Biocontrol potential of Trichoderma asperellum mutants T39 and T45 and their growth promotion of poplar seedlings
2020-05-19RuitingGuoZhiyingWangChangZhouYingHuangHaijuanFanYuchengWangZhihuaLiu
Ruiting Guo · Zhiying Wang · Chang Zhou · Ying Huang · Haijuan Fan ·Yucheng Wang · Zhihua Liu
Abstract This study investigates the biocontrol potential of Trichoderma asperellum mutants against Rhizoctonia solani, Alternaria alternata, and Fusarium oxysporum and growth promotion of Populus davidiana × P. alba var.pyramidalis (PdPap poplar) seedlings. A T-DNA insertion mutant library of T. asperellum was constructed using Agrobacterium tumefaciens-mediated transformation.Sixty-five positive transformants (T1-T65) were obtained.Growth rates of the mutants T39 and T45 were the same,39.68% faster than the WT. In toxin tolerance tests, only T39 had greater tolerance to A. alternata fermentation broth than the WT,but mutant T45 had the same tolerance as the WT to all fermentation broths.Furthermore,T39 and T45 had a greater antagonistic ability than the WT strain against R.solani and A.alternata.The inhibition rate of the mutants T39 and T45 against A. alternata are 73.92% and 80.76%, respectively, and 63.51% and 63.74%, respectively. Furthermore, the three strains increased the activities of superoxide dismutases, peroxidase, catalase (CAT)and phenylalanine ammonia lyase(PAL)in PdPap seedling leaves.CAT and PAL activity in the PdPap seedling leaves was 11.25 and 5.50 times higher, respectively, in the presence of T39 than in the control group and 12 and 6.35 times higher, respectively, in the presence of T45 than in the control group. All three strains promoted seedling growth and the root and stem development, especially mutant T45.Mutants T39 and T45 reduced the incidence of pathogenic fungi in poplar and stimulated poplar seedling growth.
Keywords Biocontrol · Trichoderma asperellum · Mutant library · Fungal disease · Poplar growth
Introduction
Many Trichoderma strains can effectively control plant diseases(Druzhinina et al.2011),but they are vulnerable to biotic and abiotic stresses in the natural environment(Mastouri et al. 2010), leading to a decline in their antagonistic capacity against pathogenic fungi. Trichoderma spp. mutants can have improved environmental tolerance and fungal antagonistic capacity over the wild type (WT).Most mutant selection for Trichoderma has focused on cellulases to generate commercially applicable cellulolytic strains. Trichoderma reesei mutants MCG77 and QM9414 produce more cellulase than the parent strain(Latifian et al.2007). Trichoderma atroviride mutant TUB F-1505 and Trichoderma citrinoviride mutant EB-104 both produce higher levels of cellulases than their respective parent strains (Jalón and Gutiérrez-López 2009; Kovács et al.2008). Among previous studies on the selection of Trichoderma for Cd resistance,cyanide hydratase,and salinity tolerance(Brotman et al.2013;Wang et al.2009a,b;Zhou et al. 2007), nine of 200 screened Trichoderma koningii mutants had higher Cd tolerance(4 times higher)and could be used to remediate Cd-contaminated soils (Wang et al.2009a, b). The T. koningii mutant TkB6 was found to secrete three times as much cyanide hydratase as the parent strain (Zhou et al. 2007). Two Trichoderma mutants(Th50M6 and Th50M11)were significantly more effective than the wild type strain at inhibiting Fusarium oxysporum(Mohamed et al. 2006). However, few have focused on Trichoderma asperellum mutants and their effect on seedling growth.
Poplar is a model forest tree (Ji et al. 2016), and Trichoderma spp. are effective in controlling poplar diseases.Trichoderma longibrachiatum controlled Cytospora chrysosperma in vitro and Cytospora canker of poplar in the field (Yi and Chi 2011). Wood decay fungi of poplar have been successfully controlled using a conidial suspension of Trichoderma strain T-15603.1 (MarkSchubert and Schwarze 2008).
Poplar tissue culture seedlings can be used for genetic engineering and are a good model material for studying the interaction between Trichoderma spp. and woody plants.Genes involved in signal transduction pathways were upregulated in poplar seedlings in response to the interaction with yeast recombinant hydrophobin rHFB2-6 from T.asperellum (Huang et al. 2015). Heterologous expression of Hsp24 from T. asperellum improved the resistance of transformed seedlings of Populus davidiana × P.alba var.pyramidalis (PdPap) to C. chrysosperma and Alternaria alternata (Ji et al. 2016). Heterologous expression of the ech42 gene from T. harzianum improved the resistance of transgenic poplar seedlings to Melampsora medusa and Cylindrocladium floridanum (Noe¨l et al. 2005).
In this study to obtain mutants of T.asperellum that had better fungal antagonistic capacity and promoted growth of poplar seedlings, we constructed a T-DNA insertional mutant library of T. asperellum using an Agrobacterium tumefaciens-mediated transformation system(ATMT).Our results provide theoretical support and a practical reference for the development of Trichoderma strains for controlling poplar fungal diseases and enhancing poplar growth.
Materials and methods
Strains, plant materials, and medium
Trichoderma asperellum ACCC30536 (T. asperellum CBS 433.97 v1.0) was obtained from the Agricultural Culture Collection of China.The genome of this strain has already been sequenced (https://genome.jgi.doe.gov/Trias1/Trias1.home.html). Rhizoctonia solani CFCC86328, A. alternata CFCC82114, and F. oxysporum CFCC86068 were obtained from the China Forestry Culture Collection Center. A. tumefaciens AGL-1, which contains the binary vector pBHt (Mullins et al. 2001) and a hygromycin B-resistance gene (hph), was provided by Prof. Fucong Zheng (South China University of Tropical Agriculture,China).
Populus davidiana × Populus alba var. pyramidalis Louche (PdPap poplar) seedlings were cultured aseptically for 15 days in liquid rooting medium [Murashige-Skoog(MS) medium with naphthalene acetic acid (NAA)0.25 mg L-1and sucrose 20 g L-1]or differential medium[MS medium with NAA 0.05 mg L-1, 6-Benzylaminopurine (6-BA) 0.5 mg L-1, sucrose 20 g L-1, and agar 8 g L-1] at 25 °C (Ji et al. 2016). Seedlings of similar height(about 5 cm) and similar number of leaves (6-8 leaves)were obtained (Huang et al. 2015).
Primers Hph-L (5′-GAAACCGACGCCCCAGCACT-3′) and Hph-R (5′-AGTGCTGGGGCGTCGGTTTC-3′)were designed to amplify the hygromycin B-resistance gene (hph) using Primer 6.0 software (PREMIER Biosoft,USA) and synthesized by Sangon Biotech (Shanghai,China) Co., Ltd.
Construction of the T-DNA insertional mutant library of T. asperellum and analysis of transformant genetic stability
T.asperellum was transformed using the A.tumefaciens as previously described (Sun et al. 2009). The 200 μM 3′5′-dimethoxy-4-hydroxy acetophenone(AS)was added to the induced and co-cultivated (pH 5.0) mediums, and 106spores/mL T. asperellum was added. Mitotic stability was determined by subculturing 80 randomly selected putative transformants five times consecutively on PDA without hygromycin B, then transferring them to PDA containing 300 μg/mL hygromycin B.The hph gene was then detected in the putative transformants using PCR, and mitotic stability was determined. The sense primer Hph-L was 5′-GAAACCGACGCCCCAGCACT-3′, and the antisense primer Hph-R was 5′-AGTGCTGGGGCGTCGGTTTC-3′.The PCR mixture contained 10 × EX Taq buffer 1 μL,dNTP 0.4 μL, DNA 1 μL, Hph-L (20 μM) 0.5 μL, Hph-R(20 μM) 0.5 μL, RNase Free ddH2O 6.4 μL, EX Taq 0.2 μL, total volume 10 μL.The PCR cycling conditions were 94 °C for 5 min; 35 cycles of 94 °C for 45 s, 55 °C for 1 min, 72 °C for 1 min.
Colony morphology and diameter of the mutant strains in PDA medium
The mutant strains were cultured at 28 °C for 5 days in PDA with 300 μg/mL hygromycin B. Mycelial discs of 5 mm diameter were cut from the edges of the PDA plates using a hole punch, placed onto new PDA plates and cultured at 28 °C for 5 days. Colony morphology was recorded, and colony diameter measured each day. The experiment was laid out in a completely randomized design with three replications.
Tolerance mutant response to toxin fermentation broths
Alternaria alternata, Rhizoctonia solani or Fusarium oxysporum fermentation broths were prepared as previously described (Vidhyasekaran et al. 1997; Dong and Wang 2011; Ji et al. 2016). The fermentation broths were filtered through 16 layers of sterile gauze,then the filtrates were transferred to 50 mL sterile centrifuge tubes, and centrifuged at 10,000 rpm for 15 min. Supernatants were collected and filtered using 0.22 μM filter, and stored at- 20 °C.
The WT was cultured at 28 °C for 5 days on PDA.Mycelial discs(5 mm diameter)were cut from the edges of PDA plates using a hole punch,placed onto new PDA,and cultured at 28 °C for 5 days. Mycelial discs were again taken from the edges of the plates and placed ontofresh PDA with 5, 10, 20, 25 or 30% concentrations of fungal pathogenic fermentation broths.The colony diameters were then measured each day. The experiment was laid out in a completely randomized design with three replications.
Finally,all 65 mutants were cultured at 28°C for 5 days on PDA,transferred to new PDA,and cultured at 28 °C for 5 days as described above.Mycelial discs were placed ontofresh PDA with fungal pathogenic fermentation broths as described above. The 30% concentrations of A. alternata,R. solani or F. oxysporum fermentation broths greatly inhibited growth of the WT. Colony diameters were measured each day during culturing. The experimental design was completely randomized with three replications.
Antifungal ability of mutant strains against three pathogenic fungi
The WT,and mutants T39 and T45 were each treated with A. alternata, R. solani or F. oxysporum. Symmetrical positions were marked on the PDA plates, and a mycelial disc(5 mm diameter)from the same part of the PDA plates was placed on the fresh PDA at the marked positions. The plates were cultured at 28 °C for 5-10 days. The experimental design was completely randomized with three replications. Mean percentage inhibition was calculated as IR = [(DC - DE)/DC] × 100, where DC is mean diameter of the control group and DE is mean diameter of the experimental group.
Growth promotion of mutant T39 or T45 to the PdPap seedlings
The PdPap seedlings were cocultured with the spores. The PdPap seedlings grown in glass tube whose diameter is 3 cm and height is 20 cm with 1/2 MS medium (liquid rooting medium). Spores were harvested after 7 days culture. Then 106spores/mL of the WT, T39 or T45 were mixed with the medium, respectively. After 15 days, the growing state of the PdPap seedlings was photographed.Leaves were removed after 0, 3, 5, 7 and 15 days to measure enzymatic activity. Superoxide dismutase (SOD),peroxidase (POD), catalase (CAT) and phenylalanine ammonia lyase (PAL) were measured according to the instructions with the kits (kits obtained from Nanjing Jiancheng Bioengineering Institute). The experimental design was completely randomized design with five replications.
Data analyses
Differences between treatments were compared using an analysis of variance(ANOVA)followed by a Duncan’s test(P <0.05). All data were analyzed using SPSS PASW Statistics v. 19.0 (SPSS Statistics, Shanghai, China).
Results
T-DNA insertion mutant library of T. asperellum and genetic stability of putative mutants
The transformation efficiency of ATMT was approximately 60 mutants per 106spores.When 80 putative transformants containing the vector pBHt1 were tested for mitotic stability of the integrated T-DNA by subculturing on PDA then transfer to PDA with 300 μg/mL hygromycin B,transformants that successfully grew under these conditions had high mitotic stability.
In the PCR of the target gene for the 80 selected putative transformants using the Hph-L and Hph-R primers, the 1005-bp PCR product was detected in 65 putative transformants, indicating the hph gene was successfully integrated into the T. asperellum genome (Fig. 1).
Colony and conidiophore morphologies of the Trichoderma mutant strains

Fig. 1 PCR detection of hph gene in partial positive transformants.The hph gene was amplified with primers Hph-L and Hph-R. Partial agarose gel electrophoresis. Lanes 1-18: 18 putative transformants;lane 19: wild-type T. asperellum; lane 20: DL2000 DNA marker.Numbers at the sides of the gel represent base pairs

Fig. 2 Colony and conidiophore morphologies of 9 mutant strains (5 days). a-j Colony morphologies of the WT and mutants T45, T39, T34,T49, T51, T44, T53, T31 and T58 on PDA; k, l Light micrographs of conidiophores of WT and T45
Of the 65 mutant strains obtained, 9 had altered morphology (T45, T39, T34, T49, T51, T44, T53, T31 and T58)compared with the WT(Fig. 2b-j).On PDA,the mycelium of the WT was initially white,with no aerial hyphae.After 48 h, approximately 108/cm2conidia were produced on highly branched, long conidiophores with twig contours and three or more ampulliform phialides in whorls with ellipsoidal and globose conidia, and green mycelium(Fig. 2a, k). The mutant T45 colony on PDA was hyaline with loose hyphae, little branching, delayed conidia formation (Fig. 2b); solitary phialides were not in whorls but often on one-celled side branches and were subulate or cylindrical (Fig. 2l). T39 on PDA had white mycelium,loose hyphae,and abundant aerial hyphae(Fig. 2c).T34 on PDA had radially arranged mycelium, green conidia, and conidia primarily produced at the edge of the mycelium(Fig. 2d).T49 on PDA had conspicuously dense mycelium,agglutinated hyphae, yellow-green conidia, and produced more conidia than the WT(Fig. 2e).The colony of T51 on PDA was thin, with an ill-defined margin, light-green conidia, and fewer conidia than the WT (Fig. 2f). T44 on PDA produced green conidia mainly in the center of the mycelium,and fewer conidia at the edge(Fig. 2g).T53 on PDA had low growing, conspicuously dense mycelium,with sparse yellow conidia (Fig. 2h). T31 on PDA had conspicuously dense mycelium and green conidia at the edge of the mycelium (Fig. 2i). T58 on PDA had radially arranged mycelium, a thick colony, and sparse light-green conidia (Fig. 2j).
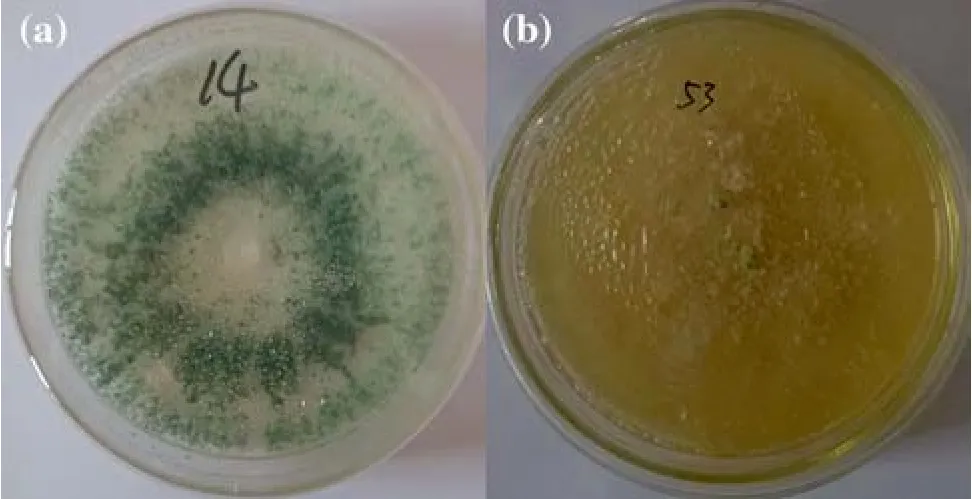
Fig. 3 Types of colony morphology. a Type I. b Type II
There were two types of colony morphology. The first was like that of the WT; white mycelium, grew rapidly with diffusing pigment and produced conidia quickly.This type of colony was defined as type I (Fig. 3a) and was similar to the WT. The other type of colony morphology was white, mycelium was yellow, conspicuously dense,slow growing, and growth was sparse after 3 days. This type of colony was defined as type II (Fig. 3b). Type I mutants were selected for subsequent experiments because their morphology was like the WT and growth was rapid.
Colony diameter of 20 mutant strains
Of the 65 mutant strains, 20 mutant strains had a growth rate that differed from that of the WT (Table 1); 12 grew slower than the WT, 8 grew faster. T39 and T45 were the fastest growers, 39.68% faster than the WT (Table 1) and were thus selected for further study.
Tolerance of mutants to three fungal pathogenic fermentation broths that inhibit the WT
Low concentrations (<10%) of R. solani, A. alternata or F. oxysporum fermentation broths promoted the growth of the WT. The growth of the WT was not inhibited by the 30% R. solani fermentation broth, but its sporulation was inhibited (Fig. 4b2). WT growth was clearly inhibited by the 30% fermentation broth from A. alternata (Fig. 4a1,b3) and F. oxysporum (Fig. 4a3, b4). Therefore, the optimal concentration of R. solani, A. alternata or F.oxysporum fermentation broths to inhibit the growth of the WT was 30%.
Only mutant T39 grew faster than the WT on PDA with 30% A. alternata fermentation broth, and its tolerance to the broth was higher than the WT. However, none of the mutants had higher tolerance than the WT to the 30%fermentation broths of R. solani or F. oxysporum.
Antagonism of mutant strains against the pathogenic fungi
The WT inhibited R. solani by 43.24% after 5 days. The WT mycelium came in contact with the A. alternata or F.oxysporum after 48 h, and quickly covered them. The antagonism of the T39 and T45 was greater than the WT against A.alternata and R.solani (Fig. 5a).The mycelium of T39 and T45 covered A. alternata and F. oxysporum after 3 days, and covered R. solani after 4 days (Fig. 5a).T45 had the highest inhibition rate(80.76%)of the mutants against R. solani (Fig. 5b).
The enzyme activities and growth of the PdPap seedlings were altered after inoculation with spores of the WT and mutants T39 and T45 in relation to the non-inoculated control. At 1 day after inoculation with spores of the WT,T39 or T45, CAT activity peaked, and PAL peaked after 7 days. Overall, the activity of CAT and PAL was 12 and 6.35 times higher, respectively, than in the control group.The spores of the T45 had significant effect on the CAT and PAL activities of the PdPap seedlings (Fig. 6).
The PdPap seedlings in response to the spores had more biomass, thicker stems and more prolific roots compared with the control group (Fig. 7). PdPap seedlings grown with the spores of T45 had the most prolific root systems(Fig. 7d).
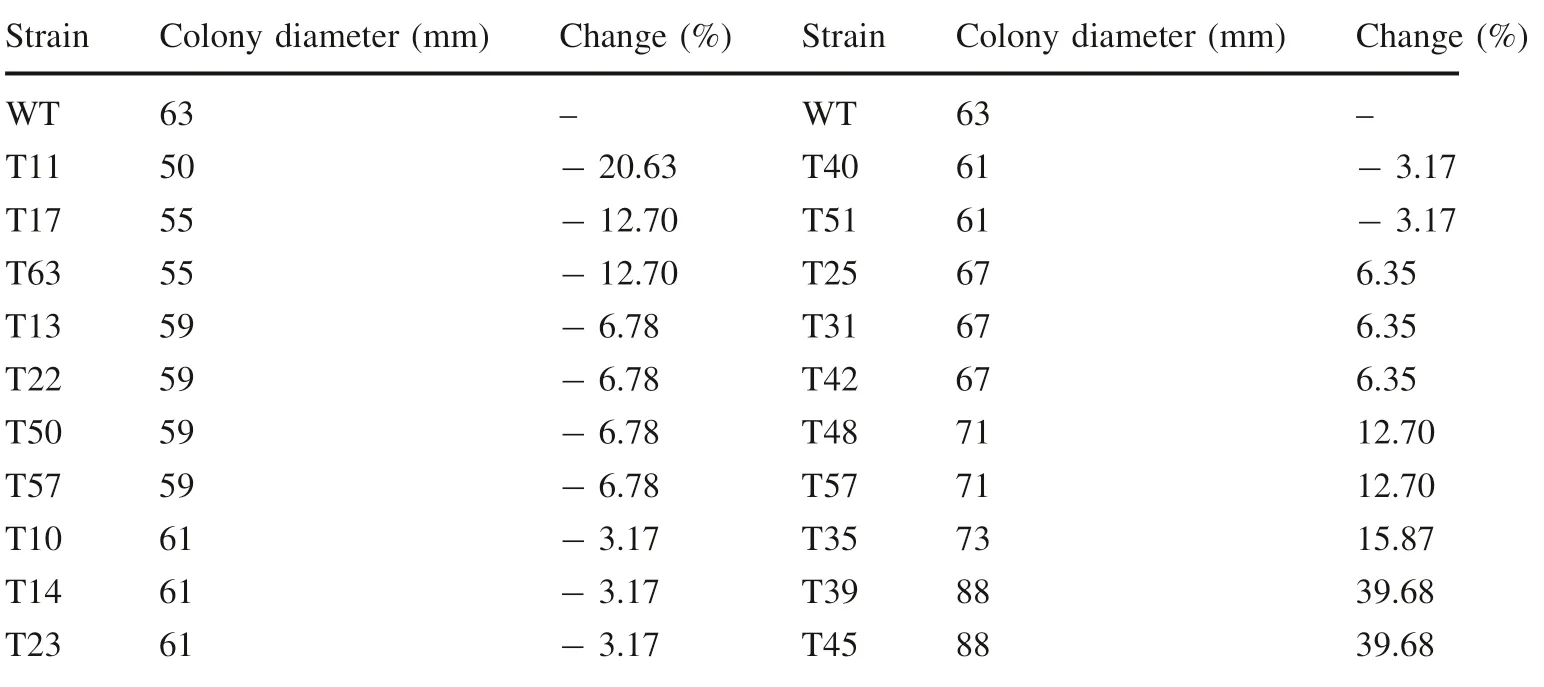
Table 1 Colony diameter and change in diameter of 20 mutant strains compared with WT after 48 h of culture

Fig. 4 Optimal concentrations of the three fungal pathogenic fermentation broths for inhibiting the WT. a Colony diameters of the WT on PDA with different concentrations of (1) R. solani, (2) A.alternata or (3) F. oxysporum fermentation broths. b Growth of WT on PDA(1)alone or with 30%fermentation broth of(2)R.solani,(3)A. alternata or (4) F. oxysporum

Fig. 5 Contact and inhibition rate of the WT, T39 and T45 in dual culture with the three pathogenic fungi (5 days).a Dual culture plates of WT,T39 or T45 with (1-3) A.alternata, (4-6) R. solani, and(7-9) F. oxysporum.b Percentage inhibition of the three pathogenic fungi by WT,T39 or T45
Discussion
Trichoderma spp. mutant libraries have been previously constructed to screen Trichoderma strains for better environmental tolerance and fungal antagonism (Michielse et al. 2005; Tsuji et al. 2003). In this study, 65 T.asperellum mutants were obtained, and their colony morphologies were either like that of the WT (type I) or were white with yellow conspicuously dense mycelium that grew slowly and sparsely (type II) (Lü et al. 2015).Because fast growth of Trichoderma spp.is a key factor for controlling fungal pathogens,only the fastest-growing type I strains (T39 and T45). T39 and T45 grew 39.68% faster than that of the WT, suggesting that they might be better antagonists than the WT.

Fig. 6 Enzymatic activities in protein extracts of P. davidiana × P.alba var. pyramidalis (PdPap poplar) seedlings leaves in control (no inoculation) and after inoculation with WT or mutant Trichoderma spores. a SOD, b POD, c CAT, d PAL. Values in each column followed by the same letter are not significantly different according to Duncan’s test (P ≤0.05)
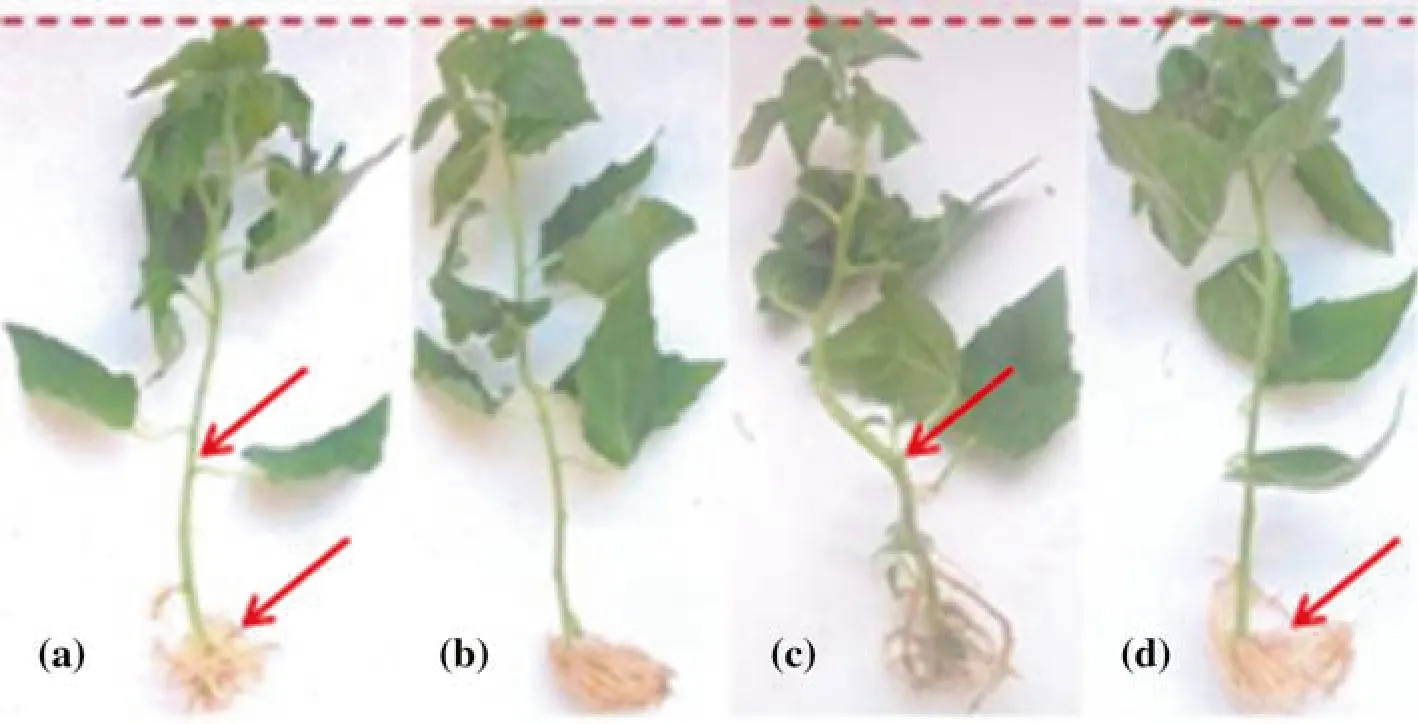
Fig. 7 Growth of PdPap seedlings 15 days after inoculation with spores of WT or mutant strains compared with the noninoculated control.a Control, b WT, c T39, d T45.Red arrowheads indicate roots and stems initiated after treatment began
Resistance tofungal pathogen toxins is another important index of fungal antagonistic capacity (Mathiyazhagan et al. 2005). Low concentration (<10%) fermentation broths of A.alternata,R.solani or F.oxysporum promoted the growth of the WT.Yet 30%A.alternata,R.solani or F.oxysporum fermentation broths clearly inhibited WT growth. Only the tolerance of T39 to 30% A. alternata fermentation broth was better than the WT.Neither mutant strain had improved tolerance in response to 30%R.solani or F. oxysporum fermentation broths.
In previous studies, mutant Th50M6 showed better antagonistic activity than the WT strain and had a maximum inhibition rate of 60% against F. oxysporum (Mohamed et al. 2006). T. harzianum mutant strain Th-14 significantly reduced the incidence of F. oxysporum-induced wilt disease in chickpea plants (Rawat et al. 2012).Our result suggests that T39 and T45 are better antagonists of A. alternata and R. solani than the WT in the in vitro antagonism tests. In particular, after 5 days T45 had the highest inhibition (80.76%) against A. alternata, while the WT inhibited growth of A. alternata by 43.24%.
Trichoderma strains are thought to increase seedling vigor by inducing physiological protection in plants against oxidative damage (Mastouri et al. 2010). SOD catalyzes the dismutation of the superoxide anion to hydrogen and molecular oxygen, the first step in active oxygen-scavenging systems.POD,CAT and PAL are also important for oxygen scavenging and in protecting cells against many environmental stresses (Mirzaee et al. 2013). Enzyme activities from PdPap seedlings treated with WT, T39 or T45 were higher than in the control group, consistent with previous studies showing that T. asperellum treatment increased activity of SOD in ‘XY335’ and ‘JY417’ maize seedlings and antioxidant enzymes in cucumbers(Brotman et al. 2013; Fu et al. 2017). In our study, CAT activity increased in contrast to the reduced CAT activity found in leaves and roots of maize after T.asperellum treatment(Fu et al. 2017). The CAT and PAL activities in the PdPap seedlings in the presence of WT,T39 or T45 spores peaked after 1 day and 7 days,respectively.Because the treatment with T45 increased CAT and PAL activities by 12 and 6.35 times, respectively, over levels in the control group, the T.asperellum mutant T45 may enhance the PdPap seedlings growth by inducing physiological protection.
Wild-type Trichoderma strains can promote seedling growth as in the case of T.harzianum strain T22 enhancing tomato seedling vigor(Mastouri et al.2010).In the present study, the mutant strains promoted seedling growth. In particular, T45 stimulated prolific root growth.
In our screen of the mutant library was constructed by random insertion of the hph gene into the Trichoderma genome, two T. asperellum mutants T39 and T45 were found to have better biocontrol potential than the WT.These mutants could be useful for promoting growth of the poplar seedlings and in controlling R. solani, A. alternata,and F. oxysporum.
杂志排行
Journal of Forestry Research的其它文章
- Do increasing respiratory costs explain the decline with age of forest growth rate?
- At what carbon price forest cutting should stop
- Mapping the risk of winter storm damage using GIS-based fuzzy logic
- Effects of seed moisture content, stratification and sowing date on the germination of Corylus avellana seeds
- Comparison of seed morphology of two ginkgo cultivars
- De novo assembly of the seed transcriptome and search for potential EST-SSR markers for an endangered, economically important tree species: Elaeagnus mollis Diels
