Contribution of neutrophils in the pathogenesis of rheumatoid arthritis
2020-05-25LingshuZhangYiYuanQiangXuZhengyuJiangCongQiuChu
Lingshu Zhang, Yi Yuan, Qiang Xu, Zhengyu Jiang, Cong-Qiu Chu,✉
1Division of Arthritis and Rheumatic Diseases, Oregon Health & Science University, Portland, OR 97239, USA;
2Rheumatology Section, VA Portland Healthcare System, Portland, OR 97239, USA;
3Department of Rheumatology and Immunology, the First Hospital of Jilin University, Changchun, Jilin 130021, China.
Abstract
Keywords: neutrophils, rheumatoid arthritis, NET formation
Introduction
Neutrophils play a pivotal role in the innate immune system for host defense in fighting microbe invasion.They migrate to the infection site as the first responders during acute inflammation. It has been well established that neutrophils kill pathogensviaphagocytosis and degranulation[1–2]. A novel third mechanism was discovered by Brinkmannet al[3]in 2004 that neutrophils utilize to kill microbes and this was named neutrophil extracellular traps (NETs). The NETs are mainly composed of histones and DNA fiber which entrap and facilitate the killing of pathogens[3]. Indeed, neutrophils undergone NET formation are identifiedin situin surgically excised appendicitis tissue in humans[4]implying NET formation involves fighting microorganismsin vivo.Since the discovery of NETs, the field of research has been extremely active and continues to expand.Contribution of NET formation to the pathogenesis of autoimmune chronic inflammation has been implicated because of extracellular extrusion of DNA and histones and other proteins that stimulate immune responses leading to the subsequent inflammatory process and cause tissue damage[5]. However,controversy and confusion have been generated due to contradictory results and divergent scientific concepts[6–7]. NET formation has been interchangeable with NETosis, a term coined by Steinberg and Grinstein to distinguish its difference from apoptosis and necrosis[8]. The initial description of NET formation for bacteria killing-appears to be more physiological,i.e., neutrophils are activated by bacteria, lipopolysaccharide (LPS), interleukin (IL)-8 or a low concentration of phorbol 12-myristate 13-acetate (PMA). The release of DNA, histones, granule proteins by neutrophils are independent of cell death.In other words, viable neutrophils form NETs to control pathogens. The neutrophil cell death referred to as NETosis was obtained following nonphysiological stimulation such as a high dose of PMA with a significantly longer duration[6–9]. Therefore, it has been suggested by a large group of scientists that NETosis should be abandoned or only be used where the demise of the neutrophil is obvious[6–7], in particular, not to be used to describe NET formation in a physiological situation.
Studies of neutrophils in RA have been focused on their role in protein citrullination in RA. NET formation (or NETosis as has been commonly referred in literature) has been linked to the citrullination of proteins in RA[10]. However, this notion has been challenged by other investigators who argue that leukotoxic hypercitrullination (LTH) and defective mitophagy of neutrophils are likely the mechanisms for the generation of citrullinated autoantigens in RA[11]. In comparison to the debatable notion regarding how neutrophils contribute to citrullination,the cytotoxic effects and inflammatory cytokine production by neutrophils in the pathogenesis of RA are generally accepted[12–14].
Neutrophils as effector cells in RA inflammation and tissue destruction
Neutrophils are the most abundant cell type present in synovial fluid in patients with RA[15]although are fewer in the synovial tissue[16–17], but are in a larger number at the cartilage-pannus junction where synovial tissue invades cartilage[18]. Neutrophils in the site of inflammation have become activated and display longevity[19]. Neutrophils in RA patients are functionally different from those of healthy individuals since neutrophils in RA blood are already primed for reactive oxygen species production[20].Among the cell types implicated in RA pathology,neutrophils have the greatest cytotoxic potential.Indeed, depletion of neutrophils significantly reduces the severity of experimental arthritis which implies the essential role of neutrophils in the pathogenesis of RA[21]. This has been confirmed by therapeutic effects in clinical practice where many efficacious agents affect neutrophils[14]although these were not originally aimed at targeting neutrophils. These agents include corticosteroids, methotrexate, tumor necrosis factor(TNF) inhibitors, IL-6 receptor blocker and JAK inhibitors[14]. The cytoplasmic granules of neutrophils contain various serine proteases which can be released upon stimulation. Immune complexes containing anticitrullinated protein antibodies (ACPA) and rheumatoid factor (RF) in RA joint can trigger neutrophil degranulation through FcγR[22]. For example, neutrophil elastase and cathepsin G process IL-33, an IL-1 family cytokine, into three bioactive forms. Moreover, these forms of IL-33 can be produced by neutrophilsin vivo[23]. Neutrophil elastase and collagenase cleave collagen, elastin and lubricin which directly involve cartilage damage[24–25]. Mice with deficiency in full activation of cathepsin G,neutrophil elastase and proteinase 3 are defective in the local production of IL-1β and TNF and are resistant to induction of experimental arthritis[26].These results provide direct evidence indicating the importance of these proteases in causing inflammation in arthritis.
Myeloperoxidase (MPO) is the most abundant cytotoxic enzyme in the azurophilic granules of neutrophils. Increased levels of MPO are present in RA plasma, synovial fluid and tissue[27–28]. In addition to its microbicidal functions, MPO interacts with vascular endothelial cells to increase endothelial permeability which is a critical process during inflammation[29]. Moreover, MPO also potently attracts more neutrophils to the site of inflammation[30]to amply the reaction. MPO triggers inflammatory cytokine production to further exacerbate the inflammation.In vitro, MPO can enhance proliferation but decrease apoptosis of RA synovial fibroblast-like synoviocytes[28]. Mice deficient in endogenous MPO significantly attenuated K/BxN arthritis and collageninduced arthritis[28].
Neutrophils regulate immune and inflammatory response in RA
Neutrophils in RA joint become activated likely by the microenvironment and the activated neutrophils are resistant to apoptosis and can survive for several days compared to the short life span of 24 hours in peripheral blood[31–32]. Besides secreting proteases,activated neutrophils act like macrophages or dendritic cells in the regulation of adaptive immune response.RA synovial fluid neutrophils secrete a number of inflammatory TNF family cytokines including TNF[33–34], B cell-activating factor (BAFF)[35]and receptor activator of nuclear factor kappa B ligand(RANKL)[36]. BAFF is a B cell growth factor that induces B cell proliferation and contributes autoantibody production in RA[37]. RANKL mediates osteoclast differentiation and is the major factor causing bone erosion in RA[38]. Activated neutrophils also express chemokines and chemokine receptors which facilitate the migration of and infiltration of neutrophils in RA joint[39–41]. Genetic deficiency or pharmacological inhibition of CCR2 abolishes neutrophil infiltration into the joints[39]. It has been shown that neutrophils can acquire the capacity of antigen presentationin vitroandex vivoto autologous memory CD4+T cells in a major histocompatibility complex Ⅱ (MHC Ⅱ) dependent manner[42]. This property of neutrophils has been observed in RA.Thus, RA joint neutrophils express MHC-Ⅱ. These MHC-Ⅱ expressing neutrophils can present antigen and stimulate CD4+T cells proliferation[43]. These results indicate that activated neutrophils gained function to regulate T and B cell function.
NET formation in RA
Enhanced NET formation in neutrophils of RA patients has been observed by many studies[44–53].Interestingly, one study suggests that the plasma level of cell-free nucleosome can be used as a biomarker for the identification of RA patients with high specificity and sensitivity[48]. Neutrophils in the peripheral blood and synovial fluid of RA patients display a propensity of spontaneous formation of NETs. Furthermore, NET formation of RA neutrophils is further enhanced by LPS stimulation when compared with those neutrophils isolated from the blood of healthy individuals or synovial fluid of patients with osteoarthritis (OA). This implies that neutrophils in RA patients have been primed to form NETsin vivowhich is evident by the presence of NET forming neutrophils in RA synovial tissue, rheumatoid nodules, and neutrophilic dermatoses. RA joint contain stimuli that are capable of inducing NET formation. These include IgA RF containing immune complex[46], purified IgG from RA plasma[54], ACPA and RF in the synovial fluid and inflammatory cytokines such as TNF and IL-17A[44]. In particular,ACPA against citrullinated vimentin which suggested to be pathogenic in RA potently induce NET formation of neutrophils from both healthy controls and RA patients. These results suggest that inducing NET formation in RA is one of the mechanisms that ACPA mediate the disease. Interestingly, TNF and IL-17A synergize in inducing NET formation of RA neutrophils,i.e., TNF priming significantly increased the NET forming induced by IL-17A[44].
Mechanisms of NET contribution to the pathogenesis of RA have been investigated. First,NETs can promote inflammatory property of fibroblast-like synoviocytes (FLS). When FLS are exposed to NETs, NETs can be internalized by FLS.FLS from both RA and OA can be stimulated by NETs and secrete significantly increased levels of IL-6[44]. The internalization is Toll-like receptor (TLR)-9 mediated. Furthermore, NET internalization induces MHC-Ⅱ upregulation by FLS. Arthritogenic NET peptides are loaded onto the MHC Ⅱ compartment and can be presented to and activate CD4+T cells[55].Most strikingly, humanized HLA-DRB*04:01 transgenic mice can produce ACPA after immunization with NETs loaded FLS. These ACPA recognize α-enolase, citrullinated fibrinogen and citrullinated vimentin which all are highly specific for RA. These ACPA production is CD4+T cell dependent. These animals also display pannus formation and cartilage degradation, although an overt clinical arthritis was not observed[55]. NET contains mainly citrullinated histones. Citrullinated H2B histones are present in high levels in RA synovial fluid in >90% of RA patients studied. These citrullinated H2B histones were able to stimulate macrophages to produce TNF and propagate neutrophil activation. Furthermore, immunization of mice with citrullinated H2B histone demonstrated that they were also arthritogenic in the setting that mice have been primed by anti-collagen type Ⅱantibodies[56]. These results may imply that citrullinated proteins contained in NETs may have triggered the immune responsein vivoin immunized mice. ACPA production is a hallmark of RA.However, how ACAP is initiated has remained elusive. NET formation has been considered to be the source of citrullinated autoantigens and may have initiated the autoimmunity to citrullinated proteins in RA[10,57]. Khandpuret aldemonstrated that induced NETs from both healthy individuals and RA patients contain citrullinated vimentin[44]. More relevant to the disease is that spontaneously formed NETs from RA patients contain citrullinated vimentin which binds to ACPA[44]. Citrullination of histones is a critical step in the initiation of NET formation and citrullinated histones comprise around 70% of all NET proteins[10].Autoantibodies against citrullinated histones are readily detected in RA sera and synovial fluid[50–51,56,58]. About 40% of recombinant monoclonal antibodies derived from ACPA positive RA patient synovial tissue display reactivity against citrullinated histones[59]. Interestingly, anti-citrullinated histones can be detected many years before RA develops in atrisk population[51,58,60]. NETs may release citrullinated histones that trigger immune response to produce antibodies against these modified proteins and subsequently ACPA recognize other citrullinated proteins are produced along with established inflammation.
Leukotoxic hypercitrullination of neutrophils and RA
Despite that these studies suggest NETs provide citrullinated proteins as antigens for ACPA production in RA, this notion has been challenged[11,61]. Andrade and colleagues suggest that a different form of cell death of neutrophils which is similar but distinctively different from NET formation is the source of citrullinated proteins in RA joint[11,61–64]. It has been demonstrated that citrullination in the RA joint is cellassociated and that a broad range of proteins are citrullinated. This pattern of citrullination was termed"cellular hypercitrullination" which is prominently induced by immune-mediated membranolytic pathways causing cell membrane pore formation, such as the action by perforin and membrane attack complex (MAC) of activated complements. Owing to its cytotoxicity in nature and resultant hypercitrullination, the membranolytic process was also termed "LTH". On the contrary, NET formation does not induce hypercitrullination observed in RA joint[64]. The so-called NET formation or NETosis in the literature was inappropriately claimed to contribute RA hypercitrullination[11,61]. Experimentally perforin and MAC caused neutrophil death trigger hypercitrullination and this process is calcium dependent. In other words, a surge of calcium influx precedes neutrophil death. Hypercitrullination is likely mediated by activation of peptidylarginine deiminase(PAD)-2, PAD3, and PAD4. These are all different from NET formation in which histone H3 citrullination is catalyzed preferentially by PAD4[65].Recently, Koniget al[62]demonstrated thatAggregatibacter actinomycetemcomitans, a pathogen causing periodontitis can cause cell death and hypercitrullination in neutrophils by secreting a poreforming toxin called leukotoxin A (LtxA). The spectrum of protein citrullination (citrullinome) of neutrophils induced by purified LtxA are markedly overlapped with citrullinome of RA synovial fluid[62].This observation is relevant to the pathogenesis of RA. In a cohort of RA patients, up to 43% have shown previousA. actinomycetemcomitansinfectionvs.11%in controls. Furthermore, the positivity of anti-LtxA antibodies (indication ofA. actinomycetemcomitansinfection) is significantly associated with the presence of ACPA and this association is more pronounced in RA patients bearing HLA-DRB1 alleles[62].
Mucosal sites as origin of RA-contribution of neutrophils
It has been hypothesized that RA may be initiated extraarticularly. This hypothesis is supported by that at-risk individuals do not display synovitis even as late as one week prior to clinical onset of arthritis[66],but ACPA and RF can exist in the circulation for over a decade before the clinical onset of arthritis[67–68]. This suggests that the autoimmune response has been in operation elsewhere precedes synovitis in the joint.Mucosal sites, in particular the lung, have been considered the sites for initiation of RA[69–70].Inflammation in the lung is evident in some at-risk individuals at pre-clinical phase of RA[71]. In at-risk population who later develop RA, ACPA can be detected in the sputum before they appear in the serum[72–73]. These autoantibodies are likely produced locally at inducible bronchus-associated lymphoid tissue[74]. The question arises here is how ACPA in the lung are developed in the first place. NET forming neutrophils may contribute. Both IgA and IgG ACPA are detectable in the at-risk first-degree relatives of RA patients and the levels of these ACPA are associated with NET levels in the sputum[73]. NET formation in the lung has been associated with smoking and inflammation and both have been associated with RA[75–76]. As the first responders,neutrophils respond primarily to pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs). It can be hypothesized that in response to the insult by PAMPs or DAMPs,neutrophils are recruited into the lung where enhanced NET formation or LTH also takes place to generate citrullination of proteins to trigger ACPA production.
The other mucosal site inflammation associated with RA is the periodontium. Clinical epidemiology studies have linked periodontitis with RA[77–80]and there are cases that periodontitis precedes joint synovitis in RA[81]. Periodontitis may breach the immune tolerance by generating citrullinated proteins for production of ACPA[82]. The major periodontal pathogensPorphyromonas gingivalisandA.actinomycetemcomitansare implicated for aberrant citrullination in periodontitis and RA.P. gingivalisis the only known human pathogen that produces PAD(designated as PPAD to distinguish it from human PAD) which exhibits a specificity for C-terminal arginine residue and generates citrullinated neoantigens of those implicated in RA[83–85]. WhileA.actinomycetemcomitanscontribute to the citrullination of proteins by means of LTH in whichA.actinomycetemcomitansmay cause pore formation of the cell membrane of neutrophils and lead to hypercitrullination[62]. Evidence presented above is compelling to indicate that the citrullination of proteins generated outside of joint may initiate the breach of tolerance and ACPA production. However,it is not clear how the autoimmunity in the mucosal site is transferred into the joint (Fig. 1).
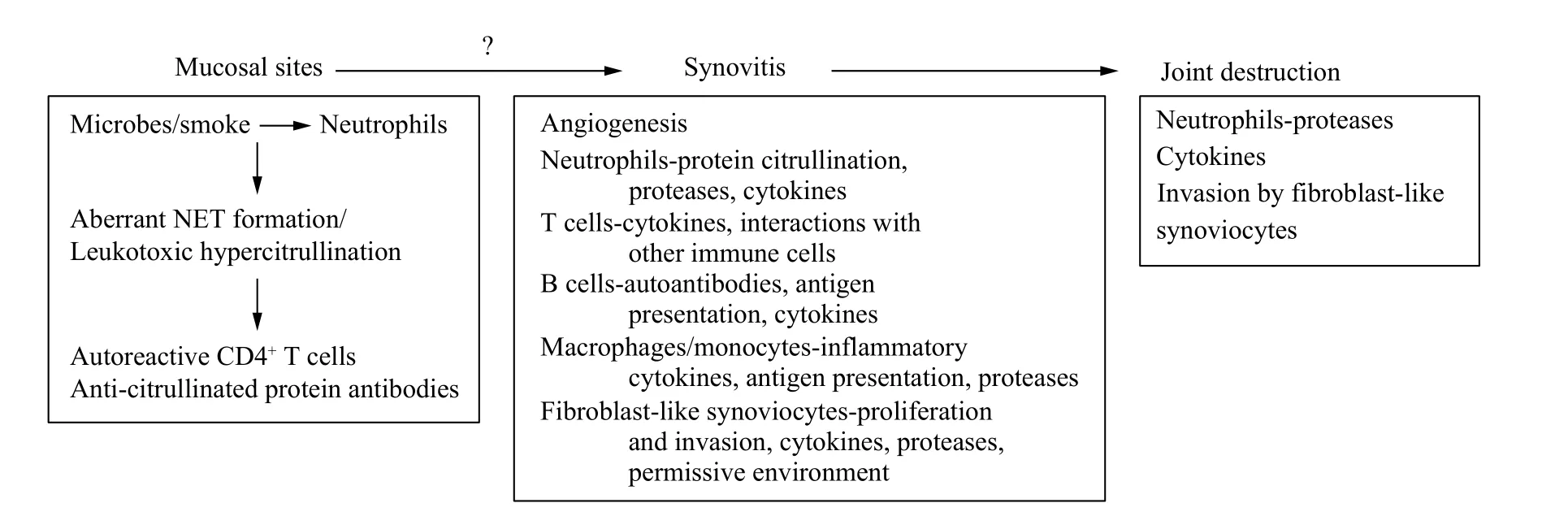
Fig. 1 Neutrophil centered etiology and pathogenesis of RA.
Concluding remarks
The cytotoxic and proinflammatory properties of neutrophils are likely mediators for RA inflammation and tissue destruction. There is also compelling evidence to support the contribution by neutrophils to citrullination in RA. However, it is debatable how neutrophils initiate citrullination in RA. Many earlier studies observed extrusion of chromatin and citrullinated proteins by neutrophils from RA patients attributed to the results of NET formation.
The initial description of NET formation which is a host defense mechanism by neutrophils that is well regulated and is not necessarily involved in neutrophil death. It is apparent the so-called NET formation or NETosis observed in RA patients may all represent an aberrant NET forming which may have been intended for host defense but was dysregulated. This may offer explanation for infection as an etiology for RA. The recently described phenomenon of neutrophils, LTH offers an alternative explanation for citrullination in RA. LTH is primarily utilized by microbes to kill neutrophils and this process results in release of a broad range of protein citrullination. The citrullination status can be amplified or maintained by MAC. The major distinction between NET formation and LTH is that NET formation is a NADPH oxidase (NOX2)-dependent process for neutrophils to kill microbes,whereas LTH is NOX2-independent process that is utilized by microbes to achieve immune evasion[11].Both processes are able to generate citrullinated proteins, but NOX2-independent process generates hypercitrullination which may be more pathogenic. In an attempt to further delineate the citrullination spectra, Chapmanet al[86]quantitatively analyzed the proteome generated by either NOX2-dependent or NOX2-independent mode. A broadly similar profile of protein citrullination can be induced by either mode. It is not clear whether the subtle difference between the protein citrullination profiles are meaningfulin vivoin terms of contribution to the disease pathogenesis in RA.
Acknowledgments
CQC was supported by Rheumatology Research Foundation Innovative and Pilot grants and VA Merit Review grant (BX002858).
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Angiosarcomatous component in gliosarcoma: case report and consideration of diagnostic challenge and hemorrhagic propensity
- Daily high-dose aspirin does not lower APRI in the Aspirin-Myocardial Infarction Study
- RNA-seq analysis identified hormone-related genes associated with prognosis of triple negative breast cancer
- The level of bile salt-stimulated lipase in the milk of Chinese women and its association with maternal BMI
- AAV-mediated human CNGB3 restores cone function in an allcone mouse model of CNGB3 achromatopsia
- Inhibitory role of peroxiredoxin 2 in LRRK2 kinase activity induced cellular pathogenesis
