Floristic diversity and carbon stocks in the periphery of Deng-Deng National Park, Eastern Cameroon
2020-05-19LouisPaulRogerKabelongBanohoLouisZapfackRobertBertrandWeladjiCedricChimiDjomoMelanieChichiNyakoYannickEnockBockoDamienMarieEssonoJullieteManchoNasangNadegeMadountsapTagnangCharlesInnocentMemviAbessoloKennethRogerMvondoS
Louis Paul Roger Kabelong Banoho · Louis Zapfack · Robert Bertrand Weladji ·Cedric Chimi Djomo,5 · Melanie Chichi Nyako · Yannick Enock Bocko ·Damien Marie Essono · Julliete Mancho Nasang · Nade`ge Madountsap Tagnang ·Charles Innocent Memvi Abessolo · Kenneth Roger Mvondo Sakouma ·Farikou Mamadou Souahibou · Florence Jeanne Sarah Palla · Tonga Ketchatang Peguy0 ·Remi Jiagho · Thierry Loic Kenmou2 · Ulrich Arnaud Choumele Kana Jumo ·Boris Armel Anyam Yi Andjik · Roger Bruno Tabue Mbobda,2,
Abstract Carbon is continuously being removed from the atmosphere by photosynthesis and stored in carbon pools(live, dead, and Soilcarbon) of forest ecosystems. However, carbon stock in dead wood and of trees with diameters at breast height(dbh)between 5 and 10 cm is often not considered in many studies carried out in the Congo Basin Forest. The relationship between tree diversity, life-forms and carbon stocks has received little attention. This study was carried out on the outskirts of Deng Deng National Park (DDNP) to determine tree diversity (dominant families, species richness and Shannon index), assess carbon stocks in the five carbon compartments (living tree,understory, fine roots, dead wood and litter) as well as to analyze the relationship between(1)carbon stocks and tree diversity; and, (2) between carbon stock and life-forms.The Shannon index of trees ≥10 cm dbh ranged from 2.6 in riparian forest to 4.3 in secondary forest;and for the tree between 5 and 10 cm,it ranged to 1.56 in riparian forest to 3.68 in the secondary forest. The study site housed 16 species, 7 genera and 3 families which are only found in trees of dbh between 5 and 10 cm. The average total carbon stock of the five compartments varied from 200.1 t ha-1 in forest residues to 439.1 t ha-1 in secondary forest. Dead wood carbon stock varied from 1.2 t ha-1 in riparian forests to 12.51t ha-1 in agroforests. The above ground carbon stocks for trees with diameter between 5 and 10 cm varied from 0.7 t ha-1 in young fallow fields to 5.02 t ha-1 in old secondary forests. This study reveals a low but positive correlation between species richness and total carbon stocks, as well as a significant positive relationship between life-forms and total carbon stocks. The findings highlight the need for more data concerning carbon content of dead wood, carbon of trees ≥5 cm <10 cm dbh and the relationship between carbon stocks and tree diversity from other areas of the Congo Basin for a good understanding of the contribution of tropical forests to climate change mitigation.
Keywords Deng Deng national park · Carbon stocks ·Land cover types · Species diversity · Dead wood
Introduction
Average global temperatures have increased as a result of anthropogenic contributions of greenhouse gases (GHGs),and has triggered many unprecedented changes in the climate system.Ocean acidification, changes in water cycles,increased snow and ice melting, rising average sea levels and climate extremes that result from climate change require significant reduction and sustainable greenhouse gases (IPCC 2013). Amongst the main causes of these changes in climate are deforestation and forest degradation that have contributed to emissions of about 32% of atmospheric CO2in the period from 1750 to 2011(IPCC 2013).Moreover, forest ecosystems, which are potential sinks for the sequestration of atmospheric carbon have become the main sources of CO2emissions into the atmosphere.Public awareness of the problem caused by global climate change and the conservation of biodiversity took place at the Earth Summit in Rio de Janeiro in June 1992 (Lescuyer and Locatelli 1999). Climate change has been debated and challenged for many years before being accepted almost unanimously (Leroux 2002).
To reduce greenhouse gas (GHG) emissions from deforestation and forest degradation, many initiatives have emerged internationally. REDD + (reducing emissions from deforestation and forest degradation and the role of conservation, sustainable management of forests and enhancement of forest carbon stocks in developing countries) is one of those initiatives. The REDD + mechanism is coordinated by the United Nations(UN)and put in place by UN-REDD. For this to be effectively and efficiently implemented, accurate estimates of carbon stocks of all forest compartments are needed (Miles and Dickson 2010;Maniatis et al. 2011; Ngo et al. 2013). However, existing data are scattered and suffer from considerable uncertainty(Loubota-Panzou et al. 2016; Bocko et al. 2017). Carbon stocks recorded in Central Africa differ between forest types according tofloristic and structural composition(Djuikouo et al. 2010; Maniatis et al. 2011; Lewis et al.2013; Fayolle et al. 2016; Bocko et al. 2017; Ifo et al.2017), soil (Gourlet-Fleury et al. 2011; Slik et al. 2013),and the types of equations used (Loubota-Panzou et al.2016). In addition, most of the existing estimates of forest carbon stocks in the Congo Basin sub regions are limited to a maximum of two or three carbon forest compartments(living tree, understory and fine roots) (Angelsen et al.2013). It appears necessary, therefore, to estimate all the carbon compartments of the forest ecosystems in Central Africa by associating a floristic and structural characterization because we cannot sustainably manage what we do not know (Bocko et al. 2017).
Protected areas have long been considered as ideal places for biodiversity conservation. With the recent need to control and monitor climate change, they are increasingly being considered as carbon storage sites. Most African countries, including Cameroon, have based their biodiversity conservation strategies on the creation and extension of protected areas(IUCN/WCMC 1994).In fact,Doumenge et al. (2001, 2003) state that the establishment of networks of protected areas representative of a region’s biodiversity remains one of the main tools for conserving this biodiversity and combating climate change. The Deng Deng National Park(DDNP)is a natural ecosystem created in 2010 to compensate for the environmental costs following the construction of the Lom Pangar hydroelectric dam. The DDNP plays an important role as a mitigation measure for climate change through the sequestration of carbon in its vegetation. Despite the importance of this Park, its biodiversity and carbon storage capacity remain unknown,while the relationship between tree diversity and the ability to store carbon is also poorly studied in Cameroon and therefore not documented(Day et al.2013).Elsewhere, despite controversies, there seems to be a relationship between floristic diversity and carbon storage in tropical forests (Day et al. 2013). Some researchers consider that forest ecosystems with high species diversity favor more efficient use of resources and result in higher net primary production (Day et al. 2013). Furthermore, an area of plant species diversity would be strongly correlated with carbon sequestration(Catovsky et al.2002;Kirby and Potvin 2007).Experimental data and direct observations of ecosystems have revealed positive relationships between tree diversity, biomass and productivity (Tilman et al.1997; Cardinale et al. 2007; Midgley et al. 2010).Thompson et al.(2011)showed that the spatial distribution of biomass and biodiversity is influenced by a range of environmental factors such as climate, soil and human activities. Nevertheless, these authors showed that it is difficult to examine the relationship between tree diversity and carbon stocks in complex ecosystems such as tropical forests.In this context,Diaz et al.(2007)showed that total biomass is correlated with functional diversity.Asase et al.(2012) noted that species diversity is positively correlated with soil organic carbon in unlogged and logged West African tropical forests. For the latter, there is no correlation between total carbon and species diversity.
Forest management offers opportunities to conserve tree diversity and carbon stocks (Feldpausch et al. 2005).However, there is little data on studies comparing tree diversity and carbon stocks in the tropical forests of the Congo Basin(Day et al. 2013).To date, most studies have been conducted in the Amazon (Feldpausch et al. 2005;Sist and Ferreira 2007). The death and subsequent decomposition of trees is an important aspect of the carbon cycle of forest ecosystems and is directly related toforest structure (Denslow 1987). The dead trunks and branches,referred to as coarse woody debris or coarse necromass above the soil, are important components of the forest carbon cycle and account for 20%to 40%of carbon storage and 12% of above ground respiration (Palace et al. 2007).Necromass is also important in nutrient cycling and provides habitat for many organisms (MacNally et al. 2001;Nordén and Paltto 2001). However, dead wood remains one of the world’s least studied key forest carbon compartments(Woldendorp et al.2002;Ifo 2010;Merganicova and Merganic 2010; Pfeifer et al. 2015). Yet its carbon stock fluctuates between 1 and 50 t ha-1(Rice et al.2004;Bocko et al. 2017) and represents about 4% to 10% of the total carbon stock of a forest ecosystem (Vogt 1991).
In order to understand the relationship between tree diversity and carbon stocks of the different land cover types in the periphery of the DDNP,which is important for conservation efforts and mitigation of the effects of climate change, the objectives of this study were to: (1) determine tree diversity (species richness and Shannon index); (2)estimate the carbon stocks in some compartments (live tree, understory, dead wood, litter, fine roots); and, (3)assess the relationship between these compartments and tree diversity at the periphery of the DDNP.
Materials and methods
Study area
This study was carried out on the peripheries of the Deng Deng National Park (DDNP) located in the eastern region of Cameroon between 5°8′and 5°32′N latitudes and 13°22′and 13°36′E longitudes, with an area of 52,347 ha. The relief is diverse and uneven; altitudes vary between 600 and 800 m a.s.l., and with an equatorial, Guinean-type climate with temperatures from 20 °C between July and August to 30 °C between January and February. The DDNpreceives 1500 to 2000 mm of precipitation per year,over an average of 135 days. The soils of the research site are essentially ferralitic soils that form in the humid tropics as the result of chemical weathering. The vegetation belongs to the equatorial forest domain of the semi-deciduous forest- types, including Malvaceae and Cannabaceae families. It covers the majority of forest biotopes and results from the progressive colonization of savannas by forests in this part of Cameroon.
Sampling design and variables description
The sampling design consists of seven transects, four km long and 25 m wide. Each transect has two km located outside the Park and two km inside the Park. The transect positioning was chosen in such a way that they were near inhabited villages and covered much of the land cover types(LCT); the two km outside the Park included certain land cover types(young fallows,agroforests and forest residues),while inside the Park covered secondary and old secondary forests and riparian forests.On each transect there were six sampling units(25 m × 25 m plots)equidistant by 650 m,totaling 42 plots representing a study area of 2.63 ha.Similar sampling approaches are commonly used in studies of African tropical forests(Hawthorne and Abu-Juam 1995;Zapfack et al.2013).Floristic and structural composition of trees at diameter at breast height(dbh) ≥5 cm 1.3 m above ground level were identified in each 625 m2plot.Absolute density, relative density, relative frequency, Importance value index (IVI), Shannon index, basal area, relative dominance,and life-forms were assessed for each plot using the formulas below:
Absolute density is the average value of the number of trees per sample unit (Jiagho et al. 2016):

where Dais stems ha-1;niis number of trees in plot i;M is total area of the plot. An extrapolation was done to get density per hectare.
Relative density (Rd), relative frequency (Rf), relative dominance (R), and Importance Value Index (IIV) were calculated for each species using the formulas(Cottam and Curtis 1956):

where NSis the number of trees of species i, TTis total number of trees, NOis the number of occurrences of the species in each plot in each LCT, SOis the sum of occurrences of all species in each LCT,BAis basal area of species I and SBAis sum of basal area of all species. Rdis relative density.

Tree diversity was assessed using the Shannon index(Shannon and Wiener 1949):

where pi= ni/n, pithe proportion of individuals belonging to the ith species in each plot,n the total number of trees,nithe number of trees of species i.
The basal area (BA) of trees per hectare was estimated from the formula:

where dbhis dbh per tree (Asase and Tetteh 2010). Once the data was obtained for each plot, an extrapolation was done per hectare.
In this study, means are provided with their standard error. However, standard error of forest residue and riparian forests were not given because only one plot was encountered for these. The classification of life-forms was according to Raukiaer adapted to tropical regions by Schnell(1971).Phanerophytes are plants with persistent aerial shoots and buds located at significant distances above the ground. Sonké (1998) synthesizes six groups of phanerophytes in a tropical rainforest. In this study, the life-forms considered are exclusively erect phanerophytes according to the current height of each observed individual. Only three life-forms have been identified, namely:
· megaphanerophytes (MgPh): trees taller than 30 m;
· mesophalerophytes (MsPh): trees between 10 and 30 m;
· microphanerophytes(McPh):trees with height between 2 and 10 m;
Species richness is the number of species per hectare.The classification system APG (Angiosperms Phylogeny Group) IV (2016) was used.
An analysis of the land cover types was done on each plot and consisted in determining the land cover type based on previous experiences,knowledge and field observations,combined with the Branthomme et al. (2009) recommended classification criteria that takes into account species composition and traces of human activities.
Estimation of carbon stocks
Carbon stocks assessed in this study cover the three forests pools (living, dead, and Soilcarbon). For the living carbon pool, two compartments were evaluated, trees (above ground and below ground components)and understory.For the dead carbon pool, two compartments were also assessed,namely dead wood and litter.For the Soilcarbon pool,only the fine root compartment was evaluated.
Estimation of above and below ground biomass for roots >2 mm diameter
The diameters obtained during the floristic inventory were used to evaluate the above ground biomass of the trees surveyed. The allometric equation developed by Chave et al. (2014) was used for the assessment of above ground biomass of each tree in each plot:

where AGBis above ground biomass (in dry mass, DM); ρ is the dry density of wood in g.cm-3; dbhis diameter at breast height in cm;and E is climatic index which depends on temperature and precipitation of each plot sampled

where TSis seasonal temperature, CWDis climatic water deficit, and PSis seasonal precipitation.
The allometric equation of Chave et al. (2014) is commonly used for carbon estimation (Fayolle et al. 2016;Tabue et al. 2016). In fact, with the richness and diversity of plants in tropical ecosystems, it is difficult to have species equations for all tree biomass estimations. To do this, we used the pantropical equation of Chave et al.(2014) which is recommended for tropical forests when species equations are missing for aboveground biomass estimation.
Specific wood density of each species was obtained from http://www.globallometree.org/data/wood-densities/and the book, Trees of Dense Forests of Central Africa(Vivien and Faure 2011).
The estimation of below ground biomass(root diameters>2 mm) was based on Mokany et al. (2006):

where DM is Dry mass. The estimation of carbon stocks(Cs) in the above ground and below ground biomass was:Cs= (AGBor Y) × 0.47 (Zapfack et al. 2013).
Collection of biomass data from fine roots, litter and understory vegetation
To estimate the biomass of fine roots (diameters <2 mm)in each 625 m2plot, Soilcaps were extracted (after removing the litter) in frames 20 cm × 20 cm × 50 cm,while avoiding stump and larger roots. By the successive flotation method in a wash basin, fine roots were extracted from the soil samples.
Litter samples were collected in blocks of 50 cm × 50 cm. Understory vegetation was sampled (cut and weighed for fresh weight) in the 1 m × 1 m blocks.
Fine roots,litter and understory samples were collected,packaged, labeled and oven-dried at the University of Yaoundé at 70 °C until a constant weight (0.01 of precision) was reached. Thus, a total of 42 samples were collected for each of the three carbon compartments (roots,litter and fine roots). In this study, soil organic carbon was not determined.
Sampling standing dead wood
The height of each piece of dead wood was measured with a clinometer and a decameter.The volume of standing dead wood was calculated using the Mund (2004) formula:

where V is volume of standing dead wood (m3tree-1);dbhis dbh (m); h height of standing dead wood (m); f is form factor (0.627) for all trees.
Sampling dead wood on the ground
Dead wood on the ground was measured by the line intersection method. It consists in considering the sum of the four sides of the plot as the sampling line with a total length of 100 m.The diameter of each piece of dead wood intersecting the sampling line was systematically measured only if: (1) it was more than 50% above the ground; and,(2) the sampling line crossed at least 50% of the diameter of the piece of dead wood (Walker et al. 2011). The following equation has been used to calculate the volume of dead wood accumulated on the ground of each plot(Warren and Olsen 1964):V is the volume of dead wood(m3ha-1);dithe diameter of each tree debris sampled (m); L the perimeter of the plot(100 m).

Carbon of dead wood
The biomass calculation for dead standing and grounded wood used the same formula:

where Dwbis dead wood biomass in (t ha-1), V is volume and φ is density.
The density of wood is 0.48 kg. DM.m-3and has been used by Ifo et al. (2015) in the absence of information on the specific densities of each decomposition class in the Congo Basin.
The change from dry mass to carbon stock uses the formula of Zapfack et al. (2013).
Statistical analyses
The Shapiro test function of the statistical software R was used to test the normality of the data. The ANOVA test compared the means with the normal errors, and when there was significant difference (P <0.05), the Tukey test was used to compare the data between the two by two land cover types (LCT). The Kruskal-Wallis test was used for data with irregular distribution errors, and when this test showed that there was significant differences (P <0.05),the Wilcoxon test allowed comparisons between two by two LCTs. Pearson correlation coefficients and Spearman tests were used to evaluate the correlation between tree diversity and carbon stock, and life-forms and carbon stock, for both normal and non-normal variables respectively.
Results
Land cover type identified
Six land cover types were identified, namely: agroforests(AFO)(two plots),forest residues(FRE),forests with trees left standing after cleaning, thinning or final felling (one plot), a riparian forest (RIF) (one plot), secondary forests(SFO) where traces of human activities are clearly visible and where most trees were juvenile or in an early growth stage (18 plots), old secondary forests (OSF) where traces of human activities are clearly visible and where most trees were mature (18 plots), and young fallows or uncultivated fields (YFA) (two plots).
Tree diversity of the study site
The inventory of trees of all LCT (dbh ≥5 cm) identified 187 species belonging to 122 genera and 43 families, with five other species unknown.
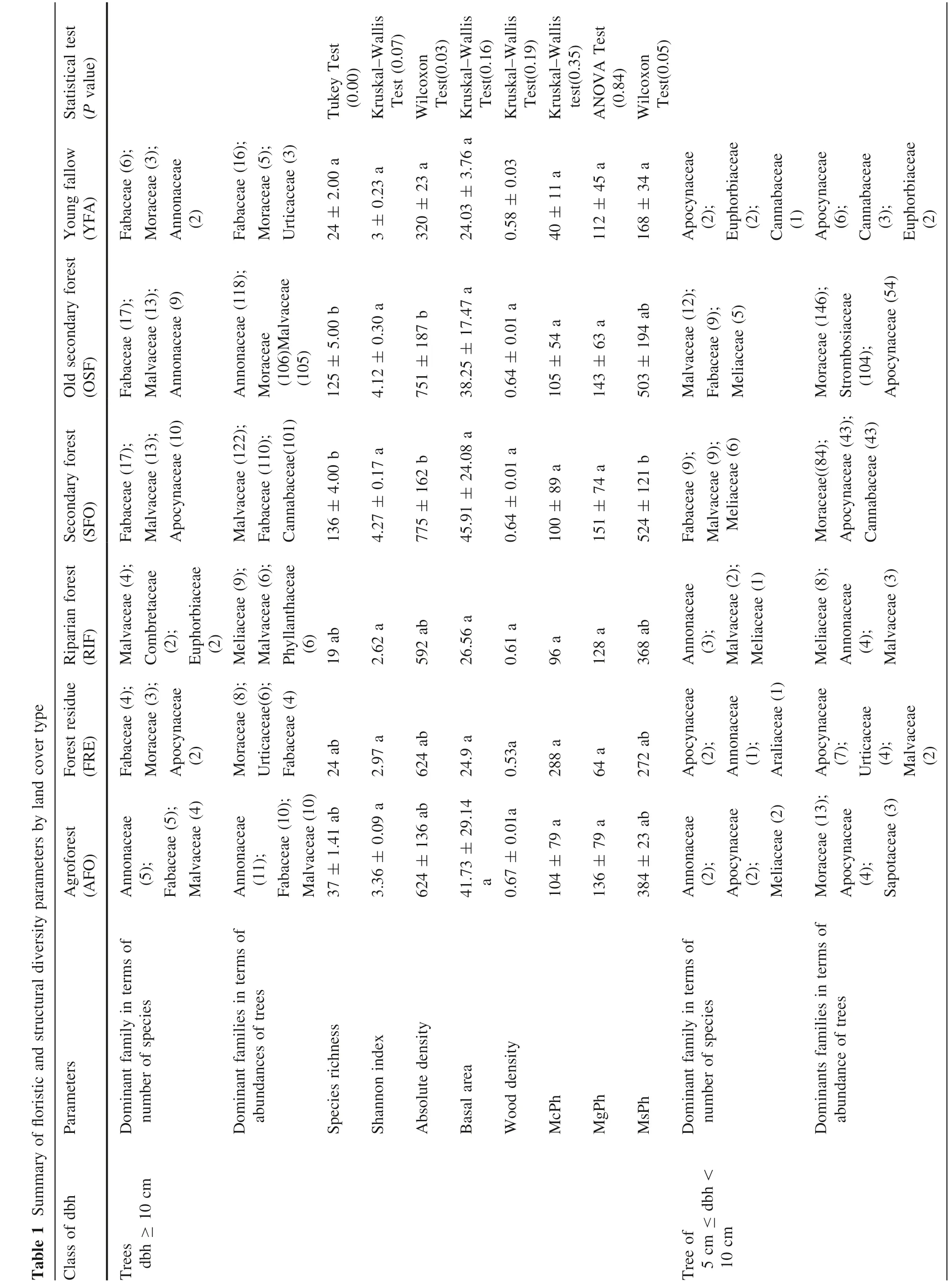
Among trees with dbh ≥10 cm (Appendix S1), 171 species belong to 115 genera in 40 families, four were unknown for all LCT. Terminalia superba Engl. & Diels(IVI = 27.73), Ficus thonningii Blume (IVI = 43.13), Trichilia welwitschii C. DC. (IVI = 32.11), Ceiba pentandra(L.)Gaertn.(IVI = 21.30),Anonidium mannii(Oliv.)Engl.& Diels (IVI = 12.68) and Albizia zygia (DC.) J.F. Mac-Bride(IVI = 34.07)appeared to be species with the highest ecological significance, respectively, in the AFO, FRE,RIF, SFO, OSF and YFA land cover types. The family Annonaceae (several genera produce edible fruit) was the most diversified in agroforests (Table 1); the Fabaceae family (legume family) was the most diversified in the forest residues, secondary forests, old secondary forests and young fallow fields, while the Malvaceae family(mallow family) is the most diverse in the riparian forest.Concerning dominance in terms of number of trees(Table 1), Annonaceae was dominant in agroforests and secondary forests, Moraceae (mulberry or fig family) was dominant in forest residues, Meliaceae in the riparian forest, Malvaceae in secondary forests, and the Fabaceae family was dominant in young fallows.
The Shannon index varied from one land cover type to another (Table 1), being relatively lower in the riparian forest (2.62) and relatively higher in the secondary forest(4.27). We did not find difference between the different LCT in term of Shannon index(Kruskal-Wallis test;P = 0.07).
Total species richness varied considerably between land cover types: 19 species in the riparian forests and 136 species in the secondary forests.The Tukey test(P = 0.00)showed that there is a significant difference between species richness in secondary forests (136 ± 5) and young fallow fields (24 ± 2), and also between old secondary forests (125 ± 5) and young fallow fields (24 ± 2). For life-forms, mesophanerophytes (MsPh) were dominant,followed by megaphanerophytes (MgPh) in all land cover types except for forest residues or microphanerophytes(McPh)- dominated MsPh and MgPh. There was a significant difference (Wilcoxon test, P = 0.05) between secondary forests and young fallow fields for (MsPh).
The inventory of species with diameter ≥5 cm and less that <10 cm dbh (Appendix S2) identified 115 species belonging to 85 genera in 34 families, with three species unknown. Morus mesozygia Stapf (IVI = 72.85), Tabernaemontana crassa Benth. (IVI = 66.46), Trichilia welwitschii C. DC. (IV I = 114.40) and Voacanga africana Stapf (IVI = 104.92) appeared to be species with high ecological significance in AFO, SFO, OSF, FRE, RIF and YFA, respectively.
The Annonaceae family had more species in agroforests and in the riparian forest; there were more species of the Apocynaceae or dogbane family in the forest residues and young fallow fields (Table 1). The Fabaceae family had more species in secondary forests. Concerning dominance in terms of number of trees(Table 1),the Moraceae family dominated agroforests, secondary forests and old secondary forests, the Apocynaceae family was dominant in the forest residue land type and young fallow fields while the Meliaceae (mahogany) family dominated the riparian forest.
The Shannon index ranged from 1.56 in riparian forests to 3.68 ± 0.64 in the secondary forests, and differed significantly among the different LCT (Kruskal-Wallis test,P =0.36). Species richness ranges from 7 in the riparian forests to 83 in secondary forests, with no significant differences between the different land cover types (ANOVA test, P >0.05). As for life-forms, microphanerophytes(McPh) are important in all LCT compared with mesophanerophytes (MsPh). There is no significant difference between land cover types in life-forms for McPh(ANOVA test,P =0.45)and for MsPh(Kruskal-Wallis test P =0.24).
Absolute density, basal areas and wood density
The average density of rees ≥5 cm dbh is 904 trees ha-1,and the average basal area is 34.98 m2ha-1for trees ≥5 cm dbh. For trees ≥10 cm dbh, density varies from 320 ± 23 trees ha-1in young fallow fields to 775 ± 162 trees ha-1in secondary forests, for an overall average of 614 ± 126 trees ha-1in the study area.The Wilcoxon test(P = 0.03) showed that there is a significant difference between secondary forests and young fallows, and also between old secondary forests and young fallow fields.Basal area varies from 24.03 ± 3.76 m2ha-1for young fallow fields to 45.91 ± 24.08 m2ha-1for secondary forests. However, there was no significant difference(Kruskal-Wallis test, P =0.16) between the different land cover types (agroforests, secondary forests, old secondary forests, and young fallow fields) basal areas. The wood density varies from 0.53 for FRE to 0.67 ± 0.01 g. cm-3for AFO. There was no significant difference (Kruskal-Wallis test, P = 0.19) between the different LCT.
For trees ≥5 cm <10 cm dbh, densities vary from 104 ± 79 trees ha-1in young fallows to 425 ± 182 trees ha-1in old secondary forests.The ANOVA test(P = 0.22)showed that there were no significant differences among the land cover types in terms of tree density as well as basal area (ANOVA Test P = 0.18). However, the basal area is low in young fallows(0.73 ± 0.31 m2ha-1)and higher in old secondary forests (2.03 ± 0.75 m2ha-1). Wood densities of trees ≥5 cm <10 cm dbh vary from 0.53 for FRE to 0.71 ± 0.01 g. cm-3for AFO. The Wilcoxon test(P =0.01) showed that there is a significant difference between AFO and YFA.
Statistical test(P value)Young fallow(YFA)Fabaceae (6);Moraceae (3);Annonaceae(2)Fabaceae (16);Moraceae (5);Urticaceae (3)Tukey Test(0.00)Kruskal-Wallis Test (0.07)Wilcoxon Test(0.03)Test(0.16)Kruskal-Wallis Test(0.19)Kruskal-Wallis test(0.35)ANOVA Test(0.84)Wilcoxon Test(0.05)24 ± 2.00 a 3 ± 0.23 a 320 ± 23 a 24.03 ± 3.76 a Kruskal-Wallis 0.58 ± 0.03 40 ± 11 a 112 ± 45 a 168 ± 34 a Apocynaceae(2);Euphorbiaceae(2);Cannabaceae(1)Apocynaceae(6);Cannabaceae(3);Euphorbiaceae(2)Old secondary forest(OSF)Secondary forest(SFO)Fabaceae (17);Malvaceae (13);Annonaceae (9)Fabaceae (17);Malvaceae (13);Apocynaceae (10)Annonaceae (118);Moraceae(106)Malvaceae(105)Malvaceae (122);Fabaceae (110);Cannabaceae(101)125 ± 5.00 b 136 ± 4.00 b 4.12 ± 0.30 a 4.27 ± 0.17 a 751 ± 187 b 775 ± 162 b 38.25 ± 17.47 a 45.91 ± 24.08 a 0.64 ± 0.01 a 0.64 ± 0.01 a 105 ± 54 a 100 ± 89 a 143 ± 63 a 151 ± 74 a 503 ± 194 ab 524 ± 121 b Malvaceae (12);Fabaceae (9);Meliaceae (5)Fabaceae (9);Malvaceae (9);Meliaceae (6)Moraceae (146);Strombosiaceae(104);Apocynaceae (54)Moraceae((84);Apocynaceae (43);Cannabaceae (43)Riparian forest(RIF)Malvaceae (4);Combretaceae(2);Euphorbiaceae(2)Meliaceae (9);Malvaceae (6);Phyllanthaceae(6)19 ab 2.62 a 592 ab 26.56 a 0.61 a 96 a 128 a 368 ab Annonaceae(3);Malvaceae (2);Meliaceae (1)Meliaceae (8);Annonaceae(4);Malvaceae (3)Table 1 Summary of floristic and structural diversity parameters by land cover type Forest residue(FRE)Agroforest(AFO)Parameters Class of dbh Fabaceae (4);Moraceae (3);Apocynaceae(2)Annonaceae(5);Fabaceae (5);Malvaceae (4)Dominant family in terms of number of species 10 cm Trees dbh ≥Moraceae (8);Urticaceae(6);Fabaceae (4)24 ab Annonaceae(11);Fabaceae (10);Malvaceae(10)37 ± 1.41 ab Dominant families in terms of abundances of trees Species richness 2.97 a 3.36 ± 0.09 a Shannon index 624 ab 624 ± 136 ab Absolute density 24.9 a 0.53a 41.73 ± 29.14 a 0.67 ± 0.01a Basal area Wood density 288 a 104 ± 79 a McPh 64 a 136 ± 79 a MgPh 272 ab 384 ± 23 ab MsPh Apocynaceae(2);Annonaceae(1);Araliaceae(1)Annonaceae(2);Apocynaceae(2);Meliaceae (2)Dominant family in terms of number of species dbh <Tree of 5 cm ≤10 cm Apocynaceae(7);Urticaceae(4);Malvaceae(2)Moraceae (13);Apocynaceae(4);Sapotaceae (3)Dominants families in terms of abundance of trees
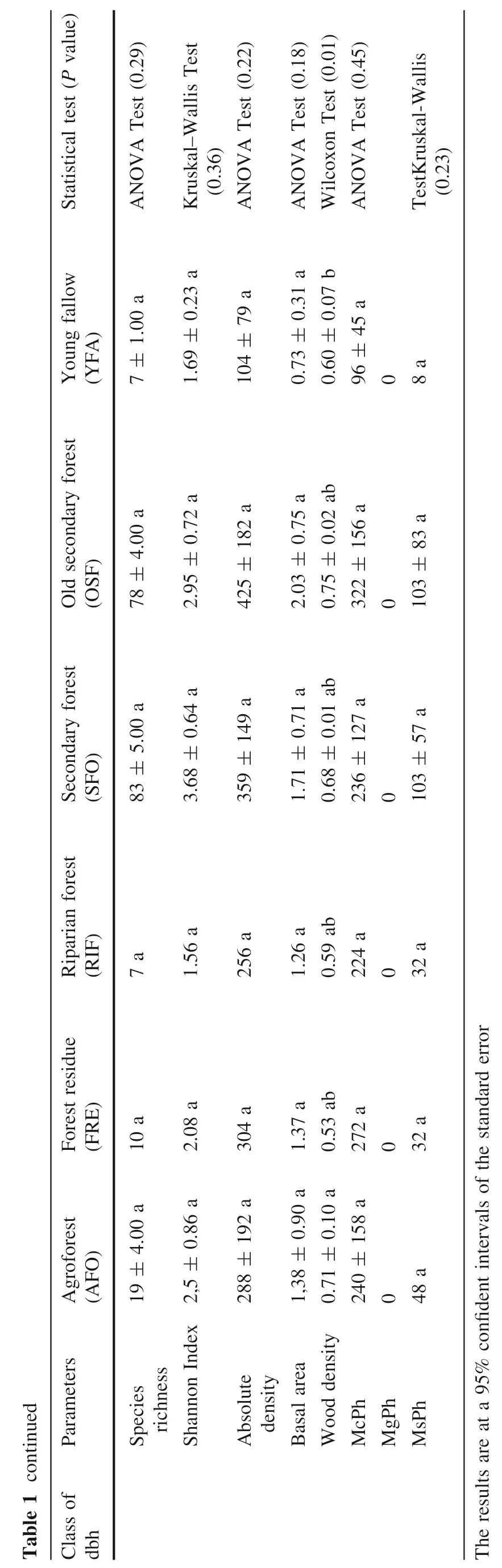
Table 1 continued Statistical test (P value)Young fallow(YFA)Old secondary forest(OSF)Secondary forest(SFO)Riparian forest(RIF)Forest residue(FRE)Agroforest(AFO)Parameters Class of dbh ANOVA Test (0.29)Kruskal-Wallis Test(0.36)ANOVA Test (0.22)ANOVA Test (0.18)Wilcoxon Test (0.01)ANOVA Test (0.45)TestKruskal-Wallis(0.23)7 ± 1.00 a 1.69 ± 0.23 a 104 ± 79 a 0.73 ± 0.31 a 0.60 ± 0.07 b 96 ± 45 a 0 8 a 78 ± 4.00 a 2.95 ± 0.72 a 425 ± 182 a 2.03 ± 0.75 a 0.75 ± 0.02 ab 322 ± 156 a 0 103 ± 83 a 83 ± 5.00 a 3.68 ± 0.64 a 359 ± 149 a 1.71 ± 0.71 a 0.68 ± 0.01 ab 236 ± 127 a 0 103 ± 57 a 7 a 1.56 a 256 a 1.26 a 0.59 ab 224 a 032 a 10 a 2.08 a 304 a 1.37 a 0.53 ab 272 a 0 32 a 19 ± 4.00 a 288 ± 192 a 1,38 ± 0.90 a 0.71 ± 0.10 a 240 ± 158 a 0 48 a Species richness Shannon Index 2,5 ± 0.86 a Absolute density Basal area Wood density McPh MgPh MsPh The results are at a 95% confident intervals of the standard error
Carbon stocks
Above ground carbon stocks of trees ≥5 cm<10 cm dbh varied between cover types (Table 2), but the differences were not significant (ANOVA test, P = 0.18). Carbon stocks were 0.72 t ha-1in young fallow fields and 5.02 t ha-1in secondary forests, for an average site value of 3.10 t ha-1(for a proportion of 0.97% of the total carbon). Below ground carbon stock of trees ≥5 cm <10 cm dbh varies from 0.15 t ha-1in young fallow fields 1.03 t ha-1in old secondary forests for an average value of 0.64 t ha-1and a proportion of 0.20% of total carbon,the difference not being significant as well (ANOVA test,P = 0.18).
Above ground carbon stocks of trees ≥10 cm dbh ranged from 151.47 t ha-1in the forest residue cover type to 339.41 t ha-1in secondary forest. The Kruskal-Wallis test shows that there was no significant difference between the different cover types for above ground carbon stock of trees ≥10 cm dbh (P >0.05). Below ground carbon stocks of trees ≥10 cm dbh ranged from 35.60 t ha-1in the forest residue cover type to 79.76 t ha-1in secondary forest. The Kruskal-Wallis test showed that there was no significant difference between the different cover types for below ground carbon stocks(P >0.05).The results for the five species with the higher IVI per LCT represent 51.1%,76.1%, 56.0% 40.5, 25.7, and 75.9% of above ground carbon respectively for AFO, FRE, RIF, SFO, OSF and YFA (Appendix S1).
Dead wood carbon stock varied from 1.24 t ha-1in the riparian forests to 12.51 t ha-1, in agroforests with no significant difference between the different LCT(Kruskal-Wallis test, P >0.05).
Fine root carbon stock ranged from 5.3 t ha-1for young fallows to 13.72 t ha-1in riparian forests with an average value of 7.81 t ha-1and 2.5%of the total carbon.The Tukey test (P <0.05) shows that there was no a significant difference in total carbon between AFO, SFO,OSF and YFA.
The carbon stock of litter ranged from 1.49 t ha-1in the forest residues to 3.1 t ha-1in riparian forests. The Kruskal-Wallis test shows that there was no a significant differences in litter carbon between the different land cover types.
Understory carbon ranged from 0.45 t ha-1in the forest residues to 1.55 t ha-1in the riparian forests.
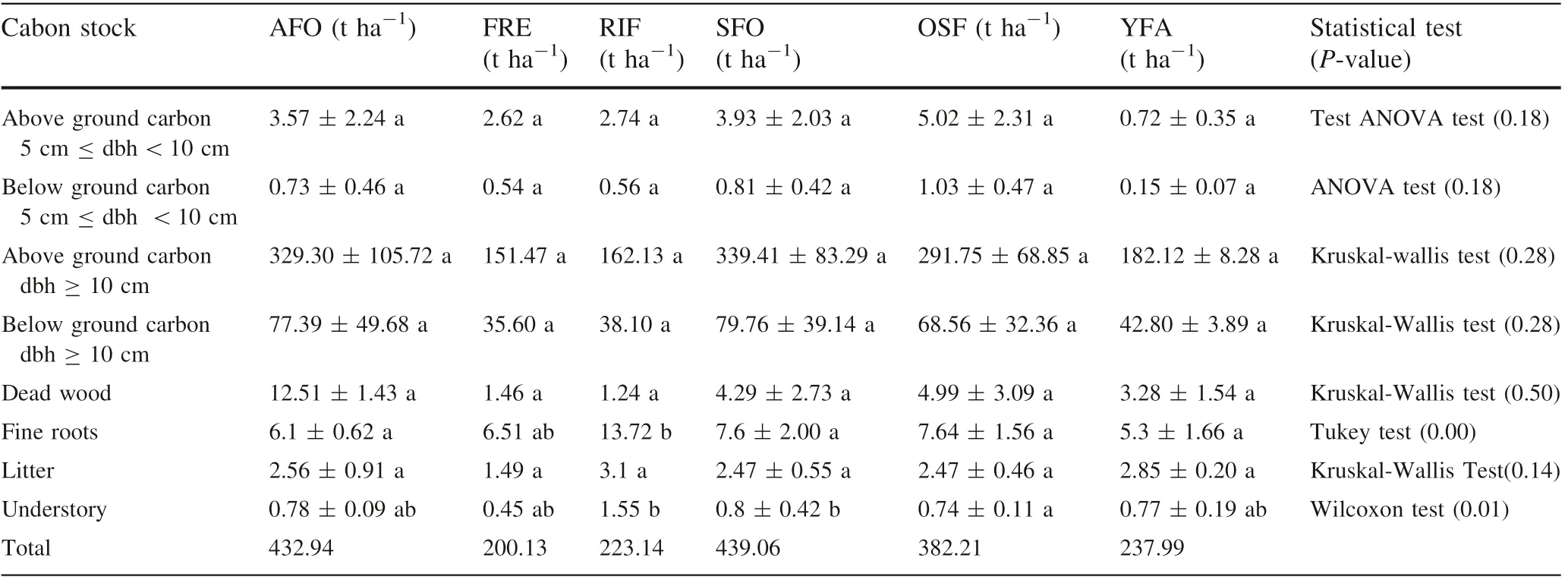
Table 2 Carbon stocks of the different compartment for land cover types

Table 3 Correlation between carbon stocks, tree diversity and life-forms for various carbon compartments
Relationship between tree diversity and carbon stocks
There was a weak, positive but insignificant correlation between the Shannon diversity index and the above ground carbon of trees ≥10 cm dbh (Table 3), the above ground carbon of trees ≥5 cm <10 cm dbh as well as total carbon(Table 3,P >0.05).There was no correlation between species richness and above ground carbon stocks for≥10 cm dbh and between 5 cm and <10 cm.We found a positive correlation between total carbon stocks, species richness (Table 3, Spearman test, P = 0.02) and life-form(Table 3, Spearman test, P = 0.00). The total carbon stock was not correlated with the Shannon index.
Discussion
Diversity and tree structure in the periphery of Deng Deng National Park
The results for trees ≥10 cm dbh and those ≥5 cm <10 cm dbh throughout the study site showed that the site is home for 187 species belonging to 122 genera distributed into 43 families. The species richness of trees ≥10 cm dbh showed that secondary forests and old secondary forests are higher than young fallows or uncultivated fields.The species richness of the secondary forests is relatively higher than that of the agroforests,forest residue land cover and the riparian forests, although statistically insignificant for the agroforests. According to Sonké (1998), floristic richness is function of climatic and pedological conditions and the capacity of species to disperse its seeds. In this study, climate and Soilconditions are the same for all land cover types. We have not studied seed dispersal but the same species are found on several cover tpyes. Thus,species richness could be explained by anthropogenic activities and the number of plots inventoried by cover type. Uncultivated, young fallows are land cover types having previously undergone anthropogenic action and are now resting. Agroforests are also based on human activities.These two land use types were only considered in two plots because of the systematic plotestablishment along transects; this could also influence species richness. Species richness and floristic diversity increased with the area until reaching a saturation threshold. Forest residue plots and riparian forests were only encountered in one plot because of the plot layout, which made it impossible to make conclusive comparative tests with these two cover types.
The results for secondary and old secondary forests(trees ≥10 cm dbh) highlight the richness of biodiversity in the tropical forest and its complexity. These results are different from those of Sonké and Couvreur (2014) who identified 312 species in 60 genera belonging to 54 families in Dja Fauna Reserve in Cameroon, and Gonmadje et al.(2011)who identified 293 species belonging to 170 genera in 60 families in Ngovayang, South Cameroon. These differences could be explained by the size of sampling plots (22.5 ha for the first authors and 5 ha for the latter)but also by the climatic parameters of these forests. Both studies are located in south and southwest Cameroon, the first being evergreen forests and the second, the Biafran forests which receive more than 2000 mm/year of rain than the Deng Deng zone. Species richness of tropical forests tends to increase with precipitation and reduced seasonality(Ter Steege et al. 2003).
The Shannon index values reflect a great diversity of species in the secondary and old secondary forests. Kent and Coker (1992) showed that forest communities considered rich are characterized by a Shannon diversity value of 3.5 or higher. The values in both secondary forests in this study are similar to those obtained by Gonmadje et al.(2011),which were 4.11 and 4.12 in the permanent plots of Lambi 2 (LAM2) and Ngovayang 1 (NGO1) in the southwest region of Cameroon, but different from the 5.7 in the heterogeneous terra firma forests on the periphery of the Dja Fauna Reserve (Djuikouo et al. 2010). The differences could be explained by the size of the sample.The Shannon Index is related to the dominance of species and is sensitive to the sample size.
The average densities of trees ≥10 cm dbh are close to those obtained by Gonmadje et al. (2011) in permanent plots in the southwest region, namely, 634 trees ha-1in LAM2 plot and 582 trees ha-1in NGO1 plot. For Pascal(2003), densities varied depending on the type of tropical rain forest but were between 450 and 750 trees ha-1(dbh ≥10 cm). On the other hand, absolute density (for secondary and old secondary forests) was higher than that of Sonké(2004),which found an average value of 536 trees ha-1. This difference could be explained by the methodology used (the small plot method allowing to maximize efforts), but also by the conditions specific to each land cover type (degree of disturbance, slope, locations, composition of the soil).Lewis et al.(2013)confirmed that tree densities in tropical forests vary according to soil types.But in this study, we may reach the same conclusion because it was conducted on the same site with soils of the different cover types the same.
The average basal area varied between land cover types,and the secondary and old secondary forest vales are consistent with those obtained in tropical forests (25 and 50 m2ha-1, Pascal 2003), and those from tropical forests of Central Africa (31.5 m2ha-1, Lewis et al.2013). However, the values obtained for this compartment varied between 0.73 ± 0.31 m2ha-1for young fallows to 2.03 ± 0.75 m2ha-1in old secondary forests. The variation between different cover types may be explained by human disturbances. In fact, old secondary and secondary forests and other cover types are characterized by the degree of human activity (Branthomme et al. 2009).
The results obtained for trees ≥5 cm >10 cm dbh confirm the need to include them. In fact, the analysis carried out on all the cover types identified 16 species exclusively in the ≥5 cm <10 cm dbh range.This shows that there are species that generally may not be considered in forest management in Cameroon. Forest management studies are most often based on trees ≥10 cm dbh).Because trees ≥5 cm >10 cm dbh may remain growing in clusters throughout their lives and are not inventoried in studies that only take account trees with diameters >10 cm(Tchouto et al.2006).They further stated that the majority of the endemic species of the Congo Basin are found in the class ≥5 cm >10 cm.
Carbon sequestration capacity of the peripheral forest of Deng Deng National Park
Quantification of carbon stocks in tropical forests has become an international priority in the implementation of the REDD + mechanism (Loubota-Panzou et al. 2016).The equation used in this study is that of Chave et al.(2014), which is an improved equation on their earlier pantropical equation (Chave et al. 2005). The latter was criticized because of the lack of data from tropical Africa in the calibration of the equation. This criticism was taken into account in their 2014 equation and,more importantly,it considered local climatic parameters. Numerous local allometric equations have been developed in recent years for the Congo Basin (Fayolle et al. 2013; Ngomanda et al.2014). These equations have been discussed and criticized at length over the number of trees studied and the extent of the study areas. Chave et al. (2014) responded to these criticisms by developing a pantropical equation that takes into account the species of global tropical forests, diameters and the wood densities which are easily measurable parameters in the field (diameter) and available in the literature (wood density). This allometric equation utilized data on 4004 trees from 58 sites.
The variations between carbon stocks in the different land cover types (although there no significant differences for above ground,below ground,dead wood and litter)may be due to the variations of tree densities of the different cover types which reflects the dominant vegetation but also by the basal areas of each cover types.In fact,densities and basal areas are more important in the secondary forest,followed by the old secondary forest,the agroforests,forest residue areas, riparian forests, and the uncultivated young fallows.However it would be best to limit comparison two by two of land cover types to secondary and old secondary forests because of their higher number of plots. The other four cover types,although with differences that may allow the conclusion that carbon stocks increase with tree density and basal area, have not been repeated enough and could bias the conclusions. This is confirmed by Bocko et al.(2017) who showed that above ground carbon stocks tend to increase with the increase of certain structural parameters such as basal area.Wood densities can also explain the variation in carbon sequestration between different cover types (Chave et al.2014). However, for this study,there is no significant difference for this parameter between cover types.Variations between different cover types also do not show the influence of this parameter because wood densities are higher in agroforests than in secondary and old secondary forests, but carbon stocks are higher in the last two than in agroforests.Above ground carbon stock values for trees ≥10 cm dbh in this study are higher than values obtained by Zapfack et al. (2013) in secondary forests(169.26 t ha-1) and uncultivated young fallows(84.74 t ha-1)in the periphery of Lobeke National Park in Cameroon. Above ground carbon values in secondary and old secondary forests are also higher than those in numerous studies in several Congo Basin forests(Djuikouo et al. 2010; Fayolle et al. 2016). The differences could be explained by the types of allometric equations used, the average density of the trees and environmental factors.Zapfack et al. (2013) used the allometric equation of Brown et al.(1989),and Djuikouo et al.(2010)used that of Chave et al. (2005). Many researchers have reported the uncertainties generated by the type of allometric equation used (Djomo et al. 2011; Fayolle et al. 2016). The basal areas may also explain the differences between our results and those obtained by Djuikouo et al.(2010),38 m2ha-1is less than those in the secondary and old secondary forests.Environmental factors may also explain the differences between above ground carbon stocks of our study and those of Djuikouo et al. (2010) and Zapfack et al. (2013).Environmental factors influence the species composition and natural selection of different species (Bocko et al.2017).
The values of dead wood carbon stocks are similar to those obtained by other researchers (Kueppers et al. 2004;Rice et al.2004;Djomo et al.2011;Ngo et al.2013).These values are, however, lower than the 9.45 t ha-1in the swamp forests of Likouala,Congo(Bocko et al.2017).The proportions in this study (Fig. 1), however, remain lower than those of Vogt (1991) showing the proportion of dead wood is 4% to 10%. This difference may be explained by the variability of phenomena that can lead to the death of trees in the tropical forest. Tree death is often caused by natural factors(natural death,winds,climatic conditions in general) and anthropogenic factors (agriculture, logging and firewood cutting). The evaluation methodology for carbon storage in dead wood requires an in depth analysis.For example, carbon stored by standing dead wood is 200 times the carbon stored by dead wood on the ground with the same diameter,mainly because of the calibration of the equations of estimation of dead wood volume. Carbon stocks of trees ≥5 cm <10 cm dbh varies between different land cover types and increases with density and basal area. The values obtained in the secondary and old secondary forests are lower than those found by Djomo et al.(2011) in the Campo Ma’an area. Thedifferences could be due to the number of stems of this diameter class per hectare. In fact, the Campo Ma’an forests are denser in terms of trees with diameters > 10 cm than forests of Deng Deng.
Evaluation of carbon stocks of ≥5 cm >10 cm dbh and dead wood reveals unvalued stocks in most studies in Congo Basin forests. These compartments together represent between 1.7% in the uncultivated young fallows to 3.7% in the agroforests of total carbon in each cover type(Fig. 1). This illustrates the importance of these two compartments in carbon sequestration in tropical forests.However, the majority of research on carbon stocks in the Congo Basin has been restricted to trees ≥10 cm dbh,fine roots,litter and understory,and have not taken into account dead wood and small trees (Zapfack et al. 2013; Tabue Mbobda et al. 2016).
Relationship between tree diversity and carbon sequestration in different compartments
There was no correlation between above ground carbon stocks of trees ≥10 cm dbh, the above ground carbon stock of trees ≥5 cm >10 cm dbh, and total carbon stocks with the Shannon index.These results are similar tothose of Diaz et al. (2007) and Asase et al. (2012). This result can be explained by the fact that the Shannon index better reflects the distribution of individuals within species than the number of species in a site.
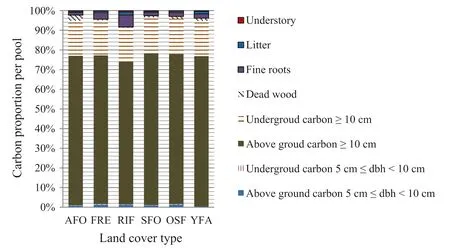
Fig. 1 Stock of different compartments by land cover type; (AFO agroforests, FRE forest residue, RIF riparian forest, SFO secondary forests,OSF old secondary forest, YFA young fallows)
There was, however, a weak positive relationship between species richness and total carbon stocks. This result is in agreement with Day et al.(2013)who showed a positive but weak relationship between species richness and total carbon stocks. Several studies have reported a significant and positive relationship in other forest types between species richness and total carbon stocks (Sagar and Singh 2006;Houle 2007;Nakakaawa et al.2009).Day et al. (2013) showed that, in some of their plots, high species richness had relatively low biomass and low species richness plots may have high biomass. However,despite this variability, there was evidence of positive correlations between biomass and species diversity. This result may be explained by the fact that a greater diversity leads to an optimization of the resources of the environment and thus of the efficiency of the species, which can translate to an increase of biomass (Day et al. 2013).Elsewhere, studies have shown that tree diversity and composition have important impacts on carbon dynamics through the relationship between plant diversity and carbon stocks (Fornara and Tilman 2008; Ruiz-Jaen and Potvin 2010).
The positive correlation between carbon stocks and lifeforms in this study are in agreement with those of Diaz et al.(2007).Diaz et al.(2007)reported that a relationship exists between functional diversity and carbon storage.Kirby and Potvin (2007) confirmed this, showing that functional traits or the presence of specific species were better predictors of ecosystem functioning than diversity and species richness. Bunker et al. (2005) suggest that carbon storage in tropical forests depends on species composition, mode, and how species are lost. The main conclusions here would be to look for the link in the functional diversity,species richness having given positive but weak correlations.
Conclusions
This study highlights the differences in floristic diversity between the six land cover types, agroforests, forest residues, riparian forests, secondary forests, old secondary forests, and young uncultivated fallows, studied in the periphery of the studied on the periphery of the Deng Deng National Park.It showed that species richness and Shannon index are higher in secondary and old secondary forests compared to other land cover types. There was no significant difference between different cover types for above ground and below ground carbon stocks. Above ground carbon stocks increased with tree density and basal area.The results for carbon stocks of trees ≥5 cm <10 cm dbh and dead wood, which represent respectively 1.7% of the total carbon of the uncultivated young fallows and 3.7%of the total carbon of the agroforest cover, show the need to assess the carbon stocks of these compartments, as they represent a significant proportion of total carbon stocks.This study also showed a positive but weak correlation between species richness and total carbon stocks, and indicates a need to consider biodiversity in greenhouse gas reduction strategies in forests and other land cover types.
AcknowledgementsThis study was funded by a Grant from the‘Organisation pour la Conservation et le Développement (OCD)’’entitled‘‘Forest Ecosystem Services’’.We gratefully thank the people of Deng Deng village for their warm welcome, their contributions as guides and helpers installing the sample plots and carrying out the inventories.
杂志排行
Journal of Forestry Research的其它文章
- Do increasing respiratory costs explain the decline with age of forest growth rate?
- At what carbon price forest cutting should stop
- Mapping the risk of winter storm damage using GIS-based fuzzy logic
- Effects of seed moisture content, stratification and sowing date on the germination of Corylus avellana seeds
- Comparison of seed morphology of two ginkgo cultivars
- De novo assembly of the seed transcriptome and search for potential EST-SSR markers for an endangered, economically important tree species: Elaeagnus mollis Diels
