AAV-mediated human CNGB3 restores cone function in an allcone mouse model of CNGB3 achromatopsia
2020-05-25YuxinZhangShanshanWangMiaoXuJijingPangZhilanYuanChenZhao
Yuxin Zhang, Shanshan Wang, Miao Xu, Jijing Pang,2,✉, Zhilan Yuan,✉, Chen Zhao,✉
1Department of Ophthalmology, the First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 210029,China;
2Department of Ophthalmology, University of Florida, Gainesville, FL 32610, USA.
Abstract Complete congenital achromatopsia is a devastating hereditary visual disorder. Mutations in the CNGB3 gene account for more than 50% of all known cases of achromatopsia. This work investigated the efficiency of subretinal (SR) delivered AAV8 (Y447, 733F) vector containing a human PR2.1 promoter and a human CNGB3 cDNA in Cngb3-/-/Nrl-/- mice. The Cngb3-/-/Nrl-/- mouse was a cone-dominant model with Cngb3 channel deficiency, which partially mimicked the all-cone foveal structure of human achromatopsia with CNGB3 mutations. Following SR delivery of the vector, AAV-mediated CNGB3 expression restored cone function which was assessed by the restoration of the cone-mediated electroretinogram (ERG) and immunohistochemistry. This therapeutic rescue resulted in long-term improvement of retinal function with the restoration of cone ERG amplitude. This study demonstrated an AAV-mediated gene therapy in a cone-dominant mouse model using a human gene construct and provided the potential to be utilized in clinical trials.
Keywords: achromatopsia, cyclic nucleotide-gated channel, CNGB3, adeno-associated virus (AAV), gene therapy, subretinal injection
Introduction
In the human retina, photoreceptor cyclic nucleotide-gated (CNG) channels play a pivotal role in phototransduction. Phototransduction relies on the function of CNG channels in both rod and cone photoreceptor outer segment plasma membranes[1–2].Structurally, CNG channels comprise two structurally related A and B subunits: the rod photoreceptor CNG channel is composed of CNGA1 and CNGB1 subunits, whereas the cone photoreceptor is composed of CNGA3 and CNGB3 subunits[2–6]. Recent studies have shown that mutations in CNGA3 or CNGB3 cause clinically indistinguishable forms of congenital achromatopsia (ACHM)[7].
ACHM or rod monochromatism is an autosomal recessive hereditary visual disorder characterized by the absence of functional cone photoreceptors in infancy, affecting one in approximately 30 000 individuals worldwide[7]. Affected individuals exhibit pendular nystagmus, poor visual acuity, and photophobia[8]. The first signs of ACHM in infancy are nystagmus and photophobia as evidenced by squinting in bright light[8]. To date, mutations in six different genes have been identified as responsible for ACHM:CNGA3,CNGB3,GNAT2,PDE6C,PDE6H,andATF6. Clinically, approximately 50% of ACHM patients are associated withCNGB3mutations, about 25% withCNGA3mutations and smaller fractions with mutations in the cone transducin or phosphodiesterase genes in European populations[7–12].Previous studies demonstrated that the CNGA3 is the ion-conducting subunit, while the CNGB3 fails to form functional homomeric channels in heterologous expression systems when expresses alone[13–14].Because the B subunits are only thought to confer specific biophysical properties to the CNG channel complex whereas the A subunits contribute to the principal channel properties[9,15]. Therefore, the A subunits are thought to be the primary subunits while the B subunits play a modulatory role for the function of both rod and cone CNG channels[9,15]. Hence,knock-out of the gene encoding Cngb3 in mouse retina would cause a moderate reduction of the cone function[16].
Recent clinic trails on patients with Leber congenital amaurosis (LCA) demonstrated the successful expression of theRPE65gene with adenoassociated viral (AAV) vectors[17–19], which provided a possible treatment for retinal degeneration including ACHM. Restoration of cone function and improvement in photopic vision were achieved in mouse and dog models of ACHM by subretinal (SR)delivery of AAV vectors[20–21]. Progresses in AAVbased gene replacement therapy to restore conemediated function in Cngb3 deficient mouse models provided a foundation for the development of clinical trials for human ACHM patients[21–22]. In these studies,AAV vectors containing human CNGB3 cDNA were delivered subretinally inCngb3-/-mice, which carry a naturally occurring mutation in theCngb3gene.
We chose theCngb3-/-/Nrl-/-mouse model because it possessed a genetically and phenotypically wellcharacterized cone degeneration. This double knockout mouse had a retinal phenotype similar to its single knock-outCngb3-/-mouse with impaired cone function and cone degeneration.Cngb3-/-/Nrl-/-mice lacked scotopic light response due to Nrl deficiency.The expression levels of cone arrestin and S-opsin were reduced inCngb3-/-/Nrl-/-mice[16]. In addition,we speculate that this mouse model will respond well to SR gene-based therapy since rescue has been achieved inCngb3-/-mice. Here, human CNGB3 expression was achieved using AAV8 (Y447, 733F)vector driven by a cone-specific promoter to test whether AAV8-(Y447, 733F)-PR2.1-hCNGB3 can rescueCngb3-/-/Nrl-/-cones when delivered to the SR space.
Materials and methods
Animals
C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, USA).Cngb3-/-[23]andNrl-/-[24]mice were kindly provided by Dr. Anand Swaroop at NEI/NIH.Cngb3-/-/Nrl-/-mice were generated as described previously[16]. All animals were maintained under standard laboratory conditions(18 °C–23 °C, 40%–65% humidity) with food and water availablead libitumin the University of Florida Health Science Center Animal Care Service Facilities a 12-hour/12-hour light/dark cycle with <15 ft-c environmental illumination. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Florida and conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and National Institutes of Health regulations.
Construction of AAV vectors
Human CNGB3 cDNA (purchased from American Type Culture Collection, USA) was cloned under the human PR2.1 promoter which has been shown to target transgene expression to mammalian L/M cones[25]to make AAV-PR2.1-hCNGB3 construct.The construct was packaged in AAV8 (Y447, 733F).The vectors were purified and tittered according to previously published methods[26].
SR injections
At postnatal day 14 (P14), one microliter of AAV8(Y447, 733F)-PR2.1-hCNGB3 vector (1013vector genome/mL) was injected subretinally into one eye of eachCngb3-/-/Nrl-/-mouse. Transcorneal SR injections[27]were performed with a 33 gauge blunt needle mounted on a 5 mL Hamilton syringe. First, an entering hole was introduced at the edge of the corneal by a 30 gauge disposable needle, then the Hamilton syringe loaded with 1 μL of viral vector mixed with fluorescein dye was glided through the cornea opening into the subretinal space with previously described methods[28–29]. The other eye remained untreated as a control. All eyes used for further evaluation had no apparent surgical complications and more than 80% of retinal detachment.
Electroretinography
Electroretinography was performed at six months following SR injections. A UTAS Visual Diagnostic System with a Big Shot Ganzfeld (LKC Technologies,USA) was used for ERG recording. All mice were anesthetized by ketamine (72 mg/kg)/xylazine(4 mg/kg) by intraperitoneal injection. The pupils were dilated with 1% atropine and 2.5% phenylephrine hydrochloride. Fifty recordings were averaged for light-adapted ERG measured light intensity of 1.4 log cd·s/m2in the presence of 30 cd·s/m2background light with inter-stimulus intervals of 0.4 seconds.Scotopic and photopic b-wave amplitudes were averaged and used to generate SEM. ERG data were presented as mean±SEM. Statistical analysis was performed with unpairedt-test and significance was defined as aPvalue of less than 0.05.
Tissue preparation and immunohistochemistry
Eyes were enucleated at six months following SR injection. Retinal sections and whole mounts were prepared according to previously described methods[28,30]. Briefly, the limbus of eyes was marked at "12 o'clock" with a hot needle immediately after sacrifice. Then eyes were enucleated and fixed in 4%paraformaldehyde overnight at 4 °C. The cornea and lens were removed from the eyes. For retinal frozen sections, the remaining eyecups were rinsed with PBS and cryoprotected 30% sucrose in PBS for 4 hours at room temperature, then embedded in cryostat compound (Tissue TEK OCT, Sakura Finetek USA,Inc., USA) and frozen at -80 °C. For immunohistochemistry, retina sections or whole mounts were rinsed in PBS and blocked in 0.3% Triton X-100, 5%BSA in PBS for 60 minutes at room temperature.Sections or whole mounts were incubated with lectin peanut agglutinin (PNA) conjugated to an Alexa Fluor 488 (1:200, Invitrogen, USA), M- or S-cone opsin(1:400, Millipore, USA), human CNGB3 (1:100,ThermoFisher, USA) primary antibodies diluted in 5%BSA in PBS overnight at 4 °C, then washed by PBS for three times, incubated with IgG secondary antibody tagged with Alexa-594 diluted 1:500 in PBS at room temperature for two hours and washed with PBS. Sections were mounted with Vectashield Mounting Medium for Fluorescence (H-1400, Vector Laboratories, USA) and coverslipped. Whole mounts and sections were analyzed with a Zeiss CD25 microscope fitted with Axiovision Rel. 4.6 software.
Results
AAV8 (Y447, 733F) vector restored photopic ERGs in Cngb3-/-/Nrl-/- mice following SR injection
It has been shown that knock-out ofNrlgene leads to no rod-mediated ERG[16]. Therefore, bothCngb3-/-/Nrl-/-andNrl-/-mice had no rod-mediated ERG response. TheNrlgene knock-out also caused a higher cone-mediated ERG response[31]. Thus we first tested the photopic ERGs at 6.5 months in untreatedCngb3-/-/Nrl-/-andNrl-/-mice (ERG traces are shown inFig. 1A). At the light intensity of 1.4 log cd·s/m2,the average b-wave amplitude record fromCngb3-/-/Nrl-/-eyes (97.86±4.92,n=5,Fig. 1B) was lower than that ofNrl-/-mouse (257.50±7.65,n=5,Fig. 1B), certified that the B subunits are not the primary subunits for the function of photoreceptor CNG channels. We also measured b-wave kinetics which was determined by b-wave implicit time of the photopic ERGs. The average b-wave implicit time which recorded from theCngb3-/-/Nrl-/-(50.72±1.67,n=5,Fig. 1C) andNrl-/-mice (51.94±1.68,n=5,Fig. 1C) had no statistical differences.
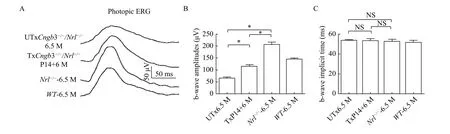
Fig. 1 Photopic electroretinogram (ERG) in treated and untreated Cngb3-/-/Nrl-/- eyes. A: Representative photopic ERG response at 1.4 log cd·s/m2 from Cngb3-/-/Nrl-/- and control eyes. B: Statistical analysis of photopic b-wave amplitudes elicited at 1.4 log cd·s/m2. C:Statistical analysis of photopic b-wave implicit times at 1.4 log cd·s/m2. Photopic ERG scale bar: all of the photopic ERG scale bars are the same (y axis: 50 μV/Div; x axis: 50 ms/Div). UTx: untreated; Tx: treated; M: months; P14: postnatal day 14; NS: no statistical difference;*P<0.05.
Since the reductions of photopic ERG in theCngb3-/-/Nrl-/-mice, we also tested the AAV treatedCngb3-/-/Nrl-/-mice at 6 months following P14 SR injections. The b-wave amplitudes of photopic ERGs which recorded from AAV treated eyes (148.00±9.47n=5,Fig. 1B) were significantly higher than that of untreated eyes (97.86±4.92,n=5,Fig. 1B) but were somewhat reduced compared to age-matchedNrl-/-mice (257.50±7.65,n=5,Fig. 1B). The b-wave kinetics still had no statistical difference between the treated (52.52±1.81,n=5,Fig. 1C) and the untreated(50.72±1.67,n=5,Fig. 1C) eyes.
AAV8 (Y447, 733F) -mediated hCNGB3 expression was detected in cones following SR injection
Human CNGB3 expression was assayed by immunohistochemistry at six months after SR vector treatment (Fig. 2). Robust outer segment (OS)CNGB3 expression was detected inCngb3-/-/Nrl-/-eyes following SR-AAV8 (Y447, 733F) treatment,whereas the contralateral untreated eyes lacked detectible CNGB3 labeling (Fig. 2, left two rows).Co-localization of human CNGB3 and cone-specific PNA (Fig. 2, right two rows) confirmed that human CNGB3 expression was localized to cone outer segments.
S-opsin expression was restored in Cngb3-/-/Nrl-/-mice by AAV8 (Y447, 733F)-mediated hCNGB3 expression following SR injections
We characterized the M- and S-opsin expression in the untreatedCngb3-/-/Nrl-/-eyes by immunohistochemistry. Immunohistochemistry of the retinal whole mounts detected no S-opsin expression (Fig. 3E)while no significant reduction of M-opsin expression was observed in the untreatedCngb3-/-/Nrl-/-eyes(Fig. 3A–D). At 6.5 months of age, S-opsin expressed with the characteristic dorsal-ventral gradient in C57BL/6J retina (Fig. 3H,LandP) but throughout theNrl-/-retinal whole mounts (Fig. 3G,KandO)[32].M-opsin expression from untreatedCngb3-/-/Nrl-/-whole mounts also showed very similar pattern as that in theNrl-/-retinas (Fig. 3AandC). M-cone distribution and expression intensity were similar in 6.5 months oldCngb3-/-/Nrl-/-and age-matchedNrl-/-whole mounts indicating thatCngb3-/-/Nrl-/-exhibited no significant M-cone degeneration at this age (Fig. 3AandC), whereas S-opsins were absent inCngb3-/-/Nrl-/-mice (Fig. 3E,IandM) indicating a progressive loss of cone photoreceptors.
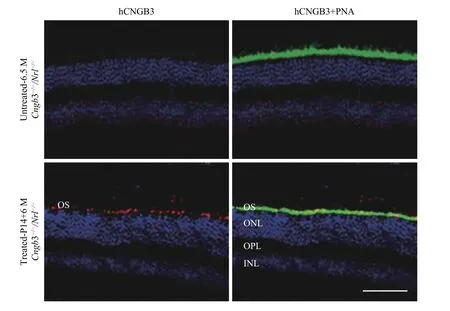
Fig. 2 Frozen retinal sections immunostained with anti-human CNGB3 antibody and cone-specific PNA. Six months post AAV8(Y447, 733F) injection, frozen retinal sections immunostained with anti-human CNGB3 antibody and cone-specific PNA showing human CNGB3 expression in outer segments of AAV treated eyes but not in untreated Cngb3-/-/Nrl-/- eye. OS: outer segment layer; ONL: outer nuclear layer; OPL: outer plexiform layer; INL: inner nuclear layer; Red: CNGB3; Blue: DAPI stained nuclei; Green: PNA (Lectin peanut agglutinin); P14: postnatal day 14; M: months. Scale bar =100 μm.
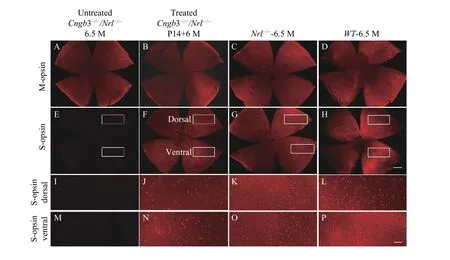
Fig. 3 Immunostaining of retina whole mounts. M-opsin and S-opsin staining distribution in AAV treated Cngb3-/-/Nrl-/- (P14+6 M),age matched untreated Cngb3-/-/Nrl-/-, Nrl-/- and C57BL/6J mice. Red: S-opsin or M-opsin staining as indicated; P14: postnatal day 14; M:months. Scale bar (A–H) =500 μm; Scale bar (I–P) =100 μm.
Expression of the S-opsin in the treatedCngb3-/-/Nrl-/-eyes was detected across the entire retinas by whole mount immunostaining and maintained at least 6 months after P14 SR injection of AAV8 (Y447, 733F) vector with therapeutic gene(Fig. 3F,JandN). Immunostaining of ventral retinal sections was also performed to check the cellular localization of S-opsin expression. In addition,S-opsin expression in the cone outer segments was evidenced by co-localization with positive PNA staining (Fig. 4). The positive S-opsin staining confirmed that SR-AAV8 (Y447, 733) treatment prevented cone degeneration (Fig. 3).
Discussion
The goal of this study was to test gene therapy using a new all-cone mouse model for treating human CNGB3-achromatopsia. In the human retina, cones account for only ~5% of the photoreceptors[33]. They are primarily concentrated in macula and responsible for day vision, central visual acuity and color vision[7].Both cone and rod CNG channels exhibit a 3:1 stoichiometry between A and B subunits[3–5,13]. The cone photoreceptor CNG channel is composed of A3 and B3 subunits, whereas the rod photoreceptor CNG channel is formed by A1 and B1 subunits[4–6,15].CNGB3 is known as the modulator. A CNGA3 homomeric channel is fully functional in heterologous expression systems[14,34]. Although CNGB3 shares a common topology with CNGA3 and possesses a poreforming region, the B3 subunit does not form a functional channel without A3 subunits[14,34].Therefore,Cnga3-/-mice show a complete loss of cone response[23], while deficiency of Cngb3 leads to residual cone response in mouse retina[35]. Fortunately,mutations in CNGB3 are clinically responsible for approximately half of all cases of ACHM patients which present moderate cone degeneration[36], to permit a relatively long period for therapy intervention. In this study, AAV delivered human CNGB3 expression in this all-cone mouse model is promising for the treatment of CNGB3-achromatopsia and other cone-specific diseases.
Nrl-/-retinas played shorter cone OS than wildtype[24,31]. Knock-out of theCngb3andNrlgene partially mimicked the fovea of ACHM patients. A subsequent study showed thatCngb3-/-/Nrl-/-mice had a retinal phenotype similar to the sum of their respective single knock-out counterparts,i.e.impaired cone function and cone degeneration[16]. Effective gene therapy for ACHM requires efficient transgene expression in cones. Here, the human CNGB3 introduced by AAVs and expressed in the cone OS and S-opsin were restored following AAV-mediated CNGB3 expression, and AAV-mediated expression of the human CNGB3 could restore light responses in theCngb3-/-/Nrl-/-mice.
The AAV serotype is one of factors that contributes to an efficient transgene delivery. Although the AAV serotype 5 transduced a significantly greater number of photoreceptor cells compared with serotype 2[37],the AAV8 had higher inherent photoreceptor transduction efficiency than AAV2 or 5[37–40], making it the most suitable vector forACHMgene therapy.Moreover, comparing with the traditional AAV vector, site-directed tyrosine to phenylanine (Y-F)mutagenesis of selected tyrosine residues on AAV capsid also increased transduction efficiency by protecting vector particles from proteasomal degradation[30,40–41].
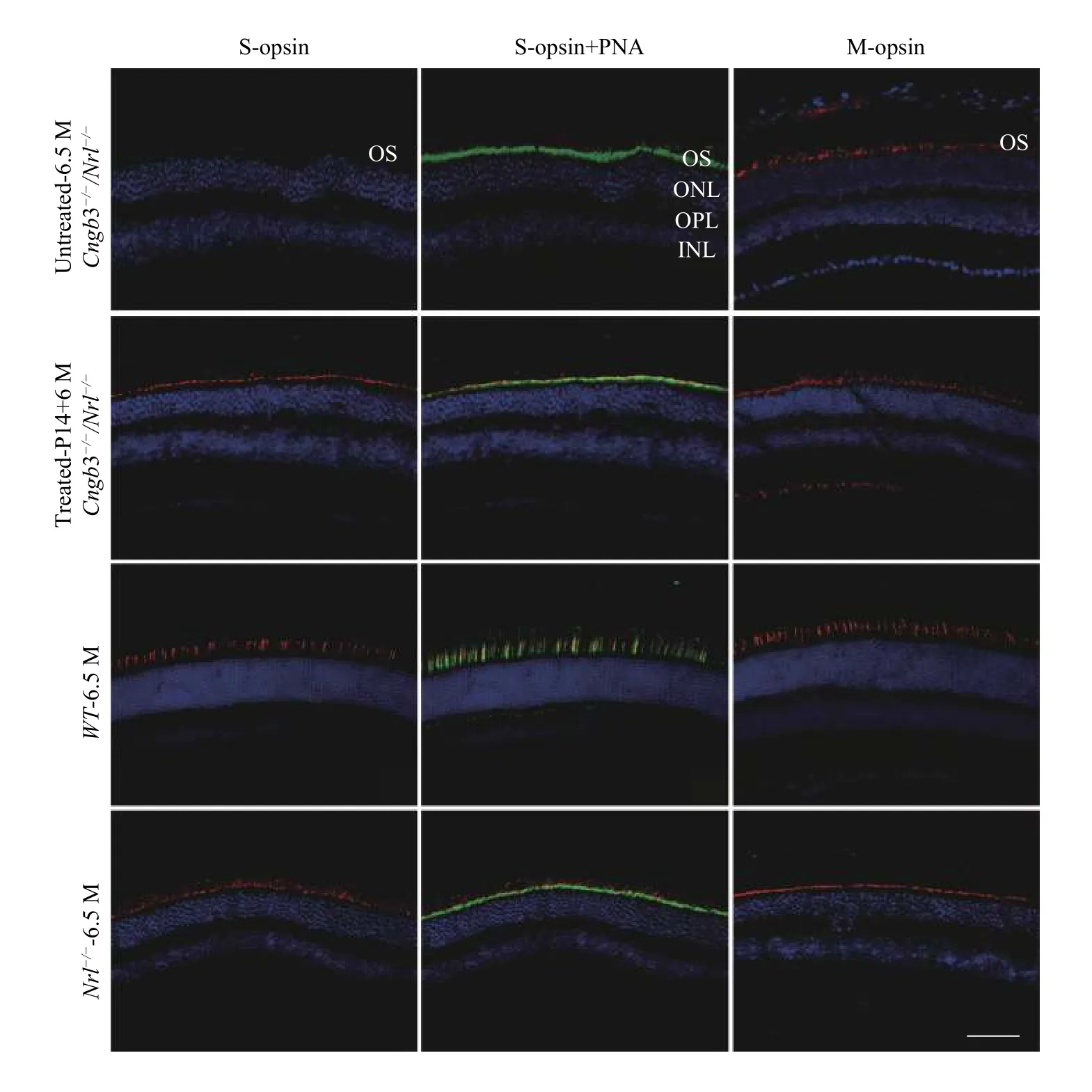
Fig. 4 Frozen retinal sections (ventral) immunostained with S- or M-opsin antibody. Frozen retinal sections (ventral) immunostained with S- or M-opsin antibody showing S-opsin or M-opsin expression in outer segments of C57 BL/6J, Nrl-/-, and AAV8 (Y447, 733F)treated eyes. No detectable S-opsin in contralateral untreated Cngb3-/-/Nrl-/- eye. OS: outer segment layer; ONL: outer nuclear layer; OPL:outer plexiform layer; INL: inner nuclear layer; Red: S-opsin or M-opsin staining as indicated; Blue: DAPI stained nuclei; Green: PNA(Lectin peanut agglutinin); P14: postnatal day 14; M: months. Scale bar =100 μm.
The specific promoter is another important factor for efficient gene therapy. The PR2.1 promoter contains 2.1 kb of nucleotides, including the locus control region (LCR) and other sequences upstream of the L-opsin coding region. This promoter was reported to direct high level of human Cngb3 expression in both L/M and S cones in mouse retinas[22]. Accordingly, we designed AAV8 (Y447,733F)-PR2.1-hCNGB3vector and obtained a robust and longer-term rescue in theCngb3-/-/Nrl-/-mouse.
Our study showed very slow cone degeneration in theCngb3-/-/Nrl-/-mice, and the photopic b-wave amplitudes were still 38% ofNrl-/-mice at the age of 6.5 months (Fig. 1B). The factor to consider was the detachment of the fragile structure of retinas after SR injection. In LCA2 human clinical trial, SR injections that detached the fovea area resulted in loss of fovea cone OS and cone cell death, and it was concluded that SR injection under the fovea may cause damage rather than benefit and should be approached with prudence[18,42–43]. Since IV delivered AAV8 (Y447,733F) could efficiently target cone photoreceptors and rescued the cone functions inCngb3-/-/Nrl-/-[44], we expect similar cone rescue inCngb3-/-/Nrl-/-mice by AAV8 (Y447, 733F)-PR2.1-hCNGB3vector. We even expect it to be utilized in clinical trials due to the reduced physical barrier to vectors intended for cones from the vitreous.
In summary, AAV-mediated human CNGB3 expression restored cone function inCngb3-/-/Nrl-/-mouse which can be served as a good model for further study of gene therapy on CNGB3-achromatopsia.
Acknowledgments
This work was supported by NIH (Grant No.EY023543 to J.P.), Jiangsu Province Foundation for Innovative Research Team (to C.Z.).
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- The current status of malignant hyperthermia
- Contribution of neutrophils in the pathogenesis of rheumatoid arthritis
- H2S protects against diabetes-accelerated atherosclerosis by preventing the activation of NLRP3 inflammasome
- Inhibitory role of peroxiredoxin 2 in LRRK2 kinase activity induced cellular pathogenesis
- The level of bile salt-stimulated lipase in the milk of Chinese women and its association with maternal BMI
- RNA-seq analysis identified hormone-related genes associated with prognosis of triple negative breast cancer
