen isotope equilibrium exchange fractionation equation was 1000lnαquartz-water=(3.38×106)T-2-3.40, whereTis the temperature in Kelvins (Claytonetal., 1972), and the average fluid inclusion temperature of each stage was used to calculate the δ18OH2Ovalue.
2.2.2 S isotope
Firstly, the sulfide samples were handled by mechanical crushing to the size between 40 to 80 mesh, and single mineral samples were selected by direct observation before testing, and subsequently screened out with purity over 99% under binoculars. Thirdly, the samples were put in an agate bowl for grinding up to 200 mesh or less. Finally, the sulfides were tested for sulfur isotope. The S isotopes were analyzed with the MAT-230 mass spectrometer in Institute of Nuclear Industrial Geology, Beijing. The SO2was obtained by using Cu2O as an oxidant, and the analyses followed standard V-CDT, with a precision of ±0.2×10-3.
2.2.3 Pb isotope
After crushing and sifting, the samples were selected 5--10 mg single mineral with purity over 99%, and size between 40 to 60 mesh, then put in an agate bowl for grinding until below 200 mesh. The samples were then taken into a Teflon cup, spiked, and dissolved in mixed acid of HF and HClO4. After the samples were thoroughly dissolved and steam-dried, hydrochloric acid was added, and the samples were dissolved and steam-dried again. Lead was separated and purified by conventional action-exchange techniques using diluted 0.5 mol/L HBr as eluent.
3 Results
3.1 Petrographic study of fluid inclusions
In this paper, fluid inclusion petrography and microscopic thermometry of pyrite-bearing quartz veins in porphyry (quartz-pyrite stage), chalcopyrite-pyrite bearing veins in skarn (chalcopyrite-pyrite-gold stage), and gold-lead-zinc mineralized veins controlled by the Beiya Formation limestone (gold-silver-galena stage) are studied, respectively.
Petrographic studies of fluid inclusions indicate that the following types of inclusions exist in the premineralization quartz-pyrite stage. L-type: gas-liquid two-phase inclusions, with a two-phase interface showing visible naked boundary at room temperature, consisting of liquid water-salt solution and water vapor. Microscopically, it is elliptical, irregular or negative crystalline shape, and its particle size is generally between 1 and 15 um. Moreover, it is rich in liquid phase (gas-liquid ratio generally between 15%--35%)(Fig.5b). D-type: this type of inclusions contains daughter minerals (mainly halite crystals), generally in circular isometric, elliptical, irregular or negative crystalline shapes, with a size of 10--15 μm. Some D-type inclusions contain cubic pyrites (Fig.5a, c). V-type: gas-liquid two-phase inclusions, this type of inclusions has two-phase interface, which consists of liquid water-salt solution and water vapor at room temperature. Microscopically, it is elliptical, irregular or negative crystal shapes with a size of 1--20 um. And it is rich in gas phase with gas-liquid ratio between 65%--85%)(Fig.5b, d).
The colorless transparent quartz in the gold-brass-pyrite stage mainly has the following inclusions types. Gas-rich two-phase inclusions (V-type) and this type of inclusions has two-phase visible interfaces at room temperature with a liquid water salt solution and water vapor. Microscopically, it is elliptical, irregular or with negative crystalline shape, and its particle size is generally 1--15 um. It is rich in gas phase with gas-liquid ratio generally from 70% to 95% (Fig.5e). Liquid-rich two-phase inclusions: this type of inclusions has a visible two-phase interface at room temperature with a liquid water salt solution and water vapor. Microscopically, it is elliptical, irregular or with negative crystalline shape, with particle size generally of 1--15 um.It is rich in liquid phase (Gas-liquid ratio is generally between 20%--45%; Fig.5e, f).
The inclusions in the gold-silver-galena stage are mainly liquid-rich two-phase inclusions (L-type). At room temperature, there is an obvious two-phase interface with a liquid water salt solution and water vapor. Microscopically, it is elliptical, irregular or with negative crystalline shape and particle size generally of 1--10 um, which is rich in liquid phase (gas-liquid ratio is generally between 10%--30%).
3.2 Microscopic thermometry results
Table 2 summarizes the microthermometric characteristics of fluid inclusions. In quartz-pyrite stage, the D-type fluid inclusions in quartz are usually homogeneous due to the disappearance of vapor at 290℃--390℃ (Fig.6a). Halite crystals dissolve at 156℃--326℃ with salinity equivalentw(NaCl) 29.92%--39.75% (Fig.6b). The L-type fluid inclusions in quartz are homogenized into liquid phase, and the homogenization temperature ranges from 317℃ to 398℃ (Fig.6a), andw(NaCl) is calculated to be 6.58%--10.74% (Fig.6b). The V-type fluid inclusions are homogenized into gas phase. The homogenization temperature ranges from 268℃ to 348℃ (Fig.6a), and thew(NaCl) ranges from 1.05% to 3.53% (Fig.6b).
The L-type fluid inclusions in quartz in the gold-chalcopyrite-pyrite stage are homogenized to liquid phase, and the homogenization temperature ranges from 284℃ to 345℃(Fig.6c), and thew(NaCl) is 4.43% --8.17% (Fig.6d).
The L-type fluid inclusions in the gold-silver-galena stage are homogenized into liquid phase. The homogenization temperature ranges from 268℃ to 348℃ (Fig.6e), and the salinityw(NaCl) ranges from 1.05% to 3.53% (Fig.6f).
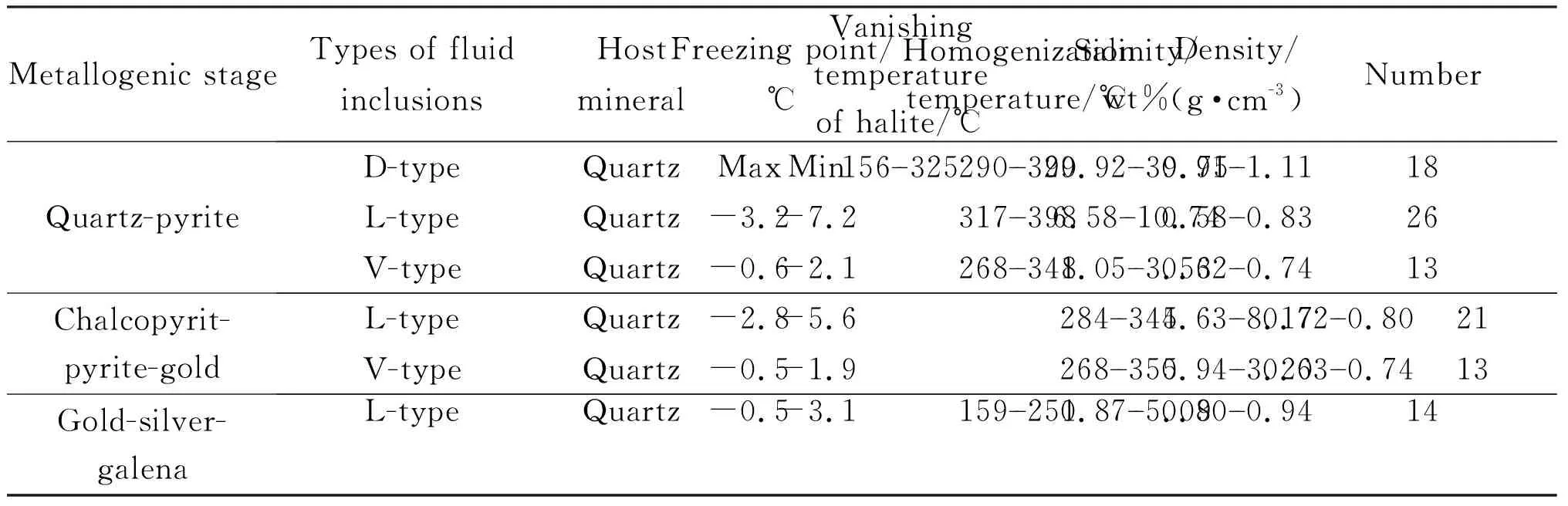
Table 2 Microscopic thermometric results of Beiya gold polymetallic deposit
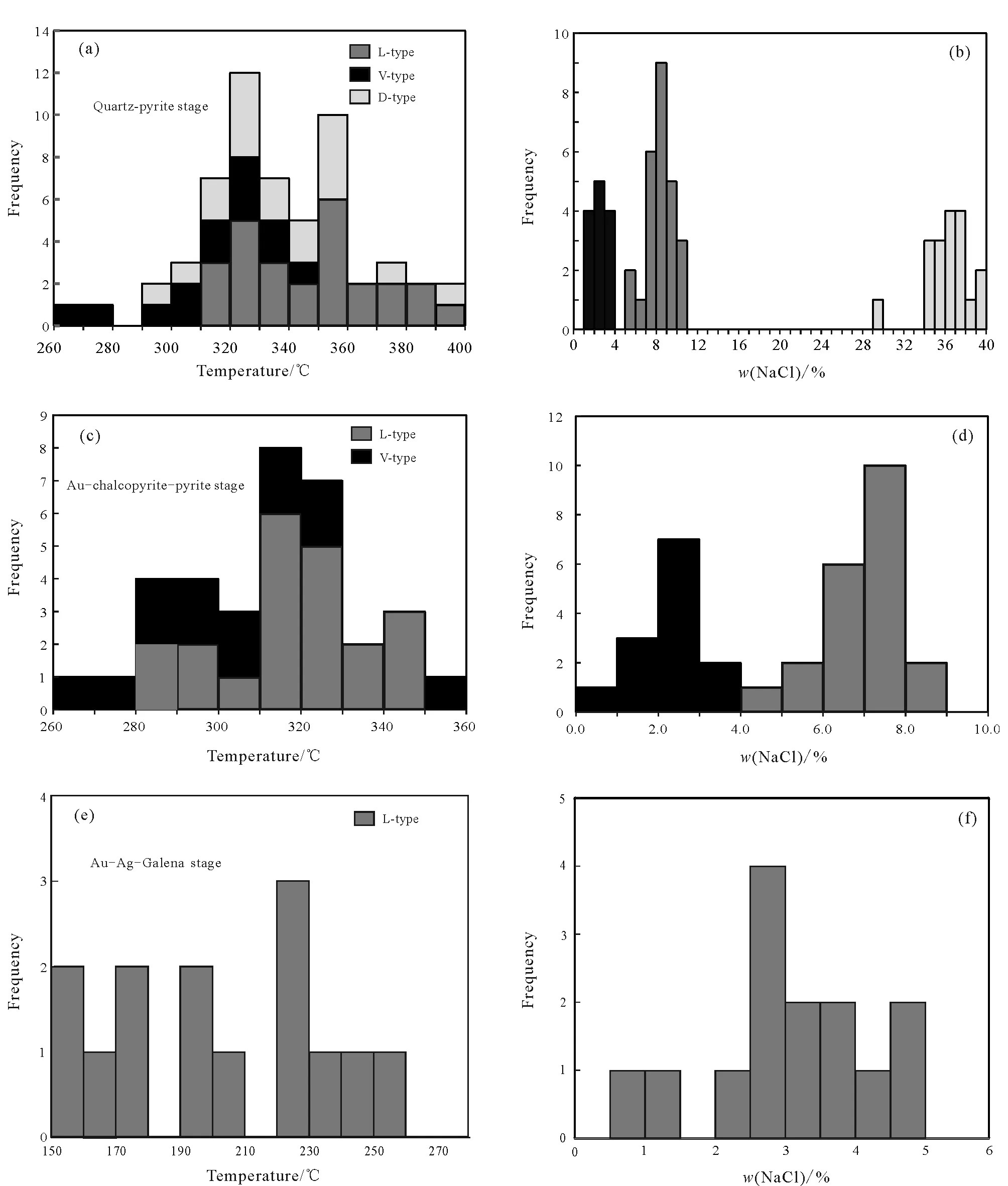
Fig.6 Statistical histograms of microscopic thermometric results (a, c, e) and salinity calculation (b, d, f ) for quartz fluid inclusions in Beiya mining area
3.3 Isotope testing
3.3.1 S isotope
Sulfur isotope analysis provides a useful tool for understanding the source of sulfur. Sulfide minerals including pyrite, chalcopyrite and galena were selected for sulfur isotope analysis. The sulfur isotope data are shown in Table 3. The δ34S of Beiya gold deposit ranges from 0.8×10-3to 1.7×10-3with obvious magmatic S characteristics.
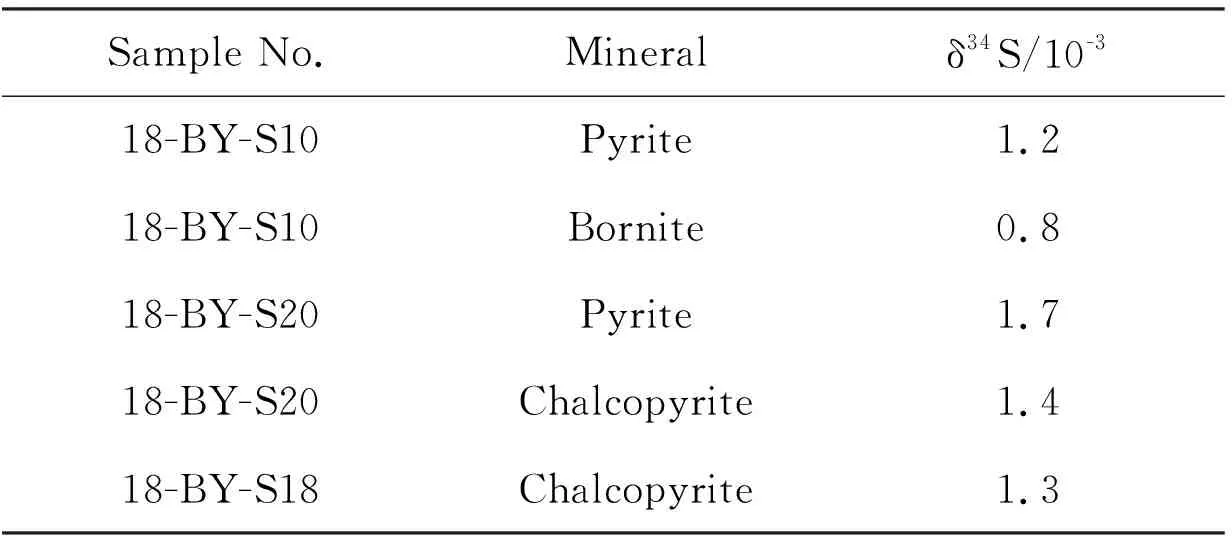
Table 3 Sulphur isotope data of Beiya gold polymetallic deposit
3.3.2 Lead isotope
Sulfide minerals including pyrite and galena were selected for Pb isotope analysis. The sulfur isotope data are shown in Table 4. The variation range of lead isotope of sulfides in Beiya mining area is relatively small, with206Pb/204Pb,207Pb/204Pb and208Pb/204Pb between 18.588--18.673, 15.645--15.694 and 38.866--39.043 (Fig.7), respectively. It is basically consistent with the ore-forming alkali-rich porphyry and amphibolite in the lower crust (Fig.7), revealing the magma source of Pb.

Table 4 Test results of lead isotope compositions of Beiya gold polymetallic deposit
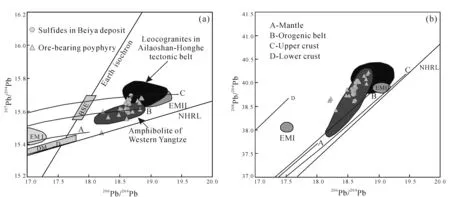
BSE,DM,EMI and EMII are revised from Zindler and Hart (1986); NHRL is after Hart (1984);mantle,orogenic belt, lower crust,upper crust are after Zartman and Doe (1981); leucogranites are from Zhang and Schärer (1999); amphibolites are from Deng et al. (1998) and Zhao et al. (2004); sulfides in Beiya deposit are from this paper and He et al. (2016); ore-bearing porphyry is after He et al. (2017).Fig.7 Diagrams of lead isotope compositions of ores and ore-bearing porphyry in Beiya deposit
3.3.3 Hydrogen and oxygen isotope
All the samples used for stable isotope analysis are the same with samples used for petrological observation and microscopic temperature analysis. The oxygen and hydrogen isotope characteristics of the fluid and host minerals are described in Table 5.
The δ18OH2Oof the Beibei quartz pyrite stage is 5.7%, δD is -81.9SMOW×10-3, and falls into the right edge of the magmatic fluid. It is considered that the fluid in this stage is magmatic fluid. The δ18OH2Oof chalcopyrite-pyrite-gold stage falls into the lower left of magmatic water. There is a tendency for magma water drifting to atmospheric water, but the addition of atmospheric water is minor (Fig.8), suggesting the gradual mixing of atmospheric water from early to late stage.

Table 5 Hydrogen and oxygen isotope testing results of Beiya gold polymetallic deposit
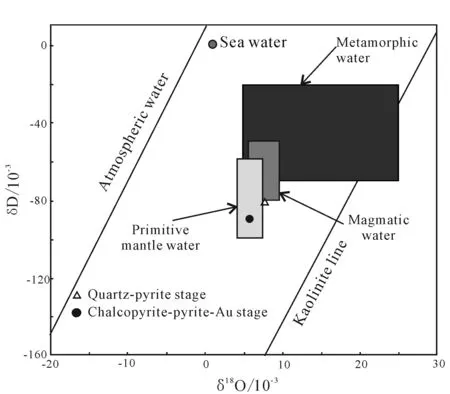
Fig.8 δ18OH2O vs δD diagram of Beiya gold polymetallic deposit
4 Discussion
4.1 Fluid evolution
Fluid inclusion thermometry shows that homogenization temperature and salinity decrease from pre-mineralization quartz pyrite stage to chalcopyrite-pyrite-gold and to gold-silver-galena stage. Ore-forming fluids should initially be high-temperature, medium-low salinity and density fluids (Richard, 2011), and the boiling takes place when fluids decompress and rise to about 2.5 km (hydrostatic pressure), forming a typical group of boiling inclusions in the pre-metallogenic stage of Beiya gold deposit. Many L-type and V-type inclusions are developed in the chalcopyrite-pyrite-gold stage, and the homogenization temperature is slightly lower than that in the quartz-pyrite stage. This is due to the cooling of the rising fluids and secondary boiling. L-type inclusions are found in gold-silver-galena stage, which is characterized by low temperature and salinity, due to the addition of atmospheric water (Fig.9).

Fig.9 Diagram of ore-forming fluid evolution of Beiya gold polymetallic deposit
4.2 Metal transporting and precipitation mechanism
Phase separation or boiling is extremely common in porphyry-skarn deposits (Ulrichetal., 2002), forming a high-density and salinity brine phase and a low-density salinity vapor phase during decompression of deep-source supercritical medium-density fluids. Since brines can dissolve large amounts of base metals to form chloride complexes, they have long been considered to be favorable ore-forming fluids (Richards, 2011). However, in many subduction-related porphyry deposits, there are always large amounts of metals in medium-low density or even gas-rich inclusions (Ulrichetal., 2002; Williams-Jonesetal., 2005). Compared with three-phase inclusions of minerals, medium-and low-density inclusions are larger and more numerous, whereas the high-density fluids are more difficult to move upward due to their high visco-sity and density (Lewis & Lowell, 2009).
Fluid inclusions in the quartz-pyrite stage in the porphyry before mineralization show that there are a large number of three-phase inclusions of daughter-minerals. There is a large number of pyrite crystals in these inclusions, but no economic ore bodies are formed, which indicates that the metals have continued to move upward along with the medium-low density fluids. Three-phase inclusions are seldom developed, indicating that brine water stays inside the porphyry because of their high density and viscosity. Medium and low density fluids rise into the contact zone between the porphyry and limestone, and then a large number of metals precipitate, forming the main copper-gold ore bodies in Beiya gold mine area. In the gold-silver-galena stage, a large amount of gas-liquid two-phase liquids were developed and gold-lead-silver-dominated metallogenic elements precipitate massively at this stage, forming hydrothermal vein type Au-Ag-Pb orebodies.
In summary, medium and low density fluids play a dominant role in the mineral transport process.
The liquid-rich and gas-rich two-phase inclusions are developed in chalcopyrite-pyrite-gold stage, suggesting the role of boiling in metal precipitation. However, large-scale boiling also took place in quartz-pyrite stage, but no Cu-Au mineralization occurred. Considering that the homogenization temperature of the chalcopyrite-pyrite-gold stage is slightly lower than that of the quartz-pyrite stage, it can be inferred that the cooling of magmatic fluid is also an important factor in metal precipitation (Catchpoleetal., 2011), which is also consistent with the fact that the solubility of sulfur and chlorine complexes in hydrothermal solution decreases with the decreasing temperature (Kouzmanov & Pokrovski, 2012). In addition, the cooling of magmatic fluid is accompanied by the change of sulfur valence. The sulfur dioxide in hydrothermal solution is decomposed into hydrogen sulfide and sulfuric acid. The solubility of sulfide decreases significantly with the increase of hydrogen sulfide concentration. Fluid pH also affects mineral precipitation. If H+of sulfuric acid can be effectively consumed, such as by argillization, most of sulfur dioxide will decompose, and the concentration of hydrogen sulfide in solution will increase (Richards, 2011). The increase of hydrogen sulfide concentration will lead to the precipitation of ore minerals. For example, the most effective mechanism of zinc-lead precipitation is the neutralization of acidic fluids during the interaction with surrounding rocks at low temperatures (Kouzmanov & Pokrovski, 2012). This is also consistent with the active chemical properties of carbonaceous wall rocks (carbonaceous wall rocks consume acid more efficiently).
Therefore, fluid cooling plays a dominant role in Cu-Au mineralization, and fluid mixing and cooling and pH reduction play a dominant role in Au-Ag-Pb mineralization.
4.3 Source of metallogenic materials
The sulfides in the Beiya deposit have well defined δ34S values (0.8×10-3--1.7×10-3; mean=1.2×10-3, n=5), which are similar to the δ34S values of sulfides from the majority of magmatic hydrothermal deposits (-3×10-3to 1×10-3) (Hoefs, 2009). Newly obtained Pb isotope data in this study, combined with previously reported results by Heetal. (2016), show a narrow range for sulfides (Fig.7), which coincides precisely with the range for the ore-bearing porphyry. Hence we can conclude that ore-forming metals are closely related to the Beiya plutons, whereas other rocks, including the Triassic limestone, have made little contribution to the mineralization.
5 Conclusions
(1) The evolution of ore-forming fluids shows a trend from high temperature, high salinity to medium-low temperature and low salinity. Magmatic water is the dominant fluid in the chalcopyrite-pyrite-gold stage, and atmospheric water is obviously involved in the late metallogenic stage.
(2) Low to medium density fluids play a dominant role in migrating and transporting minerals. Fluid cooling and boiling are the main mechanisms of gold and copper deposits. Mixing with atmospheric water and pH reduction are the main mechanisms of gold and silver polymetallic precipitation.
(3) Isotope data show that S and Pb are mainly derived from magma, not from limestone of Beiya Formation.
杂志排行
Global Geology的其它文章
- Preparation and characterization of CaO-MgO-SiO2ceramics from silver mine tailings
- Ore-controlling effect of structure and distribution of deep rock mass on Pb-Zn deposit in Qingchengzi ore concentration area,Liaoning
- Characteristics of Zhangsanying—Tongshanzi aeromagnetic anomaly zone and prospecting potential of iron deposits in northern Hebei, China
- Method for predicting cuttings transport using artificial neural networks in foam drilling
- Joint inversion of magnetotelluric and fullwaveform seismic data based on alternating cross-gradient structural constraints
