Vegetation structure and regeneration status of the moist,evergreen, afromontane Bore-Anferara-Wadera forest in southern Ethiopia
2020-05-19MesfinWoldearegayZerihunWoldu
Mesfin Woldearegay · Zerihun Woldu
Abstract In a survey of the Bore-Anferara-Wadera forest to study the vegetation structure and regeneration status of woody plant species, 112 quadrats were systematically sampled along altitudinal transects to collect vegetation data.Nested sample plots of 30 m × 30 m and 5 m × 5 m were laid for collecting data on abundance and some variables of tree and shrub size. The regeneration status of woody species was assessed by counting all seedlings within the main sample plot. Woody plant species taller than or equal to 3 m were counted and their height and DBH measured. Density, frequency, basal area and importance value (IV) of woody plant species were computed. A total of 136 vascular plant species belonging to 119 genera and 63 families were recorded. The overall Shannon-Wiener diversity value was 3.84 and evenness was 0.78. Total density of trees and shrubs with DBH >2 cm was 1047 ha-1. Size class distribution of woody species across different DBH and height classes indicated a relatively high proportion of individuals at lower classes,suggesting impacts of past anthropogenic disturbances.Analysis of population structure and regeneration status of the forest revealed various patterns of population dynamics where some species were represented by only a few mature plants, suggesting that they are on the verge of local extinction and that immediate conservation measures should be taken. The results highlight the need for joint management and conservation measures by the government,local people and other stakeholders to abate the rapid rate of deforestation and promote sustainable utilization of the forest resources in this forest in southern Ethiopia.
Keywords Anferara·Afromontane forest·Conservation·Regeneration · Structural analysis
Introduction
Ethiopia has diverse ecological habitats with considerable variation in soil, climatic and biological resources. More than 50% of the land surface with an afromontane vegetation type in Africa is found in Ethiopia (Yalden 1983;Bekele 1994). The diverse flora and fauna make it an important regional center of biological diversity and endemism (Sayer et al. 1992; Woldu 1999), but many of the genetic resources of the country are still unexplored and under continuous threat of destruction mainly due to habitat loss and fragmentation, unsustainable utilization of biological resources, invasive species and climate change(Lemenih and Teketay 2004; EBI 2014).
Although deforestation has a long history in Ethiopia,accelerated deforestation began in the early parts of the 20th century, especially in the southern and southwestern parts of the country.The forest cover of Ethiopia was about 16% of the land area in the early 1950s and rapidly declined to 3.6% in the early 1980s and 2.7% in 1989(EFAP 1994). The FAO (2010) estimated the rate of deforestation in Ethiopia as 141,000 ha a1.However,if the definition of the FAO (2001) for a forest is used, that is,vegetation with a canopy cover of more than 10% of the ground, a tree height higher than 5 m at maturity and an area coverage exceeding 0.5 ha, then the forest cover of Ethiopia rises to about 11%. However, this new definition takes into account only the height and canopy cover, with no or little concern to species composition. Selective cutting of trees in many places has decimated the diversity of the forests. According to Kelbessa et al. (1992), 120 endemic plant species of Ethiopia are threatened, underscoring the need for preserving the rich biodiversity resources of the country (Lemenih and Teketay 2004).
Although the structure and regeneration has been studied in different parts of the moist, evergreen, afromontane forests (MAFs) and dry, evergreen, afromontane forests(DAFs) of Ethiopia (e.g., Bekele 1993; Teketay 1997;Tesfaye et al. 2002, 2010; Ayalew et al. 2006; Lulekal et al.2008;Woldu et al.1989),the Bore-Anferara-Wadera Forest has not been studied in the same detail.
The MAFs represent the major natural forest fragments remaining in Ethiopia.It is one of the vegetation types that occur mostly on the wetter slopes of the higher mountains of the country (White 1983; Friis 1992; Friis and Demissew 2001;Friis et al.2011).Most of these forest fragments are important for the conservation of plant species diversity(Woldermariam 2003; Senbeta 2006; Schmitt et al. 2010),wild populations of Coffea arabica (Woldemariam et al.2002; Senbeta and Denich 2006) and various highland forest bird species (EWNHS 2001). Despite their importance,however,the MAFs in Ethiopia are rapidly declining due to deforestation (Woldemariam et al. 2002; Senbeta et al. 2005; Tadesse et al. 2008).
The present study area is also part of the Key Biodiversity Area (KBA) in the Eastern Afromontane Hotspot that contains vulnerable species of animals and plants in the highlands of southern Ethiopia(BLI 2012).Despite the high conservation priority of this area, basic information necessary to develop and implement appropriate conservation and management strategies is lacking.
For designing effective strategies to preserve these remnant forest fragments, understanding the vegetation structure and regeneration status of the species is highly important.The specific objectives of the present study were therefore to (1) determine the vegetation structure of the forest;(2)assess the composition,structure,and density of regenerating woody species in the forest;and(3)prioritize the woody species for conservation and management interventions.
Materials and methods
Study area
The Bore-Anferara-Wadera forest is found in the Guji Administrative Zone oforomia National Regional State,in southern Ethiopia(5°40′to 6°26′latitude;39°27′to 38°26′longitude; Fig. 1). The forest consists of rolling plateaus,dissected hills, valleys and plains (EWNHS 2001), generally between 1828 and 2304 m a.s.l. Three forest patches,Kilenso (1944 ha) in Bore, Anferara (20,440 ha) in Adola and Danissa (1578 ha) in Wadera were included in the study (NRSOBFED 2012).
The Anferara forest is included in the Key Biodiversity Area (KBA) of the Eastern Afromontane Biodiversity Hotspot region (BLI 2012). Vulnerable species include (1)Leptopelis ragazzii (2) Serinus xantholaemus (3) Tauraco ruspolii and (4) Ocotea kenyensis, a severely logged tree(BLI 2012).
The geology in the study area includes limestone,shale,chlorite, talc, schists, amphibolites, and quartz (Mohr 1971). Nitosols (red basaltic soils), Luvisols, Cambisols,and Fluvisols are broadly found in the highland areas and flat to sloppy terrain (NRGOBFED 2009). The study area has a bimodal rainfall with a long rainy period from late February through June and from September through October(Gamachu 1977).The mean annual temperature of the study area is 16.1 °C ranging from the mean annual minimum of 6.5 °C to the mean annual maximum of 25.3 °C. The climate diagram of the study area was constructed using climatic data from 2000 to 2014 obtained from The National Meteorological Services Agency(NMSA 2015) (Fig. 2).
The natural vegetation of the study area,which is mainly MAF (Friis et al. 2011), has more than two strata of evergreen trees that may reach a height of 30-40 m. The tree canopies typically contain a mixture of Podocarpus falcatus and broad-leaved species. Pouteria adolfi-friederici is the only emergent species in the study area in the 20-30 m high canopy layer.Other major canopy trees that reach 10-30 m include Albizia gummifera,Celtis africana,Croton macrostachyus, Ekebergia capensis, Ficus sur,Macaranga capensis, Schefflera abyssinica, Ilex mitis,Prunus africana, Ocotea kenyensis, Polyscias fulva, Syzygium guineense subsp. afromontanum and Olea capensis subsp. hochstetteri. The forest floor is dark, moist and usually dominated by the grasses and different species of ferns (Friis 1986, 1992; Hedberg et al. 2009). Ocotea kenyensis is a vulnerable tree species in Anferara forest due to overexploitation of this species for different purposes by the local people (BLI 2012). Large areas covered by this vegetation type have now changed tofarmland, secondary montane grassland, secondary montane woodland and secondary evergreen bushland (Hedberg et al. 2009).

Fig. 1 Map of Ethiopia showing the study area
Sampling design and data collection

Fig. 2 Annual climate diagram for Adola(data source:NMSA 2015)
Stratified systematic random sampling method following Kent and Coker(1992)and Muller-Dombois and Ellenberg(1974) was employed. Once the first sampling plot was established randomly at the edge of the forest at lower altitude, subsequent independent sampling plots were laid down along line transects with 400 m between each sampling plots and 750 m apart between each line transect. Nested sampling plots of 30 m × 30 m and 5 m × 5 m were established for sampling trees and shrubs,respectively,with 112 total plots(900 m2each):50 plots in the Anferara forest, 40 plots in the Kilensoforest and 22 plots in the Danisa forest. The number of plots in the different forest patches (Anferara, Kilenso, and Danisa) was determined by the size of the forest patches.
In this study,plants with a height greater than or equal to 3 m were considered as shrub or tree plants between 1.5 m and <3 m long were considered as saplings, and plants shorter than 1.5 m were considered as seedlings,following Tesfaye and Teketay (2005). In each sample plot and subplot, all woody plants were identified counted, and height and diameter at breast height(DBH)were measured.Trees/shrubs that branch at breast height or below, the diameter of each branch was measured separately, and the mean was calculated. The height of woody plants was measured using a Suunto clinometer and calibrated bamboo stick wherever topography and crown structure made it difficult to measure using the clinometer; and the circumference of woody plants at breast height(about 1.3 m)was also measured and converted to DBH. Growth forms of plants were recorded, and voucher specimens were collected and deposited at the National Herbarium (ETH) in Addis Ababa University(AAU).Specimens were identified preliminarily in the field and later authenticated at ETH using taxonomic keys in the Flora of Ethiopia and Eritrea(Edwards et al. 1995, 1997, 2000; Hedberg and Edwards 1995, 1989; Hedberg et al. 2003, 2004, 2006) and by comparison with herbarium specimens. The nomenclature of plant specimens followed the Flora of Ethiopia and Eritrea. Environmental variables such as altitude and geographical coordinates were recorded in each sample plot using Garmin 60 GPS.
Diversity analysis
Species richness is an appropriate measure of diversity and is usually expressed as a number of species per sample unit(Whittaker 1972). However, Magurran (2004) stated that species diversity within a sample or community consists of two components: species richness and the relative abundance (evenness or equitability) of species. The Shannon-Wiener diversity index is the most widely used measure of species diversity because it combines species richness with species evenness (relative abundance) (Magurran 1988;Kent and Coker 1992). The Shannon-Wiener diversity index (H′) was calculated using the following equation:

where s = the number of species, and Pi = the proportion of individuals of the ith species expressed as a proportion of total cover in the sample. The Shannon evenness index(J) was calculated using the following equation:

where H′max is the maximum level of diversity possible within a given population, which equals ln s.
The change in the diversity of species among a set of habitats is designated β diversity; that is, the number of species that differ between the two habitats. Although β diversity can be calculated using different methods, all methods determine species turnover between different sites or along environmental gradients (Perlman and Adelson 1997).
Noting a problem associated with the increasing number of species with increasing sample size, Whittaker (1972)suggested that β diversity could better be calculated from a pairwise comparison of sites.In this study,β diversity was computed using the Whittaker (1972) method.

where a is the number of shared species in two sites, and b and c are the numbers of species unique to each site.
Vegetation structure analysis
Vegetation structure analysis of the three forest patches was based on frequency, density, DBH, height, and basal area per hectare following Muller-Dombois and Ellenberg(1974). The frequency of a species was computed as the proportion of sample plots within which a species is found.Density was then computed by converting the count from all sample plots into a hectare as indicated in Kent and Coker (1992). Diameter at breast height (DBH) of each adult woody individual was simply obtained from the circumference (d = c/π).
The DBHs and heights were grouped into various DBH and height classes, and the density distribution of woody species in each class was computed(Kent and Coker 1992).The ratio of the density of individuals with DBH >10 cm and DBH >20 cm was computed to measure the size class distribution of species, following Grubb et al. (1963). The relative density of species in different DBH classes was used to obtain representative patterns of species population structures using the method of Popma et al. (1988). Basal area (BA) (in m2ha-1) of trees was computed using Eq. (4) to measure dominance, where the term dominance refers to the degree of coverage of a species as an expression of the space it occupies (Barbour et al. 1987).

The vertical structure of the woody species found in the Bore-Anferara-Wadera forest was analyzed using the IUFRO classification scheme, which categorizes a vertical structure of vegetation into upper,middle and lower storey.An importance value (IV) for each tree species was computed as indicated by Muller-Dombois and Ellenberg(1974) using a composite Eq. (5):

where

Individuals of a species with a higher IV value are dominant over individuals of species with relatively lower IV values. The maximum IV value of a species is 300.
Results
The 136 species of vascular plants recorded in the Bore-Anferara-Wadera forest represented 119 genera and 63 families.Represented by 56 species(41.18%),shrubs were the dominant growth form, followed by trees (44 species,32.35%), lianas (18 species, 13.24%) and herbs (18 species, 13.24%).
Species diversity and evenness
The overall Shannon-Wiener diversity index and evenness value of the Bore-Anferara-Wadera forest was 3.84 and 0.78, respectively (Table 1). However, the three forest patches varied in their species richness, diversity, and evenness. Danissa forest had the highest diversity and evenness, while Anferara forest had the highest species richness. On the other hand, Kilensoforest had the lowest species richness, evenness and diversity.
The magnitude of the β diversity indicates the change in species composition between adjacent forest patches along the environmental gradient. In this study, the highest β diversity was recorded between the Danissa and Kilensoforest patches (0.47) and the lowest between the Kilenso and Anferara (0.34) (Table 2).
Density of trees and shrubs
The total density of trees and shrubs with DBH >2 cm was 1047 ha-1in the Bore-Anferara-Wadera forest. Six woody species accounted for 51.23% of the total density.Conversely, the remaining 85 woody species altogether accounted for 48.77% of the total density in the forest(Table 3). The density of trees and shrubs with DBH >10 cm was 500.30 ha-1whereas that of species with DBH >20 cm was 246.63 ha-1.The ratio of the density of trees and shrubs with DBH greater than 10 cm to DBH greater than 20 cm is taken as a measure of size class distribution. Accordingly, the ratio of individuals with DBH >10 cm to DBH >20 cm was 2.03.
Diameter at breast height (DBH) and height class distribution
The size class distribution of trees and shrubs in the Bore-Anferara-Wadrea forest across seven DBH classes indicated a relatively large number of individuals in the first(542.66 ha-1) and second (257.94 ha-1) DBH classes. As the DBH class size increased, the number of individuals gradually decreased toward the successive higher classes(Fig. 3).
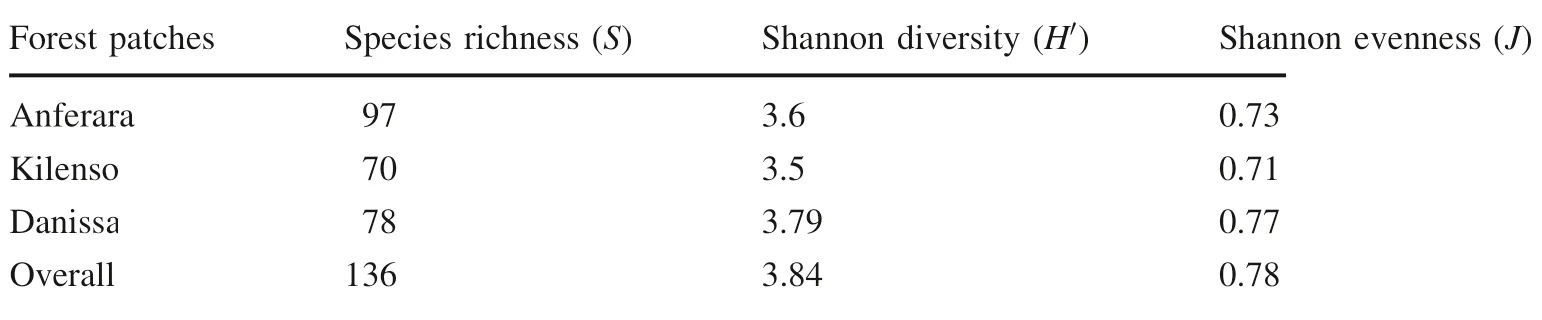
Table 1 Species richness,diversity and evenness values of the three forest patches of the Bore-Anferara-Wadera forest

Table 2 The β diversity index for species composition between the three forest patches of the Bore-Anferara-Wadera forest

Table 3 Density and percentage contribution of six woody species in the Bore-Anferara-Wadera forest
Pouteria adolfi-friederici, S. guineense subsp. afromontanum,F.sur,M.capensis,O.kenyensis,O.capensis subsp.macrocarpa,P.africana,P.falcatus,P.fulva and T.emetica were the dominant large trees(DBH >110 cm)in the Bore-Anferara-Wadrea forest.
The patterns of height class distribution of trees and shrubs revealed a high proportion of individuals in the first class followed by a rapid decline across the successive higher classes. Large proportions of individuals were aggregated in the first three height classes. About 67.07%of trees and shrubs were less than 13 m tall(height classes 1 and 2). Only a small proportion, about 17.3%, reached 23 m and above, indicating the predominance of shorter individuals (Fig. 4).
Vertical structure
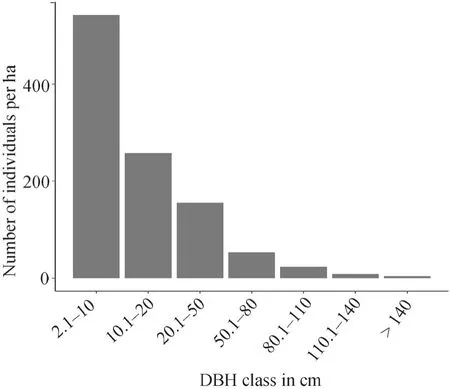
Fig. 3 DBH class distributions of trees and shrubs in Bore-Anferara-Wadera forest

Fig. 4 Relative density of trees and shrubs distributed along height classes in the Bore-Anferara-Wadera forest
According to the IUFRO classification scheme, storey is classified as upper, where tree height is greater than 2/3 of the top height; middle, where tree height is between 1/3 and 2/3 of the top height; and lower, where tree height is less than 1/3 of the top height (Lamprecht 1989). The top height for trees in the Bore-Anferara-Wadera forest was 75 m. Tree species that occupied the upper storey in the Bore-Anferara-Wadera forest included P.adolfi-friederici,S. guineense subsp. afromontanum, P. fulva, O. kenyensis,P. africana, Croton macrostachyus and O. capensis subsp.macrocarpa. A small number of individuals occupied the upper storey as the ratio of individuals to species is lower(Fig. 5, Table 4).
The middle layer of the Bore-Anferara-Wadera forest was dominated by species such as M. capensis, Psydrax schimperiana,Olea welwitschii,P.falcatus,M.ferruginea,Fagaropsis angolensis and A. abyssinicus. The lower storey was largely dominated by shrubs and small trees such as Maytenus addat, Chionanthus mildbraedii, Galiniera saxifraga, Lepidotrichilia volkensii, Ehretia cymosa,Oncoba spinosa, Celtis toka and Psychotria orophila. The highest proportion of species was concentrated in the lower storey (66.92%) followed by the middle (26.32%) and upper storey (6.77%) (Table 4).
Basal area (BA)
The total basal area of the Bore-Anferara-Wadera forest was 75.23 m2ha-1(Table 5).About 72%of the total basal area was due tofive large tree species:S.guineense subsp.afromontanum (31%), P. adolfi-friederici (19%), O.kenyensis(10%),O.capensis subsp.macrocarpa(7%)and M. capensis (5%).
About 51.70% of all individuals had DBH less than 10 cm (DBH class 1). The percentage contribution of this class to the total basal area, however, was only 2.13%.Conversely,individuals in the DBH classes >50 cm had a density of about 8.88% of the total,but they accounted for about 74.6% of the total basal area of the forest (Table 5).
Frequency

Fig. 5 Percentage density of trees that occupied the lower, middle and upper storey of the Bore-Anferara-Wadera forest
Teclea nobilis was the most frequent species in the Bore-Anferara-Wadera forest in 99% of all plots sampled, followed by Psychotria orophila (97.32%), Psydrax schimperiana(90.18%),Elaeodendron buchananii(86.61%)and S. guineense subsp. afromontanum (80.36%). The least frequent species include, among others, Dodonaea angustifolia, Ricinus communis, Ritchiea albersii, Schrebera alata, Terminalia schimperiana, each constituting 0.89%.
Importance value
The Bore-Anferara-Wadera forest is dominated by three large tree species as demonstrated by their high IV values.S. guineense subsp. afromontanum had the highest IV followed by P. adolfi-friederici, Dracaena afromontana,and Ocotea kenyensis. The first eight species with high IV contributed to about 54.91%of the total importance values,whereas the rest 83 species combined to 45.09% of the IV(Table 6).
Population structure
Analysis of the population structure of 30 common tree and shrub species revealed three general patterns in the Bore-Anferara-Wadera forest (Fig. 6a-c). The first pattern was an inverted J-shaped distribution formed by species with high frequency distribution of individuals in the lower DBH classes, followed by a gradual decrease toward the higher DBH classes (Fig. 6a). This pattern of distribution was represented by P. adolfi-friederici, Dracaena afromontana, O. kenyensis, M. ferruginea and Celtis africana. The second pattern was a bell-shaped distribution formed by species where the frequency distribution of individuals in the lower and higher DBH classes was lower than the middle classes (Fig. 6b). Species such as S. guineense subsp. afromontanum, O. capensis subsp. macrocarpa, Macaranga capensis, A. abyssinicus, and Polyscias fulva were characterized by this distribution pattern in the forest.
In the third pattern, species had an irregular distribution over DBH classes, with a small number of individuals in some DBH classes and a large number in other classes(Fig. 6c). This pattern was represented by T. emetica,Prunus africana, Ficus sur, Podocarpus falcatus and Croton macrostachyus in the forest.
The distribution of seedlings,saplings,and mature trees/shrubs of the species in the forest was characterized by four patterns. The first type includes species represented by all three stages,the second type include species represented by mature trees/shrubs only, the third type contains species represented by either sapling and mature trees/shrubs or seedlings and mature trees/shrubs and the fourth type include species represented by either seedlings only or seedlings and saplings (Fig. 7a-f).

Table 4 Density, species number,and ratio of individuals to species in the lower, middle and upper storey of the Bore-Anferara-Wadera forest
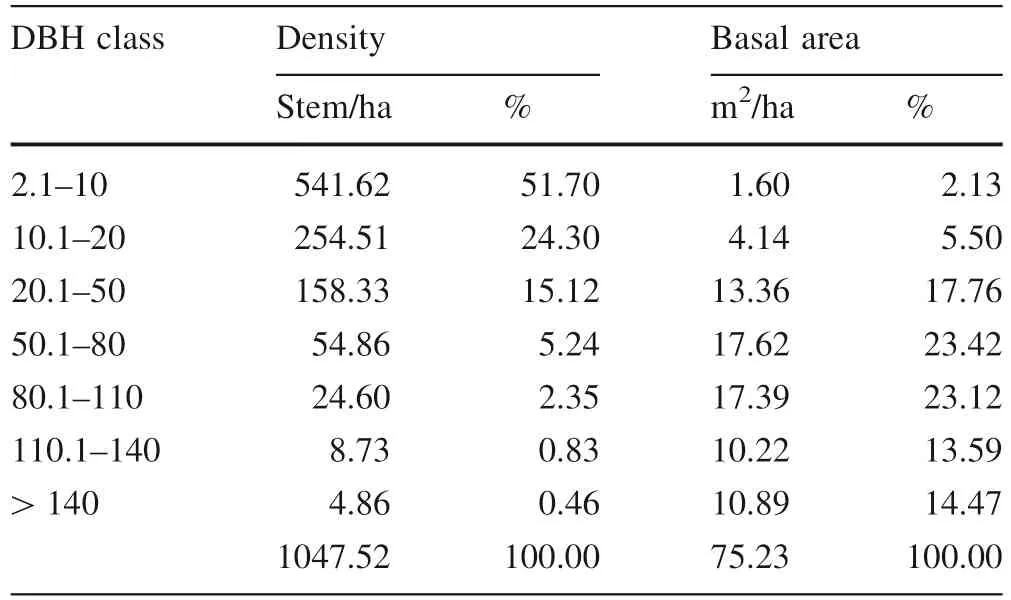
Table 5 Contribution of different DBH classes to the total density and basal area per hectare in the Bore-Anferara-Wadera forest
Regeneration status of the Bore-Anferara-Wadera forest
Seven species (10.61%) were not represented by seedlings or saplings (Fig. 7b), and only a small number of mature trees/shrubs were recorded for these species. On the other hand, two species (3.03%) had no seedlings (Fig. 7c), and three species(4.55%)had no saplings(Fig. 7d).Moreover,two species (3.03%) were not represented by saplings and mature trees/shrubs(Fig. 7e)and three species(4.55%)had no mature trees/shrubs (Fig. 7f). On the basis of these results, tree/shrub species were grouped into three priority classes for conservation: class 1 species, no seedlings or saplings;class 2 species,either no seedlings or no saplings;class 3 species,with no saplings and/or mature trees/shrubs(Table 7).
Discussion
Diversity patterns
The effects of human impacts on biodiversity can be measured based on indices of α and β diversity. The richness,diversity,and evenness of the three forest patches varied considerably.This difference is mainly a function of differences in site productivity, habitat heterogeneity and/or disturbance factors. For example, the low species richness and evenness in the Kilensoforest is due to anthropogenic disturbances, such as charcoal production,grazing, and logging, which has significantly reduced species richness. Lower evenness indicates the dominance of a few species. On the other hand, high evenness in the Danissa forest indicates little dominance by any single species but repeated coexistence of species overall plots or sites. Therefore, the implication of the evenness value is that,when evenness is high in a given forest,the location of conservation sites might not as important as in a forest with low evenness value. Several studies have highlighted the influence of human activities on species richness in the terrestrial ecosystems(Petraitis et al.1989;Maestre 2004).There is a strong relationship between disturbance and plant species richness.The low Shannon diversity index of the Kilensoforest also supports the hypothesis of a few dominant species early in succession and/or the dominance of few species due to selective cutting of preferred species(Bone et al. 1997).
β diversity is expected to be high in a fragmented tropical landscape due to the disparity of habitats (Shmida and Wilson 1985; Moreno and Halffter 2001). Therefore,habitat diversity can be recognized as the ultimate determinant of β diversity. In this study, the highest level of species turnover was found in the Kilenso and Danissa forests,reflecting the high degree of habitat diversity due to topographic and environmental variable gradients since the Danissa forest is relatively far away from Kilensoforest.Terborgh and Andresen (1998) and Pyke et al. (2001) also reported that environmental variables change with geographical distance, which leads tofloristic dissimilarity.The low value of β diversity between the Kilenso and Anferara forests might be associated with high habitat homogeneity because they are closer to each other.
Tree and shrub density
In the Bore-Anferara-Wadera forest, most plant species were represented by a small number of individuals distributed in different plots, a common pattern for most tropical forests(Valencia et al.1994).However,the Bore-Anferara-Wadera forest had a relatively high woody species density (1047 individuals ha-1) compared with other afromontane forests in Ethiopia(Burju et al.2013;Kebede et al.2014),while the tree density is lower than some of theMAF in southern and southwestern Ethiopia(Yeshitila and Bekele 2003; Hundera and Gadissa 2008), and the DAF in the northern and central parts of Ethiopia (Zegeye et al.2011). This difference could be attributed to variations in topographic gradients and habitat preferences of species forming the forest and the degree of anthropogenic disturbances (Whittaker et al. 2003).

Table 6 Importance value (IV) of trees and shrubs in the Bore-Anferara-Wadera forest
The ratio of the density of trees and shrubs with DBH >10 cm to DBH >20 cm in the Bore-Anferara-Wadera forest was higher than that of some other MAF(Ayalew et al. 2006; Hundera et al. 2007; Gurmessa et al.2012), indicating a higher predominance of small individuals in the Bore-Anferara-Wadera forest than in other forests in Ethiopia used for comparison. It is evident that the Bore-Anferara-Wadera forest has long been exposed to heavy anthropogenic disturbances as indicated by the absence of large individuals. Grubb et al. (1963) showed that a lower ratio of small to large individuals could be an indicator of a forest that developed with minimum anthropogenic disturbances,while a higher ratio indicates a predominance of small individuals that start to grow after excessive cutting or other anthropogenic cause.

Fig. 6 a-c Representative patterns of species population structures in Bore-Anferara-Wadera forest. DBH class: 1 = 2.1-10 cm,2 = 10.01-20 cm, 3 = 20.01-50 cm, 4 = 50.01-80 cm, 5 = 80.01-110 cm, 6 = 110.01-140 cm, 7 =>140 cm
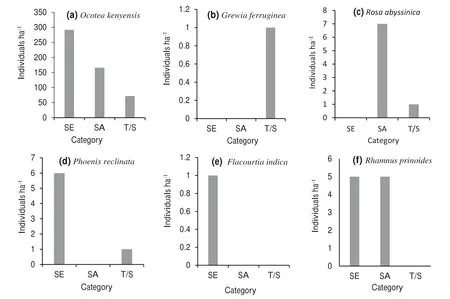
Fig. 7 a-f Number of individuals per hectare of seedlings(SE),saplings(SA)and trees/shrubs(T/S)of selected species in the Bore-Anferara-Wadera forest

Table 7 Classification of tree species in the different conservation priority classes in the Bore-Anferara-Wadera forest
DBH and height class distribution
The DBH class distributions indicate general trends in the population dynamics and recruitment processes of species in a given forest. The DBH class distribution for the trees and shrubs in the Bore-Anferara-Wadera forest had an inverted J-shape (Fig. 3), a general pattern for a normal population structure where most species have the most individuals in lower DBH classes with a gradual decrease in number as the DBH increases. This inverted J-shape across DBH classes also indicates an overall healthy,regenerating forest(Silvertown and Doust 1993).A similar pattern of population structure was reported in previous studies in both MAF and DAF of Ethiopia (e.g., Hundera et al. 2007; Yineger et al. 2008; Zegeye et al. 2011; Gurmessa et al. 2012; Burju et al. 2013).
The percentage distribution of trees in the Bore-Anferara-Wadera Forest in the DBH classes less than or equal to 50 cm was higher than in some other MAF and DAF in Ethiopia (Burju et al. 2013; Lulekal 2014), indicating a better distribution of trees in the lower DBH classes (1-3)in the Bore-Anferara-Wadera forest than in the forests compared.Hence, the result again confirms the dominance of small individuals in the Bore-Anferara-Wadera forest.The presence of a large number of small to medium-sized individuals and the relatively small proportion of large trees indicates that the Bore-Anferara-Wadera forest is in a stage of secondary development(Aye et al.2014).Height can be used as an indicator of the age of the forest. The inverted J-shape pattern in this forest showed a slight depression for the 18-23 m class, indicating selective cutting of individuals in that particular height class(Fig. 4). The decline in the number of individuals with increasing height class indicates the dominance of small individuals in the forest, a characteristic of a high rate of regeneration. For instance, about 54.96% of individuals were in the height class 3-8 m, 12.11% were 8-13 m tall,and only 3.03% reached a height of above 33 m.
Vertical structure
The highest proportion of species was concentrated in the lower storey, followed by the middle and upper storey,again confirming the dominance of small individuals and secondary development of the forest.However,the vertical structure of the forest is also influenced by the physical environment (Kent and Coker 1992) and microclimate(Grubb et al. 1963; Stoutjesdijk and Barkman 1992). The long exposure to anthropogenic factors must have contributed to the reduction of upper storey trees since most of these tree species such as Pouteria adolfi-friederici and Ocotea kenyensis are used for timber.
Basal area
The total basal area for the Bore-Anferara-Wadera forest is higher than the normal basal area for virgin tropical forests in Africa (Lamprecht 1989). According to Gentry(1988), the density of woody plants in the montane forests of Africa is relatively high compared to other tropical montane forests. Senbeta (2006)also reported higher basal area of the afromontane rainforests in Ethiopia than those reported for other tropical forests due to the high density of individuals in the forests studied. Comparison of the basal area of this forest with other afromontane forests in Ethiopia shows that it is higher than other forests in the MAF and DAF (Bekele 1993; Ayalew et al. 2006; Gurmessa et al. 2012), indicating that conservation measures,the degree of exposure to deforestation and geographical location contribute to the variation of the basal area of trees.
Measure of basal area provide a better measure of the relative dominance of woody species in a forest (Cain and Castro 1959). Here, S. guineense subsp. afromontanum contributed about 31%(23.73 m2/ha)of the total basal area of the forest, indicating that it is the most important tree species in the Bore-Anferara-Wadera forest. However, its predominance here and in other MAF in Ethiopia(Hundera and Gadissa 2008; Gurmessa et al. 2012)is due to the low market demand for its timber. A comparison of the contribution of the different DBH classes to the total basal area shows that more than 74% of the basal area was occupied by the upper DBH (above 50 cm), which contributed only 8.88%of the total individuals in the forest as a result of the presence of very few, large canopy trees.
Frequency
Frequency, that is, is the number of sampling plots with a given species in the study area, gives an indication of homogeneity and heterogeneity of the species (Lamprecht 1989). More species in lower frequency classes and fewer species in higher frequency classes indicate a high degree of heterogeneity, while fewer species in lower frequency classes and more in higher frequency classes indicate floristic homogeneity or similar species composition (Aye et al. 2014). The high percentage of species in the lower frequency classes than the higher frequency classes in the present study is thus evidence of floristic heterogeneity in the forest. The frequency of most woody species is generally low.Of the five most frequent woody species in this study, only one was dominant. Fewer occurrences of the dominant trees in Bore-Anferara-Wadera forest agrees with the assertion of Myers and Shelton (1980) that where there is a great difference in the life-form of species, the largest species is often dominant and rarely is the largest species also numerically the most common. According to Rey et al. (2000), a high frequency of a species always depends on factors that are related to habitat preferences,adaptation, the degree of exploitation and the availability of suitable conditions for regeneration.
Importance value
The importance value (IV), which reveals the ecological significance of species in a given ecosystem (Lamprecht 1989), combines data from three parameters, i.e., relative frequency, relative density and relative dominance (Kent and Coker 1992).Hence,many ecologists consider it as the most realistic aspect of a vegetation study (Curtis and McIntosh 1950). According to Muller-Dombois and Ellenberg (1974) and Curtis and McIntosh (1950), the relative ecological significance and/or dominance of a tree species in a forest ecosystem can best be identified using an IV analysis.
In the Bore-Anferara-Wadera forest, S. guineense subsp. afromontanum and P. adolfi-friederici, which had a higher basal area, also had a higher IV than the other species, suggesting that they are the most important tree species in the study area.
Population structure
The results obtained from the population structure analysis of woody species in the Bore-Anferara-Wadera forest showed three major representative patterns for the density distribution of trees and shrubs across the DBH classes.According to Popma et al. (1988), the patterns of species population structure can be interpreted as an indication of variation in population dynamics and regeneration status in the forest. The first pattern (Fig. 6a), represented by P.adolfi-friederici, indicates the presence of the highest density in the lower DBH classes with a gradual decrease in density toward the higher classes. This population pattern is an indicator of a stable population structure and healthy regeneration of the forest (Silvertown and Doust 1993;Senbeta 2006;Tesfaye et al.2010)and characteristic of a high reproductive potential and of shade-tolerant canopy trees that maintain a more or less constant rate of recruitment.There is a large probability that the death of an adult tree will be replaced by the growth of individuals from the smaller size classes and thus,plant population will be self-sustaining. Dracaena afromontana, O. kenyensis,and Psydrax schimperiana also demonstrate such an inverted J-shaped distribution, suggesting good reproduction and recruitment potential.
The second type(Fig. 6b)is represented by S.guineense subsp. afromontanum with a bell-shaped distribution pattern. It follows a Gaussian-type of distribution, having a low frequency in the first and second DBH classes, a gradual increase in the number of individuals in the middle classes,followed by a decrease in density toward the higher DBH classes. This pattern indicates poor species reproduction and recruitment(Bekele 1993;Senbeta et al.2007)and a decline in the number of large trees.Selective cutting of large individuals, mainly for timber and construction,could be the reason for the decline in their number.Senbeta(2006) also reported poor reproduction of S. guineense in the afromontane forests of Ethiopia either from most trees not producing seeds due to age or seed losses due to predation after reproduction because fruits serve as food for many animals and humans. O. capensis subsp. macrocarpa, M. capensis, and A. abyssinicus also showed this type of distribution pattern. Lulekal et al. (2008) and Gurmessa et al. (2012) reported similar results from different afromontane forests of Ethiopia.
The third type(Fig. 6c),represented here by T.emetica,is formed by species with an irregular distribution over DBH classes. Some DBH classes were poorly represented and others well represented,indicating selective removal of specific-sized individuals. Most tree species with an irregular size distribution are selectively hunted by the local people.For example,P.africana is selected for house construction at its medium size (personal communication with local people). Logging has also been extremely selective and mostly confined to a few highly valuable timber tree species in Ethiopia(Senbeta 2006)and likely a cause of distorted population structures in afromontane forests. In addition, livestock grazing and trampling (observed in the forest)of seedlings under the mother tree can cause such irregularities. Ficus sur, P. falcatus, and C.macrostachyus also show this population pattern. Similar results have been reported from different afromontane forests in Ethiopia(Hundera and Gadissa 2008;Didita et al.2010; Burju et al. 2013). Generally, the observed representative population structures of species in the Bore-Anferara-Wadera forest are indicators of the ultimate need of overall conservation activities, giving priorities to those species with poor reproduction and hampered recruitment.In addition,the absence of certain species at different DBH classes indicate selective cutting of preferred-sized individuals for various purposes by the local people and also requires attention.
Regeneration status of Bore-Anferara-Wadera forest
The regeneration status of tree/shrub species in any forest is determined by recruitment of saplings and seedlings(Dhar et al. 1997; Samant et al. 2002). In the Bore-Anferara-Wadera forest,the density of seedlings and saplings varied greatly. Species listed under priority class 1 are represented by few individuals of mature trees/shrubs,suggesting that these species might be on the verge of local extinction because of the lack seedlings and saplings to replace the adults.The major reasons for poor or hampered regeneration include unfavorable environmental factors such as rocky land and poorly developed soil, seed predation, human disturbance particularly livestock grazing and trampling and ability of a species to reproduce in the forest.On the contrary,prevention of livestock grazing and overexploitation could improve the regeneration status of woody species. Other species listed under priority class 2 were represented by seedlings and/or saplings and very few mature plants, indicating the possibility of replacement of mature plants in the future and hence show relatively better regeneration status. Similar findings were reported in different afromontane forests of Ethiopia (Shibru and Balcha 2004; Denu 2006; Kelbessa and Soromessa 2008). With regard to conservation priority, we suggest that priority should be given to the forests containing naturally and locally threatened species, especially to species with no seedlings and saplings (priority class 1) to save them from local extinction. Appropriate management practices also need to be designed for species in priority classes 2 and 3.
Conclusion
The Bore-Anferara-Wadera forest is a remnant MAF in southern Ethiopia. The Anferara forest is also one of the Eastern Afromontane Key Biodiversity Areas, which harbors vulnerable species of animals and plants. However,the forest has been severely deforested because it has not received adequate attention from appropriate governmental institutions, stakeholders or surrounding communities. In addition, the role of forests in moderating climate change and the need for preserving biodiversity have not been appreciated.
The overall diversity and evenness values of the Bore-Anferara-Wadera forest were 3.84 and 0.78, respectively.However, the three forest patches within the forest varied considerably. Analysis of the vegetation structure of most common tree and shrub species has shown the predominance of small individuals in the lower DBH and lower height classes, indicating good reproductive potential but excessive exploitation of larger trees. As a result, the developing secondary forest has a preponderance of lesspreferred trees species, which contributed to the high total basal area of the Bore-Anferara-Wadera forest(75.23 m2ha-1). The population structure of representative tree and shrub species also corroborates this selective removal of the preferred sized individuals. Moreover,assessment of the regeneration status of the forest revealed that many species lack seedlings and saplings and are represented by a few mature plants, indicating that these species are vulnerable to local extinction.
Every species in the forest has its specific role in the ecosystem as a result of the evolutionary processes that brought them into being. In general, the diverse attributes manifested by the formation of the forest into vertical structure, frequency of species, population structure, the different importance value indices, the regeneration status of the species, basal area and height class of species have allowed the system to undergo spontaneous self-organization with the potential to develop into a tall forest with richer biodiversity.This complex self-organizing system is an adaptive process that results in a quantitatively and qualitatively different form than the original undisturbed forest.It will stay in a lower state until better opportunities prevail. Heightened protection of the forest and active replacement of the lost species will help the forest to realize its original ecosystem functions and services and regain its ecological position through collaborative actions of the government, communities, and stakeholders.
AcknowledgementsWe are very grateful for financial assistance for this research from the Graduate Programs of Addis Ababa University.The first author is also very grateful to Debre Birhan University for sponsoring this study and for providing additional financial support.
杂志排行
Journal of Forestry Research的其它文章
- Do increasing respiratory costs explain the decline with age of forest growth rate?
- At what carbon price forest cutting should stop
- Mapping the risk of winter storm damage using GIS-based fuzzy logic
- Effects of seed moisture content, stratification and sowing date on the germination of Corylus avellana seeds
- Comparison of seed morphology of two ginkgo cultivars
- De novo assembly of the seed transcriptome and search for potential EST-SSR markers for an endangered, economically important tree species: Elaeagnus mollis Diels
