Biodegradation of Ammonia Nitrogen Using a Novel Candida sp.Strain N6 Immobilization
2020-05-21KaiWangYawenSunSiqiLiYingjieZhangXiquanChengandJunMa
Kai Wang,Yawen Sun, Siqi Li, Yingjie Zhang, Xiquan Chengand Jun Ma
(1. School of Marine Science and Technology, Harbin Institute of Technology, Weihai,Weihai 264209, Shandong, China;2. School of Municipal and Environmental Engineering, Harbin Institute of Technology, Harbin 150090, China)
Abstract: Due to the high efficiency of ammonia nitrogen degradation, a novel Candida sp. strain N6 was applied for wastewater treatment by using immobilization technology. Immobilization conditions and corresponding ammonia removal capacity of immobilized N6 beads were investigated.The immobilized N6 beads were applied to degrade simulated ammonia nitrogen wastewater. Results showed that the optimum formula of immobilized materials was 9% polyvinyl alcohol, 1.5% sodium alginate, and 2% calcium chloride, under which the ammonia nitrogen removal rate of the immobilized N6 beads reached 97.97%. The following immobilization conditions were observed to be optimal: the immobilization time was 24 h, the inoculum of N6 was 3%, and the pH was 8.The immobilized N6 beads exhibited excellent ammonianitrogen degradation ability in SBRs. The ammonia nitrogen removal rate was stable at 95%-99% in the SBRs. The results indicated that immobilized N6 beads possess good application prospects in the treatment of ammonia nitrogen wastewater.
Keywords: ammonia wastewater; immobilization;Candida sp.; nitrification efficiency
1 Introduction
The rapid expansion of urbanization and economic activities has brought tremendous pressure to water bodies. Compared with the past years, various pollutants are discharged into water bodies, resulting in significant deterioration of water quality. Ammonia nitrogen is a type of pollutant that causes water pollution. Domestic sewage and industrial wastewater are discharged without treatment, which will cause nitrogen pollution and have detrimental effects on human health and the aquatic environment. The ammonium ions and ammonia contained in the discharged sewage are all aerobic compounds, which can consume dissolved oxygen in the water. Nitrogen is easily utilized by aquatic plants such as algae, thereby causing eutrophication and ecological destruction[1-2]. Therefore, if the wastewater containing ammonia nitrogen is not treated in time, it will put great pressure on the environment and the normal life of human[3-4]. Among the current nitrogen containing wastewater treatment methods, the biological method is considered to be the most economical and effective for denitrification since it is low-cost, convenient, and has no secondary pollution.
Immobilized biological nitrogen removal technology is one of the most widely studied technologies in the current biological nitrogen removal process[5]. Free cells may face many problems during water treatment, such as survival, utilization of nutrients, poor adaptability, mechanical interference, and competition with other microorganisms[6]. Immobilized microbial technology has advantages in increasing microbial concentration and mechanical strength, enhancing impact load, and competing with indigenous microorganisms[7-8]. Studies reported in previous investigations have shown that the degradation process of ammonia by immobilized cells can be improved compared with that of the free cells[9]. Furthermore, the immobilized cells have wider pH and temperate range than those of free cells[10-11]. Immobilized carriers include natural polymer materials (e.g., polysaccharide, alginate, agar) and synthetic polymer materials (e.g., polyacrylamide, polyvinyl alcohol, silica gel)[12]. Immobilized microbial technology has been demonstrated by many researchers for the degradation of atrazine[13], herbicides[14], crude oil[15], heavy metals[13], etc. Studies have shown that immobilized microorganisms can also remove ammonia nitrogen from wastewater[16-20].
The traditional biological nitrogen removal process mainly relies on the nitrification and denitrification of related microorganisms. Since most of the nitrifying microorganisms are autotrophic microorganisms with extremely slow growth, nitrification has become a rate-limit step of the traditional biological nitrogen removal process. In contrast, heterotrophic microorganisms grow faster and have higher survival rates than autotrophic microorganisms, which means that they have more advantages in denitrification rate. Therefore, the selection of heterotrophic microorganisms with high ammonia nitrogen degradation efficiency is conducive to accelerating nitrification. Researchers have carried out numerous experiments on ammonia nitrogen degrading bacteria. Rodríguez et al.[21]isolated seven strains with potential nitrogen reduction in household wastewater, includingStreptomycessp.,Pseudomonasputida,Sphingomonassp., andAeromonassp. Lei et al.[22]isolated a heterotrophic nitrification-aerobic denitrification bacterium belonging toZobellellataiwanensis. Studies have shown that screening for high efficiency ammonia nitrogen degrading bacteria is one of the most effective methods for obtaining effective microbial agents.
In this study, the fungi with ammonia nitrogen degradation ability were combined with immobilization technology to treat ammonia nitrogen wastewater. A heterotrophicCandidasp.N6 was immobilized to remove ammonia nitrogen from simulated wastewater. To our knowledge, there is no report ofCandidasp. strain for ammonia nitrogen degradation. The efficiency and conditions of immobilized N6 beads on removing ammonia nitrogen were examined. The initial application of immobilized N6 beads in the laboratory scale sequencing batch reactors (SBRs) was also studied.
2 Methods
2.1 Strains
A heterotrophic bacterium named as N6 capable of removing ammonia was used in this study. The strain was originally isolated from the sludge at the offshore sewage outfall at Weihai International Beach and was identified asCandidasp.N6 based on the phylogenetic characteristics (GenBank accession number MH368485).
2.2 Culture Medium
Yeast Extract Peptone Dextrose Medium (YPD medium) used for the pre-cultivation of the fungus contains 10 g yeast extract, 20 g peptone, and 20 g glucose that were all dissolved in 1 000 mL distilled water. For ammonia nitrogen degradation studies, the simulated ammonia wastewater contains (per liter) 5.0 g glucose, 0.25 g (NH4)2SO4, 5.0 g NaCl, 0.5 g K2HPO4, 0.25 g MgSO4·7H2O, and 0.25 g FeSO4·7H2O. The glucose in the medium was sterilized by autoclaving at 115 ℃ for 15 min and other ingredients were sterilized at 121 ℃ for 20 min. The medium has an ammonia nitrogen concentration of approximately 50 mg/L.
2.3 Immobilization of Fungus
Strain N6 was inoculated into 50 mL conical flasks containing 20 mL YPD medium and was cultured in a rotary shaker at 120 r/m and 28 ℃ for 24 h. The cultured cell suspension of N6 was then centrifuged at 3 000 r/m for 10 min, after which the cells were washed with normal saline and centrifuged for three times. Polyvinyl alcohol (PVA) and sodium alginate (SA) were added to water which was then heated and stirred until complete dissolution. The obtained mixture was further sterilized by autoclaving at 121 ℃ for 20 min. The cells of N6 were added to the mixture which was slowly stirred to uniformity. By using an injector, the mixed solution was dropped into a saturated H3BO3solution containing CaCl2to form spherical beads. The pH of the solution was adjusted to 6.7 before injection. The beads were immobilized at 4 ℃. Subsequently, the immobilized N6 beads were washed with normal saline for three times and stored in normal saline at 4 ℃.
2.4 Determination of Ammonia Nitrogen Degradation Ability
Immobilized N6 beads were inoculated into 100 mL conical flasks containing 50 mL simulated ammonia wastewater medium and were cultured in a rotary shaker at 120 r/m and 28 ℃ for 5-7 days. After ammonia nitrogen degradation, the water sample was centrifuged at 5 500 r/m for 15 min. Ammonia nitrogen content was evaluated by using Nessler’s reagent spectrophotometry method. The medium without immobilized beads was used as control. Ammonia degradation rate was calculated according to the following formula
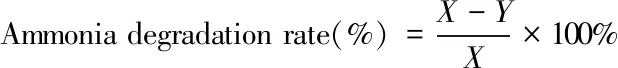
(1)
whereXandYare the ammonia nitrogen concentrations of the control and the sample.
2.5 Determination of Immobilization Conditions for Ammonia Nitrogen Removal
2.5.1ImmobilizedMaterialAdditionAmount
Immobilized materials affect the degradation capacity of N6 cells. Firstly, the levels of orthogonal array testing were determined by single factor experiments. Effects of different concentrations of immobilized materials (PVA, SA, and CaCl2) on immobilization cells were investigated by examining the physical properties (mass transfer) and the ammonia nitrogen degradation rate of immobilized N6 beads. The following experimental conditions were applied: 4%, 5%, 6%, 7%, 8%, 9%, 10% PVA, 0.5%, 1%, 1.5%, 2%, 2.5%, 3% SA, and 1%, 2%, 3%, 4%, 5%, 6% CaCl2. The mass transfer characterization method is as follows[23]: add equal amounts of diluted blue ink to different beakers and put in equal masses of immobilized beads with different parameters. After 18 h and 32 h, the solution absorbance value in the beaker was measured at 406 nm with the spectrophotometer.
Secondly, multiple parameters were optimized to immobilize N6 cells for degrading ammonia nitrogen by orthogonal array test[24]. The factors of orthogonal array test included PVA concentration, SA concentration, and CaCl2concentration. An L9(34) orthogonal array experiment was designed as shown in Table 1.
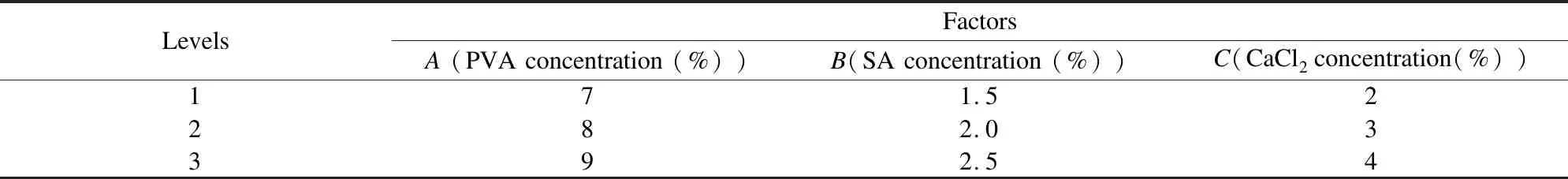
Table 1 Levels of the orthogonal array experimental design
2.5.2DeterminationofN6initialconcentration,pHvalue,andimmobilizationtime
Effects of different initial concentrations of N6 (0.5%, 1%, 2%, 3%, 4%, and 5%) on the degradation of ammonia nitrogen by immobilized N6 beads were studied. To investigate the ammonia nitrogen removal ability of immobilized N6 beads at different pH, the pH value was adjusted to 4, 5, 6, 7, 8, 9, and 10 in the simulated ammonia wastewater. Effects of different immobilization time (6 h,12 h,24 h,36 h, and 48 h) on immobilized beads were also investigated.
2.6 Stability Test of Immobilized N6 Beads
Sixty immobilized N6 beads were added to a beaker containing 100 mL deionized water which was stirred evenly with a magnetic stirrer for 24 h. The number of the remaining immobilized N6 beads in the beaker was recorded at 4, 8, 12, 20 and 24 h. The stability of the immobilized N6 beads was investigated based on the damage of the beads[25].
2.7 Ammonia Nitrogen Degradation Effect of Immobilized N6 Beads in SBRs
The ammonia nitrogen degradation effect of immobilized N6 beads under optimal immobilization conditions was investigated in SBRs. The experiment was carried out in a sequence batch operation mode with a cycle of 24 h. An air pump was used to aerate the bottom of the SBRs to control the dissolved oxygen to 3-5 mg/L. The temperature was room temperature (around 25 ℃). The immobilized N6 beads of different addition amounts (5%, 10%, 15%, 20%, 25%, and 30%) were added to the SBRs which contained fresh simulated wastewater, and the residual ammonia nitrogen concentrations were measured at the end of each cycle to obtain the optimum addition amounts of immobilized N6 beads.
3 Results and Discussion
3.1 Effect of Immobilized Materials on Immobilized N6 Beads
3.1.1SurfacemorphologyoftheimmobilizedN6beads
The immobilized N6 beads under optimal immobilization conditions are shown in Fig.1. They were pure white spheres and about 3-5 mm in diameter. Biodegradation of N6 was the main moving force to ammonia nitrogen removal, and the immobilized materials provided protection for N6 cells.
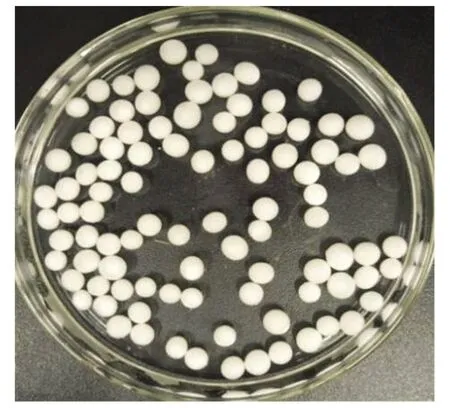
Fig.1 Surface morphology of immobilized N6 beads
3.1.2Effectofimmobilizedmaterialsconcentrationsonthepropertiesofimmobilizedbeads
PVA and SA concentrations affect the mechanical strength and pore size of the immobilized particles, while the pore size affects the diffusion of nutrients[26]. CaCl2concentration also affects the mechanical strength and the immobilization degree of immobilized beads[27-28]. To determine the effect of the concentration of immobilized materials (PVA, SA, and CaCl2) on the immobilized beads, the mass transfer (Figs.2-4)was used for characterization. The adopted immobilization conditions at this stage were 4% of the initial inoculum size, pH 7.5-7.8, and 24 h immobilization time.

Fig.2 Effect of PVA concentration on mass transfer(SA concentration: 1.5%; CaCl2 concentration: 3%)
The mass transfer of immobilized beads was represented by the absorbance change of ink color. The effect of PVA concentration on the mass transfer of immobilized beads is illustrated in Fig.2. The PVA concentrations of 6%-9% had smaller absorbance value both after 18 h and 30 h, at which the immobilized beads had the best mass transfer. It indicates that 6%-9% PVA is more suitable for immobilization.

Fig.3 Effect of SA concentration on mass transfer(PVA concentration: 8%; CaCl2 concentration: 3%)

Fig.4 Effect of CaCl2 concentration on mass transfer(PVA concentration: 8%; SA concentration: 2%)
Fig.3 shows that the immobilized beads using 2% SA exhibited the best mass transfer. During the immobilized process, the beads were easily formed at low SA concentration. With the increase of SA concentration, the solution became thicker and the immobilized beads were difficult to form.
The effect of CaCl2concentration on mass transfer is different from those of PVA and SA concentrations. As illustrated in Fig.4, the mass transfer of immobilized beads increased on the initial stage and then remained stable with the increase of CaCl2concentration. This may due to the fact that CaCl2can act as porogen[29]. When the concentration of CaCl2was more than 4%, CaCl2fully exerted its effect and the mass transfer did not increase.
3.1.3Effectofimmobilizedmaterialsconcentrationsonammonianitrogenremoval
The degradation of ammonia nitrogen in different PVA, SA, and CaCl2concentrations were investigated (Figs.5-7). The PVA concentration affected the immobilized N6 beads to degrade ammonia nitrogen, as depicted in Fig.5. Combining Fig.2 with Fig.5, it can be inferred that when the PVA concentration was less than 8%, the mass transfer property was better with the increase of PVA concentration. Meanwhile, since the mass transfer property contributed to the transfer of nutrients, the ammonia nitrogen removal rate of N6 strain increased. As the PVA concentration further increased, the interior of the immobilized N6 beads became more compact. Therefore, it was difficult for the N6 cells to contact pollutants, which resulted in a slight decrease in the ammonia nitrogen degradation rate.
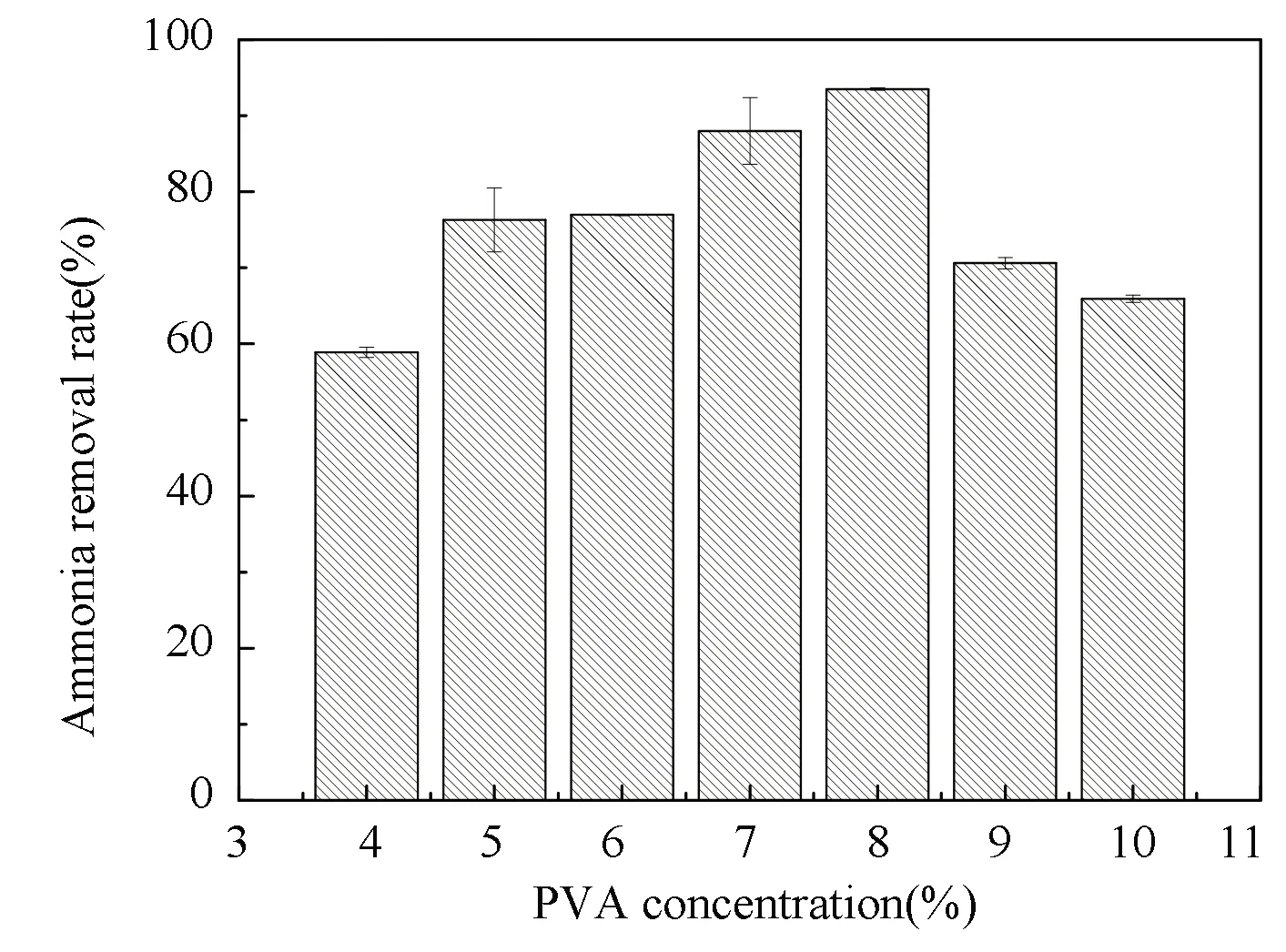
Fig.5 Ammonia removal rate at different PVA concentrations(SA concentration: 1.5%; CaCl2 concentration: 3%)

Fig.6 Ammonia removal rate at different SA concentrations(PVA concentration:8%; CaCl2 concentration:3%)
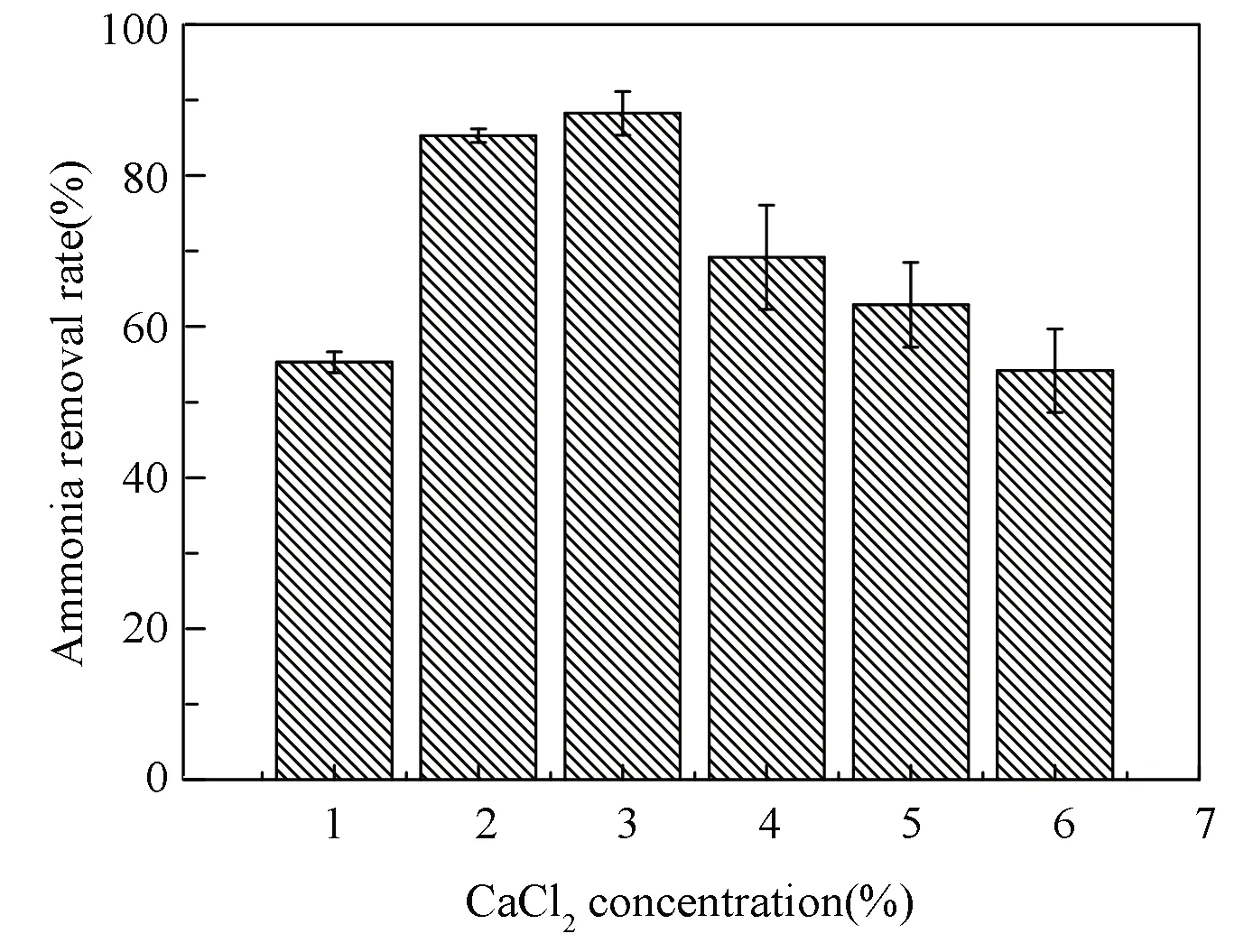
Fig.7 Ammonia removal rate at different CaCl2 concentrations(PVA concentration: 8%; SA concentration: 2%)
To determine the effect of SA concentration on the ammonia nitrogen removal ability of immobilized N6 beads, different SA concentrations were employed. Similar to PVA, the SA concentration affected the mass transfer and then the degradation of ammonia. The trend of ammonia nitrogen degradation with SA concentration was basically consistent with that of mass transfer with SA concentration. As shown in Fig.6, the ammonia removal rate reached the highest when the SA concentration was 2%.
The degradation ability of ammonia nitrogen at different CaCl2concentrations was investigated. When the CaCl2concentration was 3%, the degradation effect of the ammonia nitrogen was the best (Fig.7).When the CaCl2concentration was more than 4%, the mass transfer of the immobilized N6 beads was better (Fig.4), but the degradation ability was reduced as shown in Fig.7. The underlying cause may be that when the CaCl2concentration is low, the calcium alginate formed by the reaction of Ca2+with SA improves the internal lattice structure of the immobilized N6 beads. This structure is beneficial to the growth and reproduction of the N6 strain, which can lead to the increase of the degradation rate of ammonia nitrogen. Nevertheless, with the increase of CaCl2concentration, excessive CaCl2made salt dialysis to dehydrate the cells[30], leading to the inhibition of fungal growth and a gradual decline in the degradation rate of ammonia nitrogen.
3.1.4Optimalimmobilizationmaterials
The concentration of immobilized materials will affect the ammonia nitrogen degradation capacity of N6 cells. Table 1 shows an orthogonal array experiment which was designed to determine the optimal combination of immobilized materials. The results of the experiment are presented in Table 2. Table 3 shows the analysis of orthogonal test.
In Table 3,Kandkreflect the different of factors and levels are different to the influence of removal rates,Rrepresents the effect of the level change of factors on the degradation. The variation ranges ofkwith the change of PVA and CaCl2concentrations were 9.49 and 9.36 respectively, which were much bigger than the result of SA concentration (0.71). It indicates that PVA and CaCl2concentrations have significant influences onk, while SA concentration has weaker influence. For thekof PVA, the maximumkappeared at the concentration of 9%, whereas those of SA and CaCl2occurred at the concentrations of 1.5% and 2%, respectively. According to Table 2-Table 3, the optimal concentration combination of immobilized materials isA3B1C1, when PVA, SA, and CaCl2concentrations were 9%, 1.5%, and 2%, the immobilized N6 beads degraded ammonia nitrogen with the highest degradation rate. However, since the combination was not included in the 9 experiments, another test was carried out on the basis of the combination, and the result of the ammonia nitrogen degradation rate was 97.97%. Therefore, the optimum conditions for the immobilized materials were 9%PVA, 1.5% SA, and 2% CaCl2concentrations.
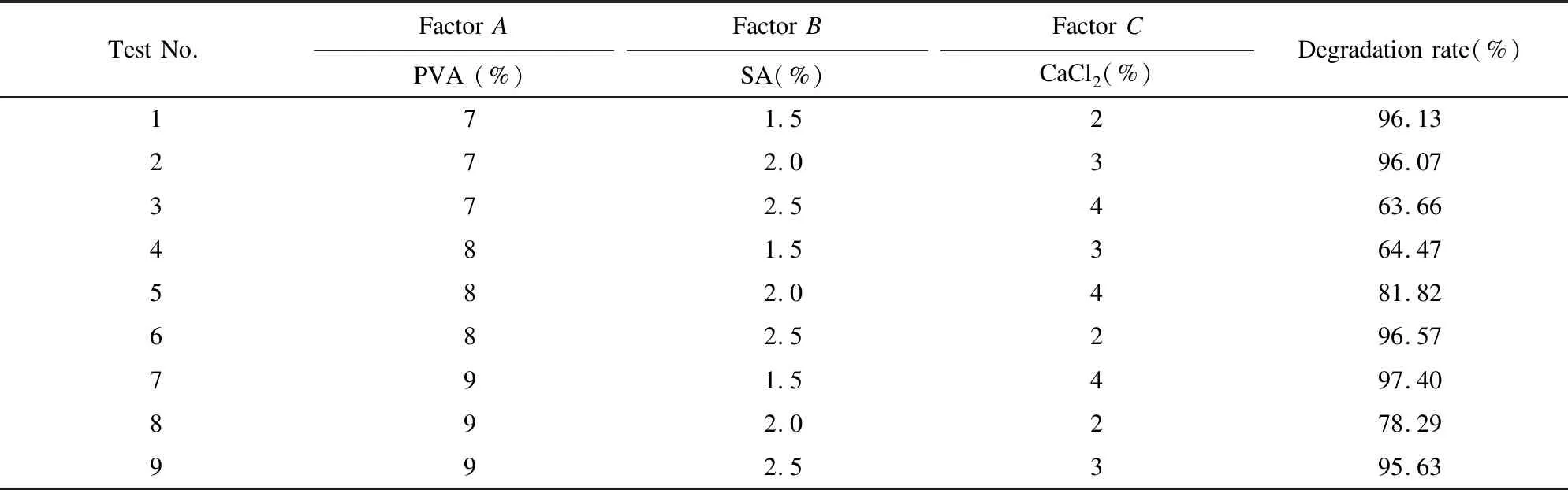
Table 2 Orthogonal array configuration and experimental results

Table 3 Analysis of orthogonal array experimental
aThe sum of degradation rate at each level of a factor;
bThe average of degradation rate at each level of a factor;
cThe range ofk;
dA: PVA concentration; B: SA concentration; C: CaCl2concentration.
3.1.5StabilityofImmobilizedN6Beads
The remaining number of immobilized N6 beads in the stability test remained constant throughout the experiment. At 4, 8, 12, 20, and 24 h, the numbers of the pellets in deionized water were all sixty. The stabilization of the immobilized N6 beads is possibly due to the high elasticity of the immobilized pellets in water.
3.2 Ammonia Nitrogen Degradation Conditions of Immobilized N6 Beads
3.2.1AmmonianitrogendegradationatdifferentinitialconcentrationsofN6
The ammonia nitrogen removal rate of immobilized N6 beads at different initial N6 concentrations is presented in Fig.8. Inoculum of N6 directly affected the degradation of ammonia nitrogen. With the increase of the initial concentration of N6, the degradation rate of ammonia nitrogen increased greatly first and then tended to grow insignificantly. When the N6 initial concentration was more than 3%, the increase of the initial concentration of N6 had little impact on the removal of ammonia nitrogen.
3.2.2AmmonianitrogendegradationatdifferentpH
The ammonia nitrogen removal ability of the immobilized N6 beads under different pH values is shown in Fig.9. Within the range of 5-10 of the pH value, the ammonia nitrogen degradation rate reached the highest at pH 8. Peracid or overbase was not conducive to the degradation of ammonia nitrogen by immobilized N6 beads.
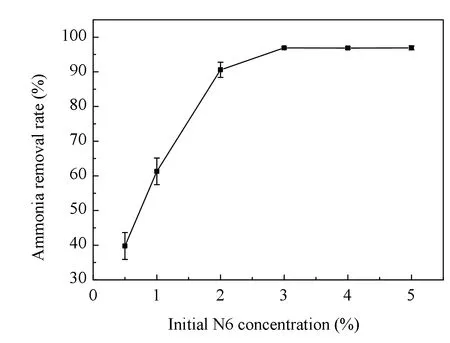
Fig.8 Effect of initial concentration of N6 on ammonia nitrogen removal rate
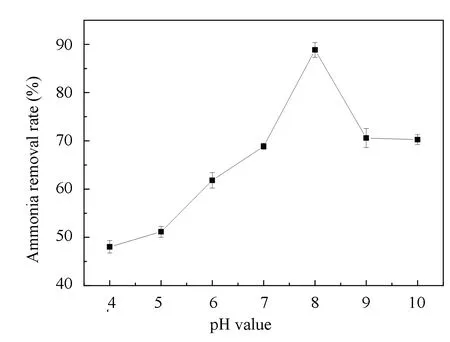
Fig.9 Effect of pH on ammonia nitrogen removal rate
3.2.3EffectofdifferentimmobilizationtimeonimmobilizedN6beads
Immobilization time has a great effect on the performance of immobilized N6 beads[31]. In the process of preparing immobilized N6 beads, the immobilization time of 6, 12, 24, 36, and 48 h was selected for testing. In this study, it was found that when the immobilization time was 6 h and 12 h, the produced immobilized N6 beads were soft and easy to break. Maybe it was because the boric acid did not fully enter the interior of the beads to react with the PVA. The immobilized N6 beads produced at other immobilization time (i.e., 24 h, 36 h, and 48 h) showed high mechanical strength during the experiments. The effect of the immobilization time on the ammonia nitrogen degradation rate is presented in Fig.10. It shows that when immobilization time was 24 h, 36 h, and 48 h, the degradation rates of ammonia nitrogen were almost the same. Therefore the optimal immobilization time was chosen as 24 h for efficiency.

Fig.10 Effect of immobilization time on ammonia nitrogen removal rate
3.3 Application of Immobilized N6 Beads in SBR
The optimal immobilization materials ofCandidasp.N6 were as follows: 9% PVA, 1.5% SA, and 2% CaCl2. The application of immobilized N6 beads was investigated under the following optimal conditions: 3% N6 initial concentration, pH 8, and 24 h immobilization time. Fig.11 depicts the ammonia nitrogen removal capacity of immobilized N6 beads in the sequencing batch experiments. Since the immobilized N6 beads were stored in normal saline at 4 ℃, when the immobilized N6 beads were added to the SBRs, strain N6 needed to adapt to the environment for a period of time, resulting in an unstable ammonia nitrogen degradation rate at this stage. After two cycles, ammonia nitrogen degradation rate of the immobilized N6 beads gradually increased with time, and finally remained stable at 95%-99%. As shown in Fig.11, when the addition amount of the immobilized N6 beads was 15%, the ammonia nitrogen degradation effect was more stable and the treatment efficiency was higher. Although 30% of the immobilized N6 beads could reach a higher ammonia nitrogen degradation rate at later stage, the ammonia nitrogen degradation effect was unstable and the economic cost was almost twice as that of the 15% dosage. Therefore the use of 15% of the immobilized N6 beads is more suitable for degradation because of cost and efficiency.
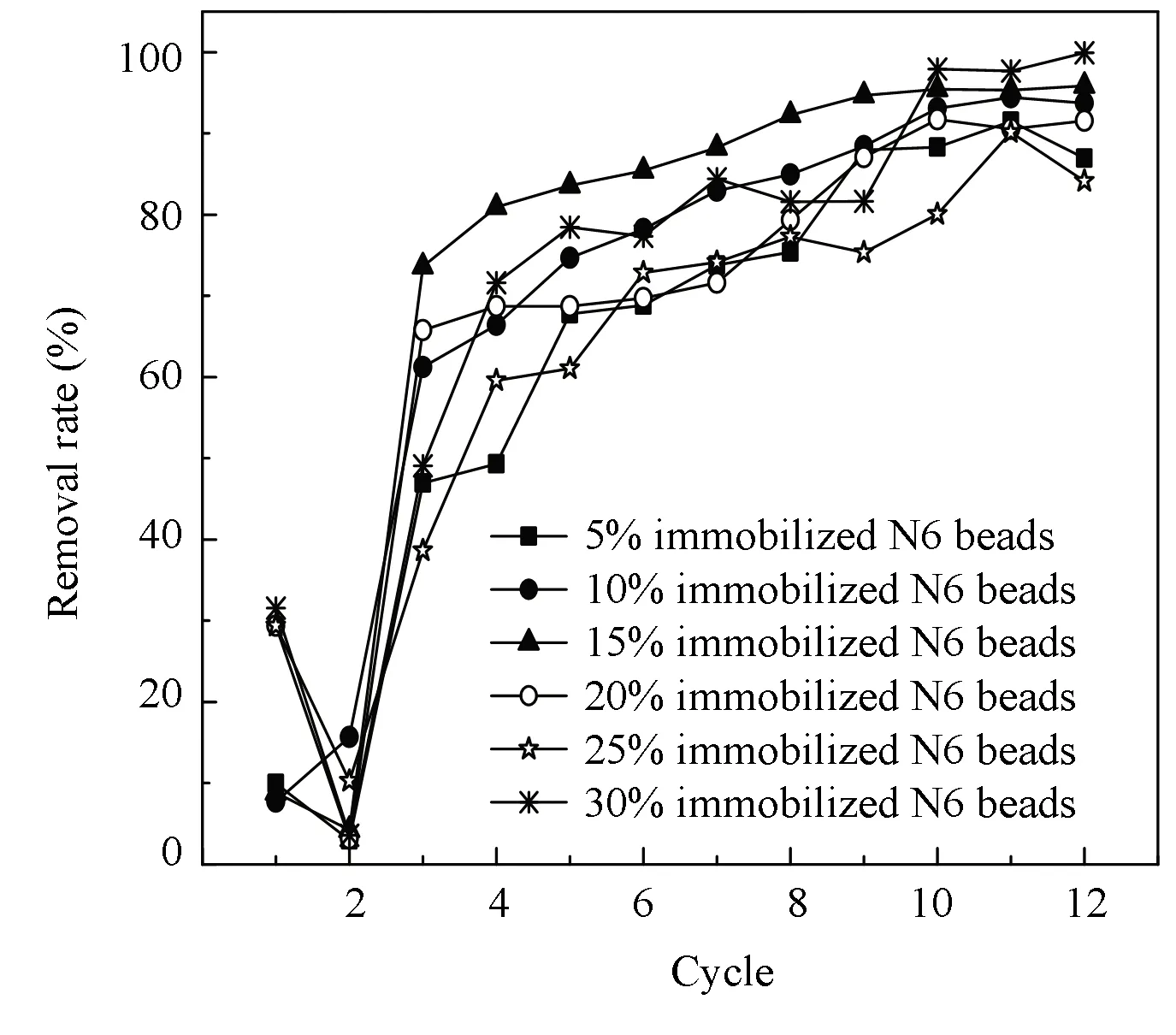
Fig.11 Ammonia nitrogen degradation rate of different immobilized N6 beads
4 Conclusions
Immobilized high efficiency strain for wastewater treatment has potential application value. In this study, the heterotrophic bacteriumCandidasp.N6 still had high ammonia nitrogen degradation ability after being immobilized by PVA, SA, and CaCl2. Immobilized materials had a great influence on the performance of immobilized N6 beads and its ability to degrade ammonia nitrogen. Physical properties and orthogonal array experiments confirmed that the concentrations of 9% PVA, 1.5% SA, and 2% CaCl2made the optimal combination of immobilized materials for the immobilization of N6 strain cells. The ammonia nitrogen degradation rate of immobilized N6 beads reached 97.97% under the optimal conditions and the beads were very stable. Furthermore, the ammonia nitrogen degradation effect was the best when the immobilization time was 24 h, the N6 inoculum was 3%, and the pH value was 8.
Immobilized N6 beads can degrade ammonia nitrogen in the SBRs. Considering costs and ammonia nitrogen treatment effects, the optimal amount of immobilized N6 beads was 15%. The ammonia removal rate of the immobilized N6 beads in SBR was stable at 95%-99%. The results exhibited practical significance for the application of immobilized N6 strain to the actual ammonia nitrogen wastewater treatment.
杂志排行
Journal of Harbin Institute of Technology(New Series)的其它文章
- Review: Energy Methods for Multiaxial Fatigue Life Prediction
- Loop Closure Detection of Visual SLAM Based on Point and Line Features
- Dynamics Analysis of Fractional-Order Memristive Time-Delay Chaotic System and Circuit Implementation
- An LCC-HVDC Adaptive Emergency Power Support Strategy Based on Unbalanced Power On-Line Estimation
- Aging Behavior and Creep Characteristics of CACB
- Effect of Crowding in Metro Carriages on Travelers’ Disutility:An Evidence from Guangzhou Metro
