Synergistic interaction between thioredoxin inhibitor 1-methylpropyl 2-imidazolyl disulfide and sorafenib in liver cancer cells
2020-07-07LinPingCaoChenZhangXiaoYuWengHaiYangXieJianWuShuSenZheng
Lin-Ping Cao , Chen Zhang , Xiao-Yu Weng , Hai-Yang Xie , c, Jian Wu , c,Shu-Sen Zheng , c , ∗
a Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310 0 03, China
b Key Lab of Combined Multi-Organ Transplantation, Ministry of Public Health, Hangzhou 310 0 03, China
c The Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Zhejiang University, Hangzhou 310 0 03, China
Hepatocellular carcinoma (HCC) is a major health problem worldwide with high incidence and mortality rate [1 , 2] . Proper treatments for HCC mainly include surgical resection, transcatheter arterial chemoembolization (TACE), radiofrequency ablation (RFA),and liver transplantation. However, many patients who are diagnosed with advanced HCC are not eligible for surgery, even the TACE and RFA does not necessarily have a good therapeutic effect.Until now, sorafenib is currently the first-line antitumor drug for the treatment of patients with advanced HCC.
Sorafenib, a multiple kinase inhibitor, significantly inhibits Raf-1 and other tyrosine kinases [3] . The main mechanism of the sorafenib-mediated effect is through inhibition of hypoxiainducible factor (HIF) pathway [4] . Although sorafenib has been verified as the first-line systematic drug for HCC treatment, especially at its advanced-stage, it failed to fulfill the anticipated goals, demonstrating limited survival benefits with the extremely low response rates [5 , 6] . The patients who are initially sensitive to sorafenib will eventually develop sorafenib resistance, tumor recurrence and progression. Moreover, high-dose sorafenib results in toxicities, including diarrhea and hand-foot syndrome [3 , 7] . In order to overcome such limitations, sorafenib combined with other drug may be more efficient and less toxic.
1-methylpropyl 2-imidazolyl disulfide (PX-12), an irreversible thioredoxin-1 (Trx-1) inhibitor, has been reported to exhibit antitumor activity [8] . Trx-1 is proved to be a proto-oncogene through promoting tumor proliferation and inhibiting the spontaneous and drug-induced apoptosis. Overexpression of Trx-1 significantly increases the expression of HIF-1 and promotes HIF-1 transactivation in cancer cells. Moreover, Trx-1 increases the expressions of vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF)and results in tumor angiogenesis [9] .
The better strategy for the treatment of HCC should include not only suppressing tumor angiogenesis, but also inducing antihypoxic effect in HCC cells. Evidences have proven that the aberrant activation of signaling pathways contributes to the initiation and progression of HCC [5] . A previous study has shown many target-drug treatments of HCC with a combination of sorafenib and other chemotherapeutic drugs or other targeted therapeutic drugs [6] . However, the efficacy of Trx-1 inhibitor PX-12, in combination with sorafenib in HCC cells is unclear.
We used Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto,Japan) to explore the efficiency of sorafenib and PX-12 on two HCC cell lines (Huh-7 and SMMC-7721, Fig. 1 ). We observed that both liver cancer cell lines have similar sensitivity to the cytotoxic effects of sorafenib, with IC-50 value higher than 6 μmol/L. However,PX-12 had different influences on these cell lines. Huh-7 showed higher sensitivity to PX-12, whereas SMMC-7721 was more resistant, with the IC-50 value higher than 25 μmol/L. It showed that both sorafenib and PX-12 can have toxic effects on HCC cells.
To examine the antitumor effects of the combined treatment of sorafenib and PX-12 in a preclinical setting, we checked the cytotoxic effects of different concentrations of sorafenib with or without PX-12 on HCC cell lines. After the 72 h of drug treatment, we used CCK-8 to detect the cell viability of these two HCC cell lines.As expected, combination of sorafenib and PX-12 reduced viability in all the cell lines more effectively than either drug alone. To further explore whether sorafenib and PX-12 have synergistic effect on HCC cells, we used the CompuSyn software to calculate combination index values of each dose, and the results indicated that sorafenib (0, 1.25, 2.5, 5, 10 μmol/L) and PX-12 (10 μmol/L and 20 μmol/L) have synergistic effect on these HCC cells, which was not cell-specific ( Fig. 1 ). These results indicate that combination of sorafenib and PX-12 can exert an enhanced cytotoxic effect on HCC cell lines.
As both sorafenib and PX-12 play significant roles in HCC cell lines, especially in cell viability, we examined the effect of these two drugs on cell death either alone or in combination. As both the drugs have different activities in HCC cell lines, we chose Huh-7 cell line for further experiments. First, we examined the effect of sorafenib alone on the cells ( Fig. 2 ), and we observed that sorafenib induced cell death in a concentration dependent manner.Then, we checked the combined effect of sorafenib and PX-12,and we found that as the drug concentration increased, the death rate of Huh-7 cell line also increased ( Fig. 2 ). Thus, we concluded that the combination of sorafenib and PX-12 can decrease cell viability through cell death pathway. Furthermore, we observed that as the drug concentration increased, the expression of PARPcleaved also increased, while the expression of BCL-2 decreased( Fig. 2 C).
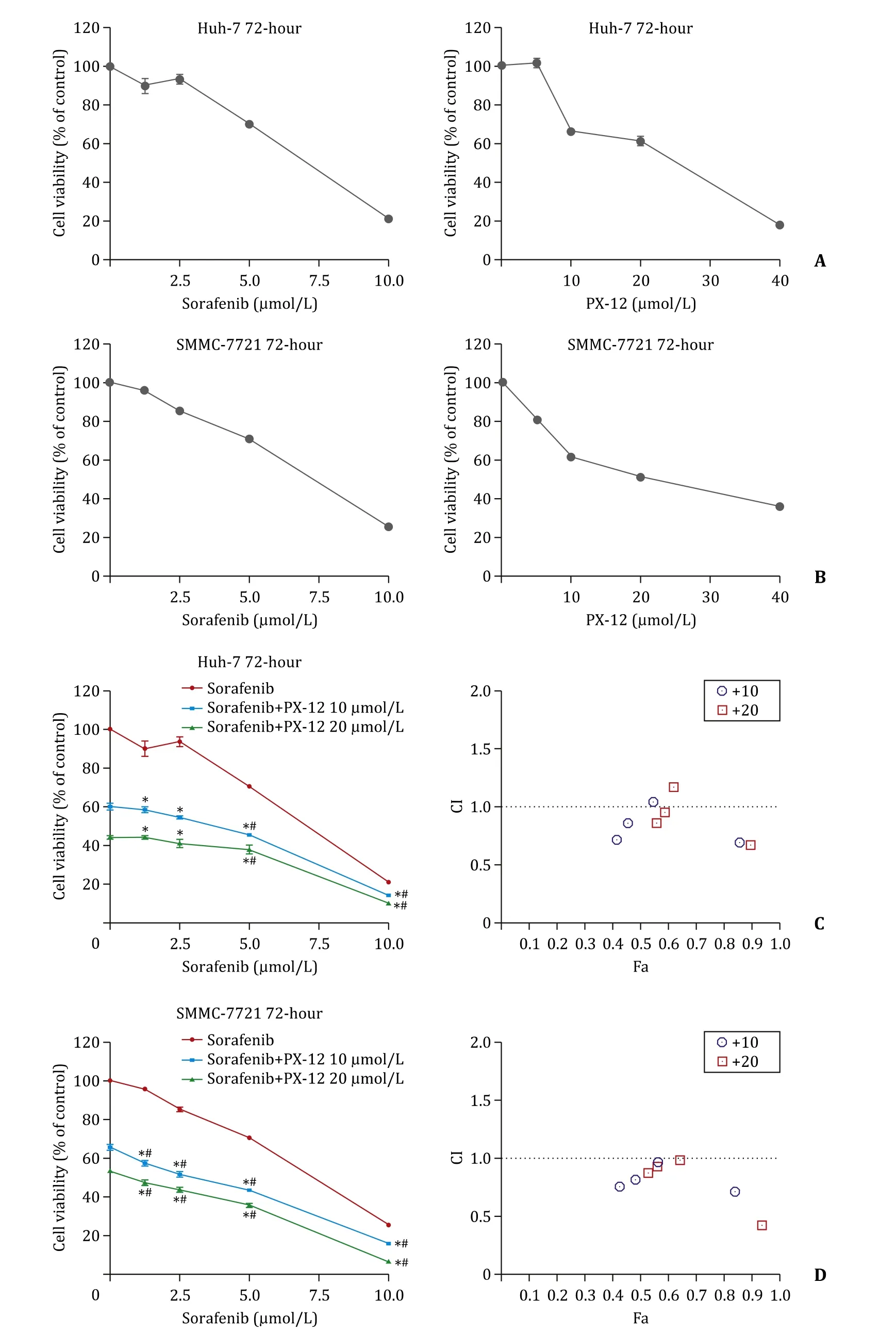
Fig. 1. A-B: Effects of sorafenib and PX-12 on cell viability. Concentration-dependent effects of sorafenib and PX-12 on cell viability ( n = 6); C-D: Synergistic interaction between sorafenib and PX-12 in hepatocellular carcinoma cells ( n = 6). ∗: P < 0.01 compared with sorafenib alone; #: P < 0.01 compared with PX-12 alone. The combination index (CI) values and the fraction affected (Fa) for each dose were used to generate the Fa-CI plots by CompuSyn software (right). CI values < 1 represent synergism.
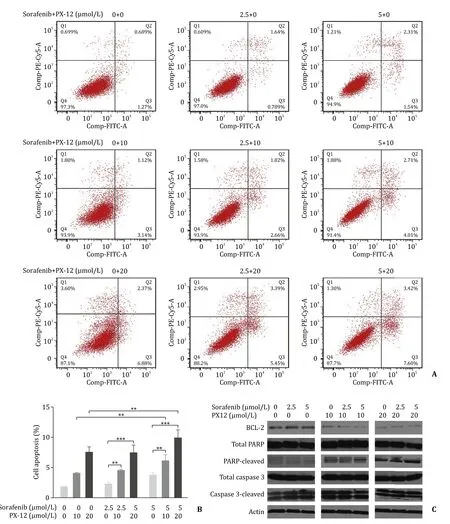
Fig. 2. A: Effects of co-treatment (72 h) on cell apoptosis in Huh-7 cell line. Cell apoptosis was analyzed by flow cytometry; B: Bar graph for flow cytometry ( n = 3;∗∗: P < 0.01; ∗∗∗: P < 0.001); C: Western blots to test the effects of different concentrations of sorafenib and sorafenib + PX-12 on apoptosis-related proteins in Huh-7 cells.Actin was as loading control.
Our study has demonstrated that PX-12 and sorafenib displayed a synergistic efficiency in killing HCC cells, and that these two drugs synergistically inhibited cell viability of HCC cells by inducing PARP related cell apoptosis. Our study showed that the combination of PX-12 and sorafenib could be a promising strategy for advanced HCC treatment. However, further animal experiments are needed and underlying mechanisms should be explored.
In conclusion, our results revealed that PX-12 and sorafenib have synergistic antitumor effect on HCC cells, which may provide a new horizon for the development of anti-HCC targeted therapies.
CRediT authorship contribution statement
Lin-Ping Cao:Investigation, Writing - original draft.Chen Zhang:Investigation, Writing - original draft.Xiao-Yu Weng:Investigation, Writing - original draft.Hai-Yang Xie:Data curation,Formal analysis.Jian Wu:Conceptualization, Funding acquisition,Writing - review & editing.Shu-Sen Zheng:Conceptualization,Funding acquisition, Writing - review & editing.
Funding
This study was supported by grants from the National Natural Science Foundation of China ( 81874228 ) and the Science and Technology Department of Zhejiang Province( 2015C03034 ).
Ethical approval
Not needed.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Transjugular intrahepatic portosystemic shunt for a patient with chylothorax in cryptogenic/metabolic cirrhosis
- Sinusoidal obstruction syndrome related to tacrolimus following liver transplantation
- Laparoscopic combined with thoracoscopic transdiaphragmatic hepatectomy for hepatitis B-related hepatocellular carcinoma located in segment VII or VIII
- Deceased donor liver transplantation for Budd-Chiari syndrome:Long-segmental thrombosis of the inferior vena cava with extensive collateral circulation
- Diagnostic value of contrast-enhanced ultrasonography for intrahepatic cholangiocarcinoma with tumor diameter larger than 5 cm
- Effect of six-stitch pancreaticojejunostomy on pancreatic fistula: A propensity score-matched comparative cohort study
