H2S protects against diabetes-accelerated atherosclerosis by preventing the activation of NLRP3 inflammasome
2020-05-25QiaoZhengLihongPanYongJi
Qiao Zheng, Lihong Pan, Yong Ji
Key Laboratory of Cardiovascular and Cerebrovascular Medicine, Key Laboratory of Targeted Intervention of Cardiovascular Disease, Collaborative Innovation Center for Cardiovascular Disease Translational Medicine, Nanjing Medical University, Nanjing, Jiangsu 211166, China.
Abstract
Keywords: H2S, NLRP3, inflammation, diabetes-accelerated atherosclerosis
Introduction
Diabetes-accelerated atherosclerosis is the most common cardiovascular complication in diabetes mellitus[1]. The incidence of atherosclerosis in diabetic patients is several times higher than that of nondiabetic patients, with the onset time being early, the process being fast and the mortality rate being high[2].At present, there is a lack of an effective treatment for atherosclerosis caused by diabetes mellitus. Therefore,it is very important to explore the pathogenesis of diabetes-accelerated atherosclerosis and find the biomolecular targets for the treatment of this disease.
Hydrogen sulfide (H2S) has been considered as a toxic gas[3], but in recent years there has been growing evidence of the physiological importance of endogenous H2S, which is now recognized as a gaseous signaling molecule in organisms–"gasotransmitter",found after carbon monoxide (CO) and nitric oxide(NO)[4]. Recent evidence indicates that H2S is an important messenger that regulates the physiological and pathological functions of the body, because of its strong antioxidant and anti-inflammatory effects[5],and thus may imply its potential protective effect on a variety of diseases, especially in the cardiovascular system[6–7]. H2S can stabilize atherosclerotic plaque,prevent ischemia-reperfusion injury[8], improve endothelial dysfunction induced by hyperglycemia[9]and protect against myocardial infarction and heart failure[6]. At present, there are few reports on the role of H2S in diabetes-accelerated atherosclerosis, and our previous studies have pointed out that H2S could promote Nrf2 activation through promoting Keap1 Ssulfhydration, thus protecting against diabetesaccelerated atherosclerosis[10]. In previous studies, we have revealed that the protective effects of H2S on diabetes-accelerated atherosclerosis were relying on the attenuation of oxidative stress and inflammation.
This study indicates that GYY4137, an H2S donor,can effectively inhibit the development of diabetesaccelerated atherosclerosis, which plays a protective rolein vitroandin vivo. Moreover, H2S can inhibit the activation of pyrin domain containing protein 3(NLRP3) inflammasome, an important inflammation signaling pathway, thus decreasing IL-1β production and eventually attenuating the pathological progress of diabetes-accelerated atherosclerosis.
Materials and methods
Cell culture and treatment
Umbilical cords of newborn babies were derived from the Third Affiliated Hospital of Nanjing Medical University. The study was approved by the Ethics Committee of the Third Affiliated Hospital of Nanjing Medical University and was performed in compliance with the Declaration of Helsinki Principles. Human umbilical vein endothelial cells (HUVECs) were isolated and cultured following the instruction of previous methods[11]. The HUVECs were cultured in endothelial cell medium (ECM, ScienCell, USA).HUVECs were treated with high glucose (25 mmol/L,Sigma, USA) and oxLDL (100 mg/L, Peking Union-Biology Co., Ltd., China), with or without GYY4137(100 μmol/L) for 24 hours.
Western blotting analysis
Total cells or aortic tissue were lysed in appropriate volumes of RIPA lysis buffer (Beyotime Biotechnology, China) (50 mmol/L Tris-HCl, pH 8.0,20 mmol/L EDTA, 1% SDS, 100 mmol/L NaCl) with the protease inhibitor cocktail (Thermo Fisher Scientific, USA), homogenized and gathered by centrifugation at 12 000gfor 5 minutes. Equal amounts of cells or aortic tissue were loaded and separated on 15% or 10% SDS poly-acrylamide gels and transferred to PVDF membranes. Western blotting analysis was performed with the primary antibodies included anti-intercellular cell adhesion molecule 1 (ICAM1) (sc-8439; Santa Cruz Biotechnology,USA), anti-vascular cell adhesion molecule 1 (VCAM1) (sc-13160; Santa Cruz Biotechnology),anti-GAPDH (AP0063; Bioworld, USA), anti-procaspase-1 (ab179515; Abcam, USA), anti-caspase-1(ab1872; Abcam), anti-IL-1β (ab9722; Abcam) and anti-NLRP3 (ab232401; Abcam), and the secondary antibodies included Peroxidase-AffiniPure Goat Anti-Rabbit IgG (H+L) (111-036-003; Jackson, USA),Peroxidase-AffiniPure Goat Anti-Mouse IgG (H+L)(111-035-003; Jackson). Proteins were visualized by enhanced chemiluminescence substrate (Tanon,China).
Small interfering RNA transfection
NLRP3 expression was silenced by using siRNA against NLRP3 which was purchased from Genepharma (China). HUVECs were transfected with siRNA oligonucleotide following the instruction of the Lipofectamine 3000 reagent (Invitrogen, USA).
RNA isolation and quantitative real-time PCR(qRT-PCR)
The whole RNA was isolated from cells or the aortic tissues with Trizol reagent (Takara, Japan).Total mRNAs were reverse-transcribed into cDNA for subsequent analyses by using the Revert Aid First Strand cDNA Synthesis kit (Vazyme, China),according to the instruction. qRT-PCR was carried out in triplicate with the resulting cDNAs using SYBR Green Premix (Takara) and ABI 7500 Real-time PCR System (ABI, Carlsbad, USA). Primers used for qRTPCR were purchased from Thermo Fisher Scientific.Sequences of primers involved in this paper for qRTPCR were as follows: Human TNF-α, Forward primer(5′→3′): CCTCTCTCTAATCAGCCCTCTG, Reverse primer (5′→3′): GAGGACCTGGGAGTAGATGAG;Human IL-1β, Forward primer (5′→3′): ATGATGG CTTATTACAGTGGCAA, Reverse primer (5′→3′):GTCGGAGATTCGTAGCTGGA; Human IL-6,Forward primer (5′→3′): ACTCACCTCTTCAGAAC GAATTG, Reverse primer (5′→3′): CCATCTTTGG AAGGTTCAGGTTG; Human MCP1, Forward primer (5′→3′): CAGCCAGATGCAATCAATGCC,Reverse primer (5′→3′): TGGAATCCTGAACCCA CTTCT; Human ICAM1, Forward primer (5′→3′):TGACCGTGAATGTGCTCTC, Reverse primer(5′→3′): TCCCTTTTTGGGCCTGTTGT; Human VCAM1, Forward primer (5′→3′): AATGCCTGGG AAGATGGTCG, Reverse primer (5′→3′): GATGT GGTCCCCTCATTCGT; Human GAPDH, Forward primer (5′→3′): GGAGCGAGATCCCTCCAAAAT,Reverse primer (5′→3′): GGCTGTTGTCATACTTC TCATGG; Mouse ICAM1, Forward primer (5′→3′):GCTACCATCACCGTGTATTCG, Reverse primer(5′→3′): TGAGGTCCTTGCCTACTTGC; Mouse VCAM1, Forward primer (5′→3′): ACTCCCGTC ATTGAGGATATTG, Reverse primer (5′→3′): TGA CAGTCTCCCTTTCTTTGAG; Mouse β-actin,Forward primer (5′→3′): GGACTGTTACTGAGCTG CGTT, Reverse primer (5′→3′): CAACCAACTGCT GTCGCCTT.
Animal treatment
MaleLdlr-/-mice (specific pathogen free, SPF), on a C57BL/6 background, were derived from Model Animal Research Center of Nanjing Medical University. Streptozotocin (STZ) [60 mg/(kg·day)]was intraperitoneally injected intoLdlr-/-mice at the age of 8 weeks for 5 days without interruption. Five days later, mice were performed with GYY4137 (a H2S donor) and were fed with a high-fat diet (D1-2108C) for 1 month. GYY4137 [133 μmoL/(kg·day)]or saline was intraperitoneally injected. The animals were kept under standard animal room conditions[temperature (21±1) °C; humidity 55%–60%; 12–hour light : dark cycles]. The mice were anesthetized with 3.5% isoflurane and maintained with 2.0% isoflurane during the surgery. All animal experiments were carried out in accordance with the guidelines approved by the Institutional Animal Care and Use Committee of Nanjing Medical University.
Hematoxylin-eosin staining
Hematoxylin-eosin staining was conducted in the light of the instruction (Beyotime Biotechnology).Briefly, after 5 μm sections were deparaffinized and rehydrated, sections were dipped in hematoxylin solution for 10 minutes. Then, the sections were dipped in 1% acid ethanol (1% HCl in 70% ethanol)for 5 times and then washed in ultra-pure water for 20 minutes. The sections were then dipped in eosin solution for 3 minutes, and then dehydrated in graded ethyl alcohol and washed in xylene. Finally, the handled slides were then checked and photographed under a microscope (Olympus, Japan).
Masson's trichrome staining
After deparaffinization and rehydration, using graded ethyl alcohol, sections were washed in ultrapure water for 1 minute and then dipped in Weigert's iron hematoxylin working solution (Beyotime Biotechnology) for 10 minutes. Sections were then washed under warm running water for 10 minutes before being twice washed in ultra-pure water. The sections were next dipped in Biebrich scarlet-acid fuchsin solution for 10–15 minutes. Wash the sections in ultra-pure water for 2 times. The sections were then differentiated in phosphomolybdic-phosphotungstic acid solution for 10 –15 minutes while waiting for the collagen no longer turned red. Sections were then transferred directly to the aniline blue solution and tarnished for 5–10 minutes before being washed in ultra-pure water, then washed in ultra-pure water and differentiated in 1% acetic acid solution for 2 –5 minutes. Finally, sections were washed with ultra-pure water, dehydrated in a gradient of ethanol and washed in xylene. The handled slides were then checked and photographed under a microscope (Olympus).
Statistical analysis
All data are expressed as mean±standard error of mean (mean±SEM). Gray value of the bands was analyzed by ImageJ software. Distribution of the data was first analyzed using the Kolmogorov-Smirnov test for analysis of normality. Depending on the number of experimental groups and factors to be compared,statistical analysis was performed with Student'st-test or one-way ANOVA followed by post hoc test(Bonferroni). Otherwise, Johnson transformation was applied to transform the data.P<0.05 was considered statistically significant. Statistical analysis was done with GraphPad Prism 5 (GraphPad Software Inc, San Diego, USA).
Results
H2S protects against diabetes-accelerated atherosclerosis in diabetic Ldlr-/- mice
To determine the effect of H2S on the endothelium of diabetes-accelerated atherosclerosis mouse models,mice were injected intraperitoneally with GYY4137 or saline for 4 weeks. Meanwhile, they were kept on a high fat diet (HFD) to accelerate atherogenesis. The plaque area and collagen deposition in the aortic roots of mice's hearts were performed. As expected,treatment of diabeticLdlr-/-mice with H2S significantly reduced atherosclerotic plaques and collagen deposition compared with theLdlr-/-mice(Fig. 1A). We also found a significant decrease in the expression of intercellular cell adhesion molecule 1(ICAM1) and vascular cell adhesion molecule 1(VCAM1), both at mRNA levels (Fig. 1B) and protein levels (Fig. 1C). Collectively, these results revealed that exogenous H2S decreased atherosclerotic lesions and ICAM1 and VCAM1 expression in diabetes-accelerated atherosclerosis mouse models.
H2S reduced the expression of ICAM1 and VCAM1, and inflammation factors in vitro
To further investigate the protective effects of H2S,we established a HUVECs model treated with high glucose (25 mmol/L) and oxidized low density lipoprotein (oxLDL, 100 mg/L)in vitro, which could replicate some of the features of endothelial cells in the diabetes-accelerated atherosclerotic mouse models. We examined the expression of adhesion molecules ICAM1 and VCAM1 (Fig. 2AandB) as well as inflammatory factors of tumor necrosis factorα (TNF-α), interleukine-1β (IL-1β), interleukin 6 (IL-6), monocyte chemoattractant protein 1 (MCP1)(Fig. 2C) in the HUVECs of high glucose and oxLDL treatments. The increase of ICAM1 and VCAM1 induced by high glucose and oxLDL were reversed by H2S. The same changes were observed in inflammatory factors. These results demonstrated that H2S could reduce inflammationin vitro.
H2S decreased the activation of NLRP3 inflammasome in vivo and in vitro
The above results suggested that the inflammatory response plays a vital role in the pathological process of diabetes-accelerated atherosclerotic. Therefore, we detected the activation of caspase-1 and IL-1β, and the expression of NLRP3 (Fig. 3A) which could reflect the inflammatory infiltration in the aortic tissues of diabeticLdlr-/-mice. Moreover, diabetic mice demonstrated an obvious increase in caspase-1 and IL-1β as well as the expression of NLRP3, compared with the non-diabetic control. In addition, the treatment of diabeticLdlr-/-mice with GYY4137 could also reverse these changes. We next verified thesein vitrowith the same results (Fig. 3B). Together, these experiments indicated that H2S decreased the activation of NLRP3 inflammasome in diabetesaccelerated atherosclerotic cells and mouse models.
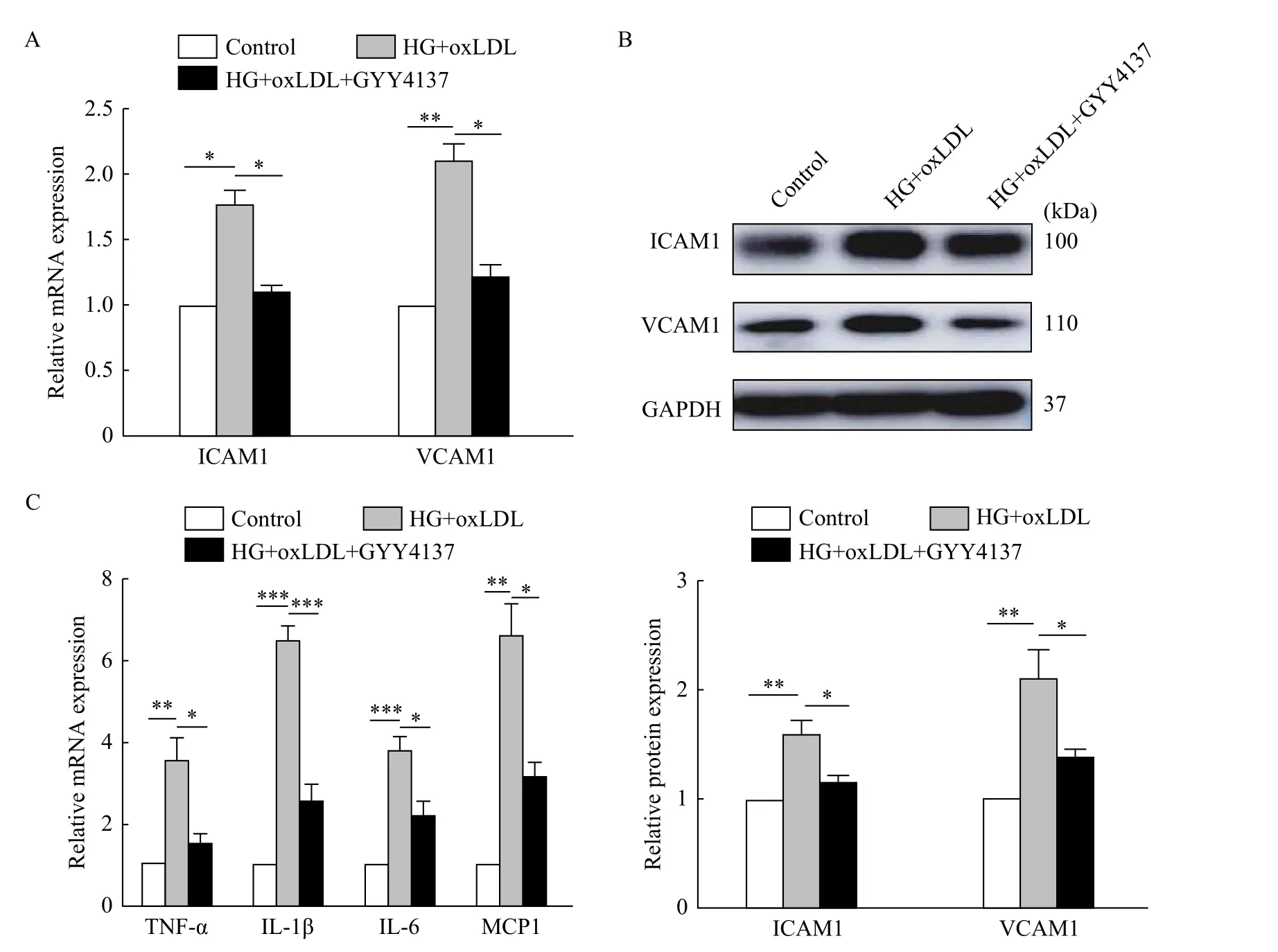
Fig. 2 H2S reduced the expression of ICAM1 and VCAM1, and inflammatory factors in vitro. HUVECs were treated with high glucose and oxLDL, with or without GYY4137 for 24 hours. A: Total mRNAs were extracted and gene expression levels of ICAM1 and VCAM1 were determined by qRT-PCR (*P<0.05, **P<0.01, n=3). B: Total proteins were extracted and analyzed by Western blotting assay to determine the level of ICAM1 and VCAM1 expression (*P<0.05, **P<0.01, n=3). C: Total mRNAs were extracted and gene expression levels of TNF-α, IL-1β, IL-6 and MCP1 were determined by qRT-PCR (*P<0.05, **P<0.01, ***P<0.001, n=3). HG: high glucose.
Down-regulation of NLRP3 attenuated endothelial injury in vitro
The above results suggested that H2S may participate in diabetes-accelerated atherosclerotic by inhibiting inflammasome. To further verify this, we knocked down the expression of NLRP3 with siRNA(Fig. 4A), and then detected the expression of adhesion molecules ICAM1 and VCAM1 (Fig. 4B).Silencing of NLRP3 significantly reduced the expression of ICAM1 and VCAM1 in high glucose and oxLDL-treated HUVECs compared with that of control. In conclusion, H2S can protect endothelial injury from high glucose and oxLDL treatment by inhibiting NLRP3 inflammasome.
Discussion
Diabetes-accelerated atherosclerosis is closely related to oxidative stress and inflammatory response[12]. High glucose-induced the increase of superoxide may be a key event leading to atherosclerosis[13–16], so suppressing oxidative stress is an important measure to attenuate atherosclerosis caused by diabetes. Our previous research also reported that the anti-oxidative stress of H2S could effectively delay the progression of diabetesaccelerated atherosclerosis caused by high glucose and oxLDL[10]. Moreover, GYY4137 is expected to become a drug for the treatment of diabetesaccelerated atherosclerosis caused by oxidative stress with its effective release of H2S at a physiological concentration.
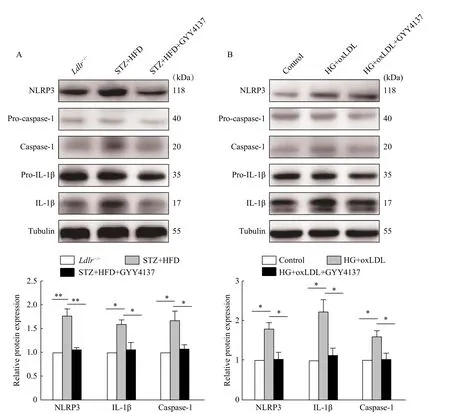
Fig. 3 H2S inhibited the activity of NLRP3 inflammasome in vivo and in vitro. A: Total proteins was extracted from the aortic tissues and analyzed by Western blotting assay to determine the levels of pro-caspase-1, caspase-1, pro-IL-1β, IL-1β and NLRP3 expression(*P<0.05, **P<0.01, n=3). B: HUVECs were treated with high glucose and oxLDL, with or without GYY4137 for 2 hours. The control group means without any treatment. Total proteins were extracted from cells and analyzed by Western blotting assay to determine the level of procaspase-1, caspase-1, pro-IL-1β, IL-1β and NLRP3 expression (*P<0.05, n=3). STZ: streptozotocin; HFD: high fat diet; HG: high glucose.
In our previous studies, we found that significant lipid plaques formed in the aortic roots of theLdlr-/-mice of high fat diet and STZ-induced diabetesaccelerated atherosclerosis, and H2S could effectively inhibit plaque formation. Additionally, vascular oxidation is effectively reduced by activating the Nrf2 signaling pathway[10,17–18]. We also found that macrophages infiltration was evident in high fat and STZinduced diabetes-accelerated atherosclerosis mouse models, suggesting that the inflammation also plays a role in the pathological process. H2S treatment could significantly reduce the extent of macrophages infiltration and inhibit the inflammatory response.Based on this finding, we have now studied and demonstrated that H2S could not only protect against atherosclerosis in diabetes through inhibiting oxidative stress, but also prevent the progression of the disease through the attenuation of inflammatory response.
GYY4137, a H2S donor, could inhibit inflammation-related diseases such as peritonitis and acute ventilation by inhibiting the activation of NLRP3 inflammasome and reducing caspase-1 activation and IL-1β secretion[19–20]. GYY4137 also protects against amyloid beta-peptide induced neuronal injuryviaattenuating inflammatory response[7]. GYY4137 attenuates myocardial ischemia and reperfusion injury by reducing oxidative stress and apoptosis in rats[21]. In cardiovascular diseases,H2S regulates the inflammatory response of macrophages by inhibiting the activation of NLRP3 inflammasome, reducing caspase-1 activation and IL-1β secretion. Additionally, NLRP3 inflammasome is closely related to endothelial function[22]. However,the role of NLRP3 in diabetes-accelerated atherosclerosis has not been reported. The above reports suggested that H2S may reduce inflammation by reducing the activation of NLRP3 inflammasome and play a key role in the protection of diabetesaccelerated atherosclerosis.
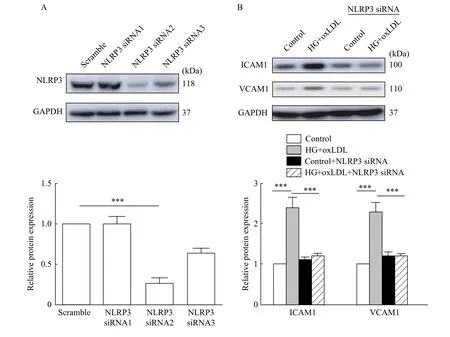
Fig. 4 Silencing NLRP3 suppressed ICAM1 and VCAM1 expression in vitro. A: HUVECs were transfected with scramble or NLRP3 siRNA for 48 hours. Total proteins were extracted and analyzed by Western blotting assay to determine interference efficiency. B: HUVECs were transfected with control siRNA or NLRP3 siRNA for 24 hours and then treated with high glucose and oxLDL (***P<0.001, n=3). Total proteins were extracted and analyzed by Western blotting assay to determine the level of ICAM1 and VCAM1 expression (***P<0.001, n=3).HG: high glucose.
To construct type 1 diabetes,Ldlr-/-mice were intraperitoneally injected with STZ and kept on a HFD to induce diabetes-accelerated atherosclerosis mouse models. Moreover, endothelial cells were treated with high-glucose and oxLDL to mimic diabetesaccelerated atherosclerosis induced cell injury[23]. In the endothelial injury, we found that the expression of ICAM1 and VCAM1 was significantly increasedin vivoandin vitro. At the same time, we tested the mRNA expression levels of various inflammatory factors. The expression of inflammatory factors increased in high-glucose and oxLDL-treated endothelial cells, while significantly declined after H2S pretreatment. Thus, H2S plays an important role in inhibiting inflammation of diabetes-accelerated atherosclerosis. It is worth noting that we have demonstrated that high glucose and oxLDL can induce the activation of NLRP3 inflammasome, leading to the activation of caspase-1 and the production of the proinflammatory cytokine IL-1β. H2S could significantly inhibit these effects (Fig. 5). The silencing of NLRP3 significantly reduced caspase-1 activation, IL-1β production, ICAM1 and VCAM1 expression in high glucose and oxLDL-treated endothelial cells.
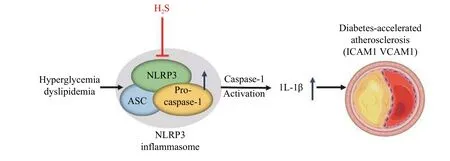
Fig. 5 GYY4137 can effectively protect against the development of diabetes-accelerated atherosclerosis through inhibiting inflammasome activation.
Taken together, our experiments demonstrated that H2S delayed the progression of diabetes-accelerated atherosclerosis inLdlr-/-mice and improved high glucose and oxLDL-induced endothelial cell injury.H2S reduced the activation of NLRP3 inflammasome in endothelial cell injury and diabetes-accelerated atherosclerosis mouse models. H2S donors have therapeutic potential for cancer, erectile dysfunction,peptic ulcer disease, Parkinson's and Alzheimer's diseases, acute and chronic inflammatory diseases,atherosclerosis, arterial and pulmonary hypertension and heart failure, among other diseases[24]. Several drugs (such as statins[25], aspirin[26], and metformin[27])are also found to regulate H2S production, but the mechanisms and clinical significance are not fully understood. Our findings provide new evidence for the treatment of cardiovascular diseases by H2S donors.
Acknowledgments
This work was supported by grant from National Nature Science Foundation of China (Grant No.81820108002).
杂志排行
THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- The current status of malignant hyperthermia
- Contribution of neutrophils in the pathogenesis of rheumatoid arthritis
- Inhibitory role of peroxiredoxin 2 in LRRK2 kinase activity induced cellular pathogenesis
- AAV-mediated human CNGB3 restores cone function in an allcone mouse model of CNGB3 achromatopsia
- The level of bile salt-stimulated lipase in the milk of Chinese women and its association with maternal BMI
- RNA-seq analysis identified hormone-related genes associated with prognosis of triple negative breast cancer
