Diagnostic value of contrast-enhanced ultrasonography for intrahepatic cholangiocarcinoma with tumor diameter larger than 5 cm
2020-07-07ShuYunTinDongXuYuJunWngYongHongYuYuYngTinAnJing
Shu-Yun Tin Dong Xu Yu-Jun Wng Yong-Hong Yu Yu Yng Tin-An Jing
a Department of Ultrasonography, Tongde Hospital of Zhejiang Province, Hangzhou 320012, China
b Department of Ultrasonography, Zhejiang Cancer Hospital, Hangzhou 320022, China
c Department of Radiology, Tongde Hospital of Zhejiang Province, Hangzhou 320012, China
d Department of Pathology, Tongde Hospital of Zhejiang Province, Hangzhou 320012, China
e Department of Ultrasound Medicine, the First Affliated Hospital, Zhejiang University School of Medicine, Hangzhou 310 0 03, China
Herein we reported 5 patients of mass-type intrahepatic cholangiocarcinoma (ICC) misdiagnosed as liver abscess by contrast-enhanced CT, the diagnosis was confirmed through contrast-enhanced ultrasound (CEUS) imaging and pathology.
From January 2015 to January 2018, five patients (1 male and 4 females) were confirmed as ICC by surgical resection or biopsy in Tongde Hospital of Zhejiang Province, with average age of(74.2 ±5.6) years. These 5 ICC patients were misdiagnosed as liver abscess by contrast-enhanced CT and later on, diagnosed by CEUS and pathology. The clinical data of the patients were shown in Table 1 .
Contrast-enhanced CT was performed by using dual-source CT(Siemens Somaton Definition Flash, Forchheim, Germany) scanners,and all patients underwent intravenous infusion of 2 mL/kg contrast agent Ultravist 370 (Scherning AG, Berlin, Germany) by an automated injector at a rate of 2.5 mL/s. After an unenhanced scan through the liver, contrast-enhanced CT scans were obtained in the arterial, portal venous and late phases. All these 5 patients were tumors with low density, and 3 patients with "sign of tumor shrinkage" showed unclear boundary and continuous slow enhancement in low density area on contrast-enhanced CT scanning ( Fig. 1 A and B). The other 2 patients showed clear boundary,edge ring enhancement and internal continuous enhancement on contrast-enhanced CT ( Fig. 2 A and B). These 5 patients were misdiagnosed as liver abscess by contrast-enhanced CT.
The patients were examined by Italian Esaote MyLab90 ultrasonic diagnostic instrument (Esaote, Genoa, Italy) with probe frequency of 3.5 MHz. The CnTI mode was used to dynamically observe the whole process (including arterial, portal venous and late phases) of CEUS scanning. A 2.4 mL volume of SonoVue(Bracco, Milan, Italy) was injected into the cubital vein in bolus,followed by a 5 mL volume of saline flush. All these 5 patients showed low echo mass with clear boundary and uneven internal echo, and a small amount of scattered blood flow signal was seen through the color Doppler instrument ( Figs. 1 C and 2 C). According to CEUS examinations, 3 of these 5 patients showed peripheral rim hyperenhancement and strip-like enhancement inside the tumor from the arterial phase to the late phase, and showed hypoenhancement inside the tumor during the portal-venous and late phase ( Fig. 1 D-F). The other 2 patients showed peripheral rim hyperenhancement and strip-like enhancement extending to the central portion of the tumor, and showed no enhancement inside the tumor from the arterial phase to the late phase ( Fig. 2 D-F).
Surgical or puncture specimens of tumors were stored in formalin. The routine histopathological examination and immunohistochemical staining were performed. The pathological results of 5 patients showed typical adenocarcinoma structure with glandular tubular distribution. The cancer cells were well-formed,columnar or pleomorphic, rich in cytoplasm and were arranged into unequal and irregular acinar-like structures, with a marked increase in mucus in the cellular components. The fibrous tissue was less in the margin of tumor, while in the central region, the tumor cells were less and fibrous tissue was rich; the distribution of blood vessels was sparse; and most of them were embedded in interstitial fibers ( Figs. 1 G-J and 2 G-J).
Some studies has demonstrated that tumor size is an important factor that affects the enhancement pattern of ICCs on CEUS [1 , 2] .The 5 ICC patients hah tumor size larger than 5 cm, which shows two enhancement patterns on CEUS. In comparison with the pathological result, we considered the amount of artery density,microvessel density, fibrous stroma, and necrosis in ICC tumors may be responsible for the differences in the enhancement pattern of ICCs on CEUS. These 5 ICCs were mainly composed of malignant tumor cells, fibrous tissue, coagulative necrosis and mucin. The proportion and distribution of various components in different regions of the tumor were different. ICCs with marked tumor cells at the periphery displayed peripheral rim hypoenhancement; ICCs with marked fibrous stroma and necrosis in tumor displayed only sparse filiform or punctiform internal enhancement on CEUS; ICCs with hypoenhancement had tumor cells and fibrous stroma with almost equal proportions in tumor.

Fig. 1. Case 1 was performed by contrast-enhanced CT, CEUS and pathology. A : Contrast-enhanced CT showed that the tumor in the arterial phase was a low-density area with unclear boundaries, and an enhanced density could be seen inside the tumor; B : contrast-enhanced CT showed that the tumor in the delayed phase was a density of continuous enhancement, and the range of the low-density area was smaller than that of the arterial phase, and the degree of internal enhancement was more obvious than that of arterial phase (yellow arrow); C : gray scale ultrasound showed the right liver with clear border hypoechoic tumor; D : in arterial phase, peripheral rim hyperenhancement (blue arrow) was shown, and strip-like enhancement in the central portion of the tumor was shown (green arrow); E : in the portal phase, hypoenhancement in the central portion of the tumor (blue arrow) was noted, and peripheral rim hypoenhancement (green arrow) was more obvious; F : in the late phase, hypoenhancement in the central portion of the tumor (blue arrow) and peripheral rim hypoenhancement (green arrow) was noted; G : regular pathology showed irregular glandular arrangement in the tumor (HE staining, ×100); H : CK19 ( + ) was detected by immunohistochemical staining ( ×100); I : HEP (-) was detected by immunohistochemical staining ( ×100); J :AFP (-) was detected by immunohistochemical staining ( ×100).

Fig. 2. Case 2 was performed by contrast-enhanced CT, CEUS and pathology. A : Contrast-enhanced CT for the mass in the arterial phase showed a low density area with unclear boundaries, and internal enhancement (yellow arrow); B : contrast-enhanced CT showed that the enhanced density of tumor was continued to strengthen in the delayed phase, and the range of the low density area was smaller than that in the arterial phase. Internal density enhancement was more obvious than that in the arterial phase (yellow arrow); C : gray scale ultrasound showed clear border hypoechoic tumor in right liver; D -F : from the arterial phase to the late phase peripheral rim hyperenhancement was shown (blue arrow); strip-like enhancement extending to the central portion of the tumor was shown (green arrow); G : regular pathology showed irregular glandular arrangement in the tumor (HE staining, ×100); H : CK19 ( + ) was detected by immunohistochemical staining ( ×100); I : HEP ( −) was detected by immunohistochemical staining ( ×100); J : AFP ( −) was detected by immunohistochemical staining ( ×100).
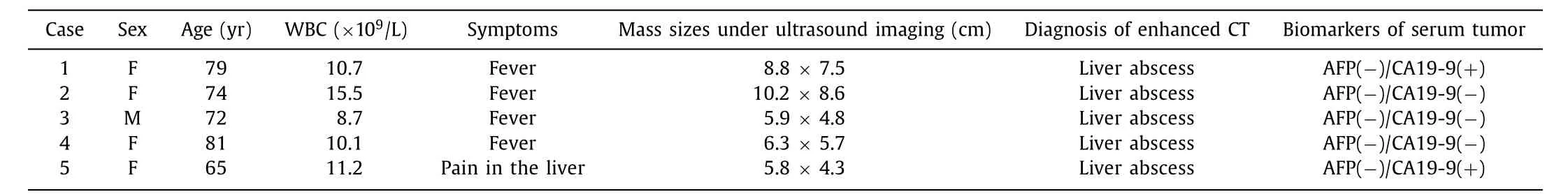
Table 1 Clinical data of ICC patients.
The larger ICCs had more marked necrosis and fibrous stroma in the tumor, which was prone to cystic necrosis and hemorrhagic infection, and the imaging features of these ICCs are similar to those of liver abscesses on contrast-enhanced CT, which was also the reason why 5 ICC patients in our study were misdiagnosed on contrast-enhanced CT. However, the features of ICCs in different phases of CEUS were different from those of contrast-enhanced CT, especially in late phase, which might be due to the different metabolic patterns of contrast agents used in two methods in the human body. The ultrasound contrast agent sulfur-hexafluoride filled microbubbles (SonoVue, Bracco, Milan, Italy) has been widely used in the diagnosis of liver lesions, because the SonoVue is only distributed intravascularly without any passage into the interstitial space, which contributes to its wide application for diagnosing hepatic lesions and allows the analysis of the entire liver parenchyma [3] . In comparison, the contrast agent used for CT and MRI can leak into the interstitium of lesions, thereby resulting in long-lasting enhancement [4] . We further summarize the different features of CEUS and contrast-enhanced CT in ICCs, and continue to accumulate experience in order to reduce misdiagnosis and improve the accuracy of diagnosis.
The experience with ICC was much more limited and different.The enhancement patterns of ICC on CEUS described in different articles varied noticeably [1] . In the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) guidelines, typical features included rim-like hyperenhancement and central hypoenhancement, and the additional features include nonenhancing regions and inhomogeneous hyperenhancement in the arterial phase [5] . Based on the diversity of CEUS patterns for ICC,and for the reason that CEUS images of some ICCs can be confused with hepatocellular carcinoma, CEUS was not included as one of the diagnostic methods for liver diseases in the 2011 practice guideline released by the American Association for the Study of Liver Diseases (AASLD) [6] . This study summarized 5 ICCs which were larger than 5 cm and easily misdiagnosed as liver abscess on contrast-enhanced CT. Due to the small number of tumors and limitation of this study, further research on multi-center and large sample are warranted.
CRediT authorship contribution statement
Shu-Yuan Tian:Conceptualization, Data curation, Investigation,Writing - original draft.Dong Xu:Conceptualization, Funding acquisition, Investigation, Writing - review & editing.Yu-Jun Wang:Investigation.Yong-Hong Yu:Data curation.Yue Yang:Investigation.Tian-An Jiang:Conceptualization.
Funding
This study was supported by a grant from the Major Research Project of Natural Science Foundation of Zhejiang Province(NO. LSD19H180 0 01 ).
Ethical approval
This study was approved by the Ethics Committee of Tongde Hospital of Zhejiang Province.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Transjugular intrahepatic portosystemic shunt for a patient with chylothorax in cryptogenic/metabolic cirrhosis
- Sinusoidal obstruction syndrome related to tacrolimus following liver transplantation
- Synergistic interaction between thioredoxin inhibitor 1-methylpropyl 2-imidazolyl disulfide and sorafenib in liver cancer cells
- Laparoscopic combined with thoracoscopic transdiaphragmatic hepatectomy for hepatitis B-related hepatocellular carcinoma located in segment VII or VIII
- Deceased donor liver transplantation for Budd-Chiari syndrome:Long-segmental thrombosis of the inferior vena cava with extensive collateral circulation
- Effect of six-stitch pancreaticojejunostomy on pancreatic fistula: A propensity score-matched comparative cohort study
